Introduction
Onychomycosis constitutes frequent fungal infections of the nails. It is commonly seen in dermatological practice worldwide. The clinical picture though variable is characterized by alterations in the nail architecture such as changes in color, thickness, onycholysis and onychodystrophy.1 In most cases they are caused by filamentous fungi like dermatophytes or yeasts of the genus Candida.2 However, in a small fraction of cases, the etiological agent comprises of non-dermatophyte molds, belonging to several genera and species.1,3
The prevalence of non-dermatophytic onychomycosis varies widely, depending on the geographical location or the climate, but is more frequent in hot and humid tropical areas.3 They are often considered contaminants or secondary pathogens that invade nails previously damaged by trauma or disease. However, in some cases they actually act as primary pathogens to humans, as has been seen for Nattrassia mangiferae, otherwise better known as a plant pathogen. The objective of this case report is to present a rare case of onychomycosis caused by N. mangiferae in a diabetic adult residing in Mumbai, Maharashtra.
Case Report
A 55-year-old diabetic patient, presented with blackish discoloration of the toe nails bilaterally of three years duration. His diabetic state had been under control on oral hypoglycemic drugs. On clinical examination, he was found to have onycholysis and onychodystrophy, with no apparent thickening of ungual bed. The microscopic examination of nail clipping after treatment with 40% KOH revealed the presence of septate hyaline hyphae. The material was cultured in Sabouraud's dextrose agar (SDA) slant tubes with and without cycloheximide and cornmeal agar. SDA without cycloheximide grew woolly brown colonies after about 7 days of incubation at room temperature. There was no growth on SDA with cycloheximide. Greyish black colonies grew on cornmeal agar. Lactophenol cotton blue (LCB) mount showed numerous cylindrical or globose, one-to-two-celled barrel-shaped arthroconidia with dark walls (Fig. 1 and Fig. 2). The fungus was identified as N. mangiferae based on the cultural characteristics and the arrangement of arthroconidia on LCB mount. The isolate was found to be sensitive to amphotericin B and itraconazole. The patient was placed on oral itraconazole for 12 weeks and assessed subsequently. The patient exhibited significant improvement. Itraconazole was continued for further 12 weeks, after which the lesions showed complete therapeutic resolution.
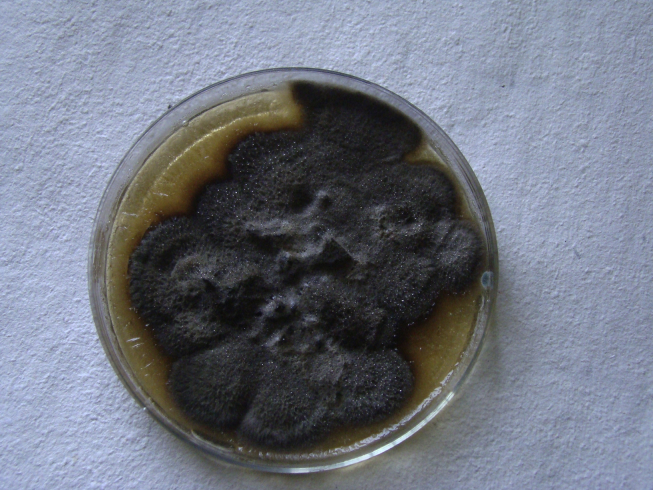
Fig. 1.
Growth on cornmeal agar showing typical brownish-black colonies.
Fig. 2.
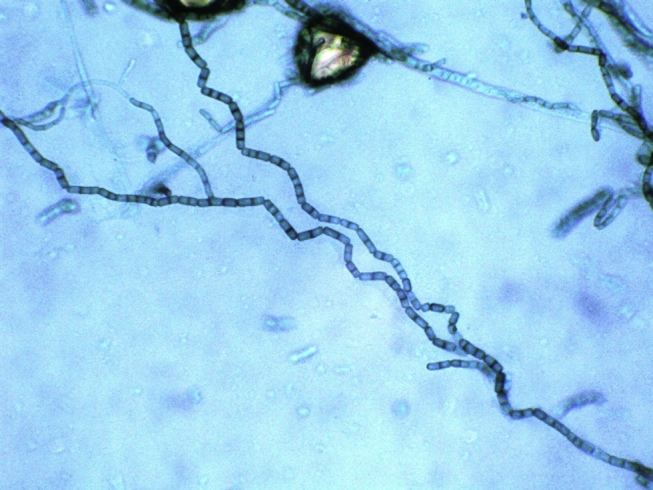
Lactophenol cotton blue mount showing chains of one-to-two-celled darkly pigmented arthroconidia.
Discussion
N. mangiferae, formerly known as Hendersonula toruloidea is a phaeoid coelomycete described by Nattrass in 1933.4 It is a well recognized plant pathogen of a very wide variety of trees. The role of N. mangiferae as etiological agent of onychomycosis has been known since 1970, when Gentles & Evans isolated it from cases of dermatomycoses and onychomycosis.1 They named the isolated fungus as H. toruloidea. Later, Campbell & Mulder reported a patient with identical disease to which they named the etiological agent Scytalidium hyalinum.1,5 Afterward, genetic studies showed that the two species were phylogenetically very close to each other and the name Scytalidium, comprising the species S. hyalinum and Scytalidium dimidiatum were then considered the most appropriate taxonomic designation.1 Lacaz et al reported two cases of onychomycosis, one of them in an HIV positive patient, caused by S. dimidiatum. They also made a taxonomic review of this specie, presenting it as a synanamorph of N. mangiferae.6
Subsequently, several authors reported that in addition to superficial infections, N. mangiferae and its synanamorph S. dimidiatum could also cause subcutaneous abscesses, fungemia, abdominal abscess, endophthalmitis and maxillary sinusitis.4
Case reports describing N. mangiferae as a cause for cutaneous mycosis have been published in Brazil.1 Two such cases of onychomycosis have been described in green tea leaf pluckers from North-eastern India by Barua et al.7
From the clinical point of view, the lesions caused by N. mangiferae are often similar to those caused by dermatophytes. The affected nails display dystrophy and onycholysis, as it was also observed by us in this case report. Microscopy of nail clipping exhibits long, sinuous hyphae of irregular diameter and less refractile than that of dermatophyte hyphae. Occasionally, the hyphae fragment into structures of different sizes to form globose arthroconidia. They may form pycnidia on longer incubation.
Baban et al8 and Liony et al9 in their studies reiterate that superficial infections caused by N. mangiferae are often misdiagnosed. The presence of hyaline mycelium in direct microscopic examination, the poor confirmation of laboratory findings, the use of inappropriate culture media for the development of this fungus and the lack of pathognomonic characteristics of the lesions contribute to this fact.4 The laboratory diagnosis of N. mangiferae infections, as in other non-dermatophyte mold infections must be confirmed by the repetition of examination with a new collected specimen.1 This fungus being sensitive to cycloheximide, grows very poorly or does not grow at all in the media routinely used for the isolation of dermatophytes in cutaneous specimens.
The treatment of N. mangiferae infection is generally ineffective as the fungus does not respond well to antifungals traditionally intended for onychomycosis, although infection remission after the use of itraconazole has been observed for some patients. Similarly, in our case, the lesions of the patient, after a course of itraconazole for about 24 weeks were completely cured.
On detailed search for similar references from India, we could cite only one case report of onychomycosis caused by N. mangiferae.7 The other case reported was N. mangiferae causing fungal keratitis by Kindo et al.10 Our case thus highlights the rarity of isolation of such fungus and also the importance of recognizing dematiaceous fungi as agents of onychomycosis using appropriate fungal culture techniques.
Conflicts of interest
All authors have none to declare.
Acknowledgments
We acknowledge the technical support rendered by Sub BD Rao, Laboratory Technician, Mycology section, Dept of Microbiology, AFMC, Pune.
References
- 1.Pontarelli L.N., Hasse J., Galindo C.D.C., Coelho M.P.P., Nappi B.P., Santos J.I.D. Onychomycosis by Scytalidium dimidiatum: report of two cases in Santa Catarina, Brazil. Rev Inst Med Trop Sao Paulo. 2005;47(6):351–353. doi: 10.1590/s0036-46652005000600008. [DOI] [PubMed] [Google Scholar]
- 2.Maleszka R., Adamski Z. Clinical and diagnostic aspects of dermatophyte onychomycosis. Mycoses. 1998;41:67–72. doi: 10.1111/j.1439-0507.1998.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 3.Ellis D.H., Watson A.B., Marley J.E., Williams T.G. Non-dermatophytes in onychomycosis of the toe nails. Br J Dermatol. 1997;136:490–493. [PubMed] [Google Scholar]
- 4.Godoy P., Reyes E., Silva V. Dermatomycoses caused by Nattrassia mangiferae in Sao Paulo, Brazil. Mycopathologica. 2004;157:273–276. doi: 10.1023/b:myco.0000024176.74949.ce. [DOI] [PubMed] [Google Scholar]
- 5.Campbell C.K., Mulder J.L. Skin and nail infection by Scytalidium dimidiatum sp nov. Sabouraudia. 1977;15:161–166. [PubMed] [Google Scholar]
- 6.Lacaz C.D.S., Pereira A.D., Heins-Vaccari E.M. Onychomycosis caused by Scytalidium dimidiatum. Report of two cases. Review of the taxonomy of the synanamorph and anamorph forms of this coelomycete. Rev Inst Med Trop Sao Paulo. 1999;41(5):319–323. [PubMed] [Google Scholar]
- 7.Barua P., Barua S., Borkakoty B., Mahanta J. Onychomycosis by Scytalidium dimidiatum in green tea leaf pluckers: report of two cases. Mycopathologia. 2007;164(4):193–195. doi: 10.1007/s11046-007-9024-9. [DOI] [PubMed] [Google Scholar]
- 8.Baban B., Choolaei A., Emami M., Shidfar M., Rezaei S. The first survey of Hendersonula toruloidea as a human pathogen in Iran. J Int Med Res. 1995;23:123–125. doi: 10.1177/030006059502300206. [DOI] [PubMed] [Google Scholar]
- 9.Liony C., Joly P., Balguerie X., Fusade T., Lauret P. Cutaneous and nail infections caused by Hendersonula toruloidea. [Article in French]. Ann Dermatol Venereol. 1993;120:226–228. [PubMed] [Google Scholar]
- 10.Kindo A.J., Anita S., Kalpana S. Nattrassia mangiferae causing fungal keratitis. Indian J Med Microbiol. 2010;28:178–181. doi: 10.4103/0255-0857.62504. [DOI] [PubMed] [Google Scholar]