Abstract
The metabolic activity of organisms can be measured by recording the heat output using microcalorimetry. In this paper, the total alkaloids in the traditional Chinese medicine Radix Aconiti Lateralis were extracted and applied to Escherichia coli and Staphylococcus aureus. The effect of alkaloids on bacteria growth was studied by microcalorimetry. The power-time curves were plotted with a thermal activity monitor (TAM) air isothermal microcalorimeter and parameters such as growth rate constant (μ), peak-time (Tm), inhibitory ratio (I), and enhancement ratio (E) were calculated. The relationships between the concentration of Aconitum alkaloids and μ of E. coli or S. aureus were discussed. The results showed that Aconitum alkaloids had little effect on E. coli and had a potentially inhibitory effect on the growth of S. aureus.
Keywords: Aconitum alkaloids, Escherichia coli, Staphylococcus aureus, Microcalorimetry
1. Introduction
Radix Aconiti Lateralis (RAL) (also known as monkshood or aconite), which is the daughter root of Aconitum carmichaeli Debx. (Ranunculaceae), is one of the most useful herbal medicines. It has been reported that RAL Preparata have some pharmacological activities for the treatment of heart failure, congestion, colds, neuralgia, polyarthralgia, rheumatism, and gout (Wadsö, 2002; Zhao et al., 2006; Chen et al., 2008). Up until now, the most investigated pharmaceutical ingredients in RAL Preparata were a series of alkaloids, which are also found to be the major toxic ingredients in the medicine. Inappropriate use of RAL Preparata could result in unwarranted toxicity (Ameri, 1998; Singhuber et al., 2009; Wang et al., 2009). However, there are few studies on the effect of alkaloids on bacteria. Therefore, the aim of this study is to investigate the effect of alkaloids extracted from RAL Preparata on bacteria.
In recent years, microcalorimetric techniques have been widely applied in biochemistry, biophysics and other fields (Wadsö, 1995; 1997; 2001). These techniques have also increasingly been used to study the interactions between drugs and microorganisms. Microcalorimetry is a highly sensitive, quantitative, and versatile tool for assessing the activity of microorganisms. Further advantages of this method are that it is simple and non-invasive vis-a-vis the sample. As a result, the power-time curves of bacteria can be continuously plotted in real time (Critter et al., 2001; Yan et al., 2007). It can provide abundant thermodynamic and kinetic information, such as the growth rate constant (μ), inhibitory ratio (I), generation time (T), minimal inhibitory concentration (MIC), peak-height (Hm), peak-time (Tm), and total heat (Qtot). Microcalorimetry therefore appears to be a suitable technique for studying microbial activity (Xi et al., 2002; Wu et al., 2005).
In this paper, we investigate the antimicrobial activity of alkaloids extracted from RAL Preparata on Escherichia coli and Staphylococcus aureus growth using microcalorimetry. Aconitum alkaloids were obtained using the water extraction method and determined with aconitine reference substance by ultra-violet (UV). The metabolic power-time curves for E. coli and S. aureus growth in different concentrations of extractions were obtained and compared with bacterial growth curves without the addition of samples. The relationships between the thermokinetic parameters of E. coli growth and sample concentrations were discussed, and the correlations between the antimicrobial effects were determined.
2. Materials and methods
2.1. Instrumentation
A thermal activity monitor (TAM) air device (Thermometric AB, Sweden), an 8-channel heat-conduction calorimeter for heat-flow measurements in isothermal conditions, was used to assess the metabolism of E. coli and S. aureus. The device was equipped with eight twin calorimetric channels with one side for the sample and the other for a static reference. Measurements were made in sealed 24-ml glass ampoules between 5 °C and 90 °C. The error in the temperature measurements was ±0.02 °C. The detection limit was 2 µW and the baseline stability was 2×106 µW over a period of 24 h (Kong et al., 2008). The heat output was recorded continuously using a PLW32 recorder in real time.
2.2. Materials and methods
The bacteria E. coli (CMCC(B)44102) and S. aureus (CMCC(B)26003) were provided by the Shandong Institute for Drug Control, China. They were routinely cultured in a Luria-Bertani (LB) culture medium, which contained 10 g of peptone, 5 g of yeast extract, and 5 g of NaCl per liter (pH 7.0–7.2). The LB culture medium was sterilized by autoclaving at 121 °C for 20 min. The Aconitum alkaloids were provided by Jiangyou Zhongba Ltd. (Sichuan, China).
The purified Aconitum alkaloid test samples were prepared according to the following procedure. The powdered commercial RAL Preparata (10.0 g) was extracted at boiling conditions with 180 and 90 ml of water. The combined filtrates were concentrated to approximately 120 ml by rotary vacuum evaporation. The concentrated filtrate was then fractionated overnight with 60% ethanol at 4 °C. The supernatant was concentrated and extracted with chloroform five times to obtain the Aconitum alkaloids, which were then dried under vacuum. The average yield of Aconitum alkaloids was determined by UV spectroscopy at 490 nm. According to the standard curve established by the aconitine reference substance (A=0.0181c+0.1353, r=0.9989, where A is the absorbance and c is the concentration of aconitine reference substance), the content of Aconitum alkaloids in RAL Preparata was 81.2%.
The microcalorimetric measurements were made with the ampoule method. The LB culture medium containing the bacteria was placed in 20-ml glass ampoules. Then, different concentrations of Aconitum alkaloids were mixed into each ampoule to a final volume of 10 ml. One ampoule without Aconitum alkaloids was used as the blank control. The ampoules were then sealed and placed in the microcalorimeter. The temperature was maintained at 37 °C. The power-time signals were recorded every minute until the recordings returned to the baseline measurement (Yang et al., 2008).
3. Results and discussion
3.1. Power-time curves
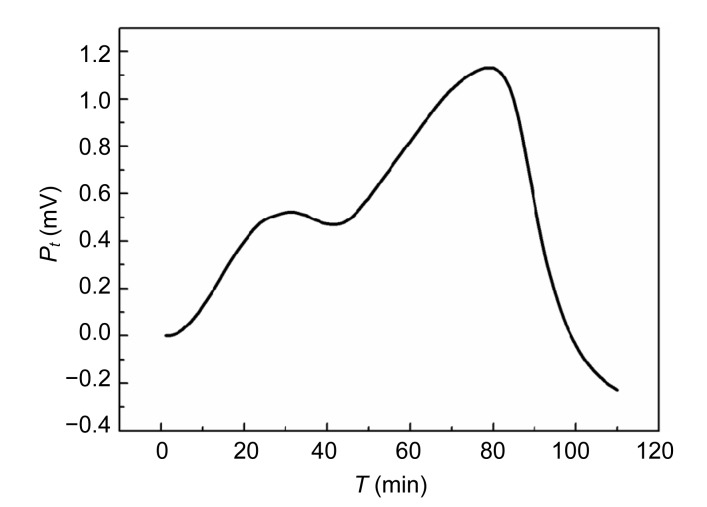
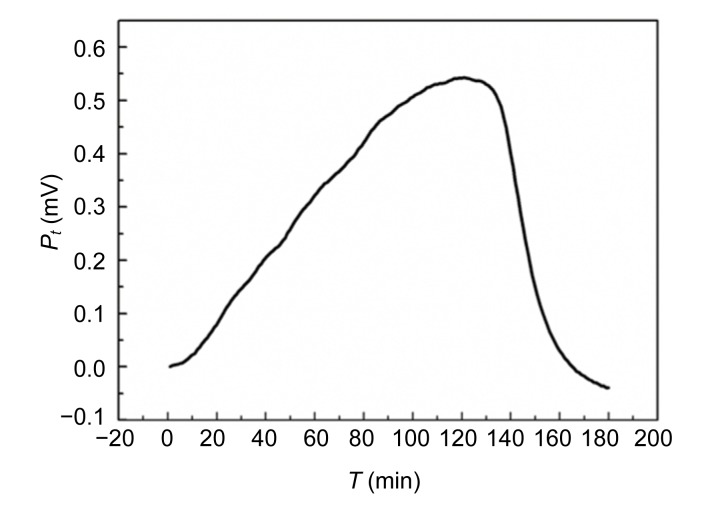
The power-time curves of the growth of E. coli and S. aureus without Aconitum alkaloids are shown in Figs. 1 and 2. The shapes of the two curves are different. The E. coli growth curves can be divided into four stages: the first growth, first decrease in metabolic activity, second growth, and second decrease in metabolic activity stages. The output power was stronger than that of S. aureus in the first 30 min. The power-time curves of the S. aureus growth process were comparatively simple with only an increased growth stage and a decreased metabolic stage. In the exponential phase (the first rapid growth stage), the bacteria were in a good environment with sufficient nutrients and limited metabolic products. The shapes of the power-time curves made it possible to study the effect of drugs on the bacteria. The exponential growth phases of E. coli and S. aureus with the addition of different concentrations of Aconitum alkaloids are shown in Figs. 3 and 4, respectively. In the exponential growth phase, S. aureus had a distinct downward trend with increases in the Aconitum alkaloid concentrations, and a high concentration of samples needed more time to reach the same heat output. However, a concentration-dependent delay was not clearly observed in the exponential growth phases of E. coli with the Aconitum alkaloid samples.
Fig. 1.
Power-time curve of E. coli without Aconitum alkaloids
Fig. 2.
Power-time curve of S. aureus without Aconitum alkaloids
Fig. 3.
Exponential phase of E. coli with different concentrations of Aconitum alkaloids
Fig. 4.
Exponential phase of S. aureus with different concentrations of Aconitum alkaloids
3.2. Thermokinetics
In the exponential growth phase, the power-time curves for E. coli and S. aureus obey the following law:
| dNt/dt=μNt−βNt2, | (1) |
where Nt is the bacterial number at time t, μ is the growth rate constant, and β is the bacterial static-rate constant. The integral of Eq. (1) is
| Nt=K/(1+αe−μt), | (2) |
where K is the maximum density and α is integral constant. If the power produced by every bacterium is P, then PNt=KP/(1+αe−μt), making Pt=PNt and Pm=PK (Pt is the power output at time t and Pm is the maximum power output). This leads to the following relationship:
| Pt=Pm/(1+αe−μt). | (3) |
Eq. (3) is the logistic equation. If the values of Pt and t can be obtained from the bacterial growth curves, the rate constant μ can then be calculated.
The inhibition ratio, I, is an excellent index of the inhibition of different Glycyrrhiza extracts on the metabolism of E. coli. The inhibition ration can be defined as:
| I=(μ0−μc)/μ0×100%. | (4) |
The enhancement ratio, E, is an excellent index of the inhibition of Aconitum alkaloids on E. coli and S. aureus, and can be defined as:
| E=(μc−μ0)/μ0×100%, | (5) |
where μ0 and μc are the growth rate constants of the bacteria without and with Aconitum alkaloids, respectively.
The corresponding values of μ, Tm, Pm, I, E and the calculated results are shown in Table 1.
Table 1.
Parameters of E. coli and S. aureus growth at different concentrations of Aconitum alkaloids
| Bacteria | c (mg/ml) | μ (min−1) | r | Tm (min) | Pm (mW) | I (%) | E (%) |
| E. coli | 0 | 0.3522 | 0.9975 | 77 | 1.1346 | ||
| 2.0 | 0.3599 | 0.9973 | 78 | 1.1187 | 2.19 | ||
| 4.0 | 0.3667 | 0.9980 | 76 | 1.1507 | 4.12 | ||
| 6.0 | 0.3623 | 0.9976 | 75 | 1.1032 | 2.87 | ||
| 10.0 | 0.3557 | 0.9972 | 77 | 1.1195 | 0.99 | ||
| 12.0 | 0.3474 | 0.9973 | 78 | 1.1032 | 1.36 | ||
| S. aureus | 0 | 0.2455 | 0.9990 | 122 | 0.5414 | ||
| 2.0 | 0.2604 | 0.9979 | 129 | 0.5045 | 5.50 | 6.07 | |
| 4.0 | 0.2526 | 0.9991 | 145 | 0.5467 | 16.29 | 2.89 | |
| 8.0 | 0.1876 | 0.9988 | 154 | 0.5488 | 22.71 | ||
| 10.0 | 0.1675 | 0.9973 | 158 | 0.5411 | 29.80 | ||
| 12.0 | 0.1577 | 0.9978 | 163 | 0.5321 | 34.68 |
c: concentration; μ: growth rate constant; r: correlation coefficient; Tm: peak-time, Pm: maximum heat output; I: inhibitory ratio; E: enhancement ratio
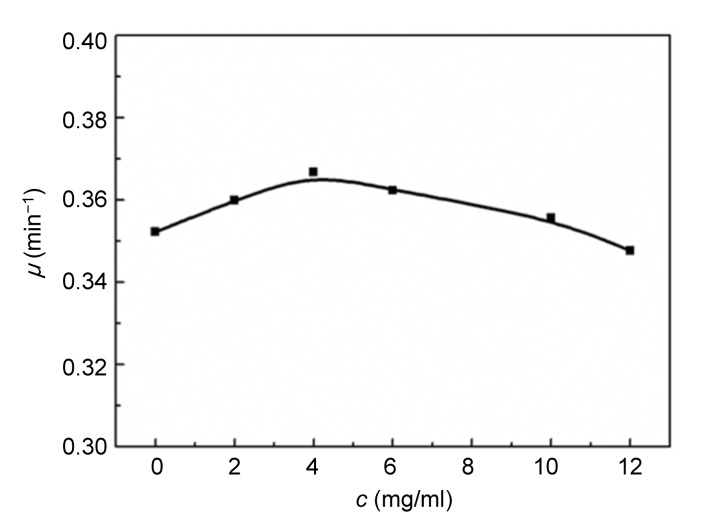
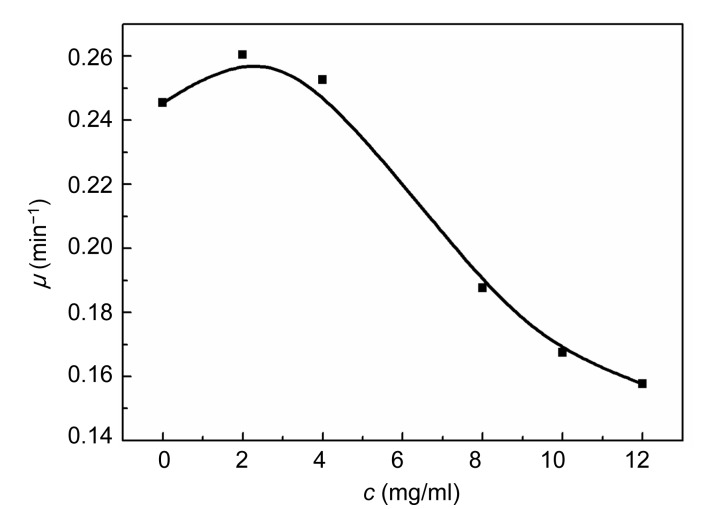
3.3. Relationships between growth rate constant (μ) and concentration (c)
The relationships between μ and c are demonstrated in Figs. 5 and 6. The rate constant, μ, of E. coli changed slightly with an increase in the concentration of Aconitum alkaloids. It could be inferred that Aconitum alkaloids had little influence on E. coli growth. Fig. 6 shows that μ for S. aureus growth increased with a low concentration of Aconitum alkaloids and decreased as the concentration was increased. This reveals that Aconitum alkaloids promoted S. aureus growth at low concentrations and inhibited growth at higher concentrations. When the concentration of Aconitum alkaloids was between 0 and 12 mg/ml, the relationship between μ and c was
| μ=0.0002c3−0.0050c2+0.0173c+0.2452 (r=0.9990). | (6) |
Fig. 5.
Relationship between growth rate constant (μ) and concentration (c) of Aconitum alkaloids for E. coli
Fig. 6.
Relationship between growth rate constant (μ) and concentration (c) of Aconitum alkaloids for S. aureus
According to Eqs. (4) and (5), the inhibitory (I) and enhancement (E) ratios were calculated, as shown in Table 1. From the data, we concluded that Aconitum alkaloids had a minimal effect on E. coli growth and a potent antibacterial activity against S. aureus.
3.4. Relationships between peak-time (Tm) and concentration (c)
As seen in Table 1, Aconitum alkaloids had no effect on the first Tm of E. coli growth, while an increasing trend was observed in the values of Tm for S. aureus growth as c was increased. This indicates that Aconitum alkaloids had a concentration-dependent inhibitory effect on S. aureus.
4. Conclusions
In this research, the microcalorimetric method was used to study the effect of different concentrations of Aconitum alkaloids on the growth of E. coli and S. aureus. By recording the heat output, the metabolic activity of the bacteria was evaluated, and the effect of extracts was investigated. By the kinetic and thermodynamic information from the microcalorimetric method, a series of kinetic parameters, such as the rate constant μ, peak-time Tm, maximum power output Pm, inhibitory ratio I, and enhancement ratio E, were obtained. We also discussed the relationship between the growth rate constant (μ) and the concentration of Aconitum alkaloids (c). The rate constant of E. coli growth showed little change with increase in the concentration of Aconitum alkaloids. However, the growth rate constant of S. aureus increased and then decreased as the concentration of Aconitum alkaloids was increased. The increased concentration of Aconitum alkaloids changed the Tm of E. coli slightly, but the Tm of S. aureus was prolonged compared with the control. This indicated that the Aconitum alkaloid treatment slowed the growth and metabolism of S. aureus. We could draw the conclusion that Aconitum alkaloids had no effect on the growth of E. coli but a potential inhibitory effect on S. aureus.
This work has shown that microcalorimetry is a useful technique, which can be applied to the study of microbial growth. It also offered useful information for research and the application of the traditional herbal medicine RAL and its active ingredients.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 21203112) and the Natural Science Foundation of Shandong Province (No. ZR2012BQ002), China
Compliance with ethics guidelines: Yan-bin SHI, Lian LIU, Wei SHAO, Ting WEI, and Gui-mei LIN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ameri A. The effects of Aconitum alkaloids on the central nervous system. Prog Neurobiol. 1998;56(2):211–235. doi: 10.1016/S0301-0082(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen JH, Lee CY, Liau BC, et al. Determination of aconitine-type alkaloids as markers in fuzi (Aconitum carmichaeli) by LC/(+)ESI/MS3 . J Pharm Biomed Anal. 2008;48(4):1105–1111. doi: 10.1016/j.jpba.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Critter SAM, Freitas SS, Airoldi C. Calorimetry versus respirometry for the monitoring of microbial activity in a tropical soil. Appl Soil Ecol. 2001;18(3):217–227. doi: 10.1016/S0929-1393(01)00166-4. [DOI] [Google Scholar]
- 4.Kong W, Zhao Y, Shan L, et al. Microcalorimetric studies of the action on four organic acids in radix isatidis on the growth of microorganisms. Chin J Biotechnol. 2008;24(4):646–650. doi: 10.1016/S1872-2075(08)60033-3. [DOI] [PubMed] [Google Scholar]
- 5.Singhuber J, Zhu M, Prinz S, et al. Aconitum in Traditional Chinese Medicine–a valuable drug or an unpredictable risk? J Ethnopharmacol. 2009;126(1):18–30. doi: 10.1016/j.jep.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Wadsö I. Microcalorimetric techniques for characterization of living cellular systems. Will there be any important practical applications? Thermochim Acta. 1995;269-270:337–350. doi: 10.1016/0040-6031(95)02673-8. [DOI] [Google Scholar]
- 7.Wadsö I. Isothermal microcalorimetry near ambient temperature: an overview and discussion. Thermochim Acta. 1997;294(1):1–11. doi: 10.1016/S0040-6031(96)03136-X. [DOI] [Google Scholar]
- 8.Wadsö I. Isothermal microcalorimetry in applied biology. Thermochim Acta. 2002;394(1-2):305–311. doi: 10.1016/S0040-6031(02)00263-0. [DOI] [Google Scholar]
- 9.Wadsö L. Isothermal microcalorimetry. Current problems and prospects. J Therm Anal Calorim. 2001;64(1):75–84. doi: 10.1023/A:1011576710913. [DOI] [Google Scholar]
- 10.Wang J, van der Heijden R, Spijksma G, et al. Alkaloid profiling of the Chinese herbal medicine Fuzi by combination of matrix-assisted laser desorption ionization mass spectrometry with liquid chromatography–mass spectrometry. J Chromatogr A. 2009;1216(11):2169–2178. doi: 10.1016/j.chroma.2008.11.077. [DOI] [PubMed] [Google Scholar]
- 11.Wu YW, Gao WY, Xiao XH, et al. Calorimetric investigation of the effect of hydroxyanthraquinones in Rheum officinale Baill on Staphylococcus aureus growth. Thermochim Acta. 2005;429(2):167–170. doi: 10.1016/j.tca.2005.03.008. [DOI] [Google Scholar]
- 12.Xi L, Yi L, Jun W, et al. Microcalorimetric study of Staphylococcus aureus growth affected by selenium compounds. Thermochim Acta. 2002;387(1):57–61. doi: 10.1016/S0040-6031(01)00825-5. [DOI] [Google Scholar]
- 13.Yan D, Jin C, Xiao X, et al. Investigation of the effect of berberine alkaloids in Coptis chinensis Franch on Bacillus shigae growth by microcalorimetry. Sci China Ser B. 2007;50(5):638–642. doi: 10.1007/s11426-007-0094-9. [DOI] [Google Scholar]
- 14.Yang L, Xu F, Sun L, et al. Microcalorimetric studies on the antimicrobial actions of different cephalosporins. J Therm Anal Calorim. 2008;93(2):417–421. doi: 10.1007/s10973-007-8680-9. [DOI] [Google Scholar]
- 15.Zhao C, Li M, Luo Y, et al. Isolation and structural characterization of an immunostimulating polysaccharide from fuzi, Aconitum carmichaeli . Carbohyd Res. 2006;341(4):485–491. doi: 10.1016/j.carres.2005.11.032. [DOI] [PubMed] [Google Scholar]