Summary
The cellular basis of age-related tissue deterioration remains largely obscure. The ability to activate compensatory mechanisms in response to environmental stress is an important factor for survival and maintenance of cellular functions. Autophagy is activated both under short and prolonged stress and is required to clear the cell of dysfunctional organelles and altered proteins. We report that specific autophagy inhibition in muscle has a major impact on neuromuscular synaptic function and, consequently, on muscle strength, ultimately affecting the lifespan of animals. Inhibition of autophagy also exacerbates aging phenotypes in muscle, such as mitochondrial dysfunction, oxidative stress, and profound weakness. Mitochondrial dysfunction and oxidative stress directly affect acto-myosin interaction and force generation but show a limited effect on stability of neuromuscular synapses. These results demonstrate that age-related deterioration of synaptic structure and function is exacerbated by defective autophagy.
Graphical Abstract
Highlights
-
•
Autophagy in muscle decreases with age, and rescue prevents muscle loss
-
•
Autophagy inhibition in muscles shortens animal lifespan
-
•
Autophagy is required to maintain a normal muscle-nerve interaction
-
•
Autophagy impairment induces oxidation of contractile proteins, causing weakness
The cellular basis of age-related tissue deterioration remains largely obscure. Autophagy is often activated under stress conditions and declines with age. Carnio et al. now report that specific autophagy inhibition in muscle has a major impact on neuromuscular synaptic function and consequently on muscle strength, ultimately affecting the lifespan of animals. Autophagy inhibition also exacerbates several aging phenotypes, confirming that autophagy is required for healthy aging.
Introduction
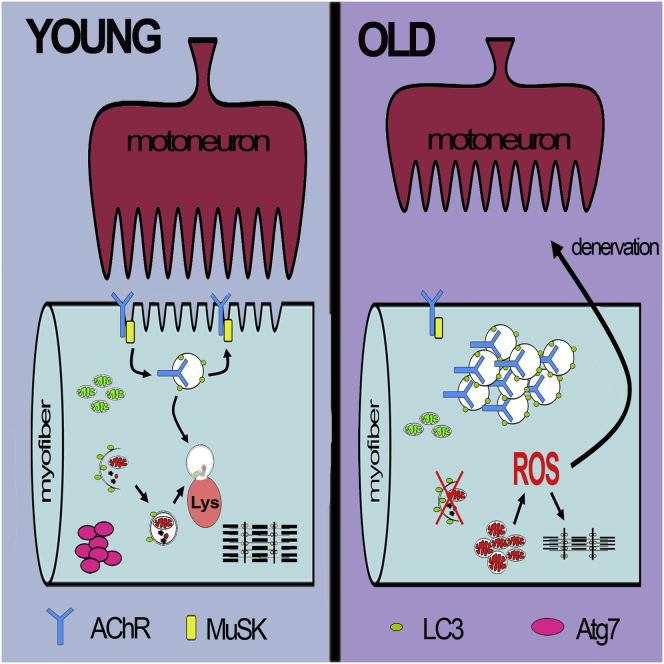
Aging is accompanied by numerous tissue phenotypes. Changes that occur in postmitotic organs are considered hallmarks of aging. A common feature of aged organisms is functional alteration and gradual loss of muscle mass and force. A deficit of muscle innervation due to motor-neuron loss is believed to have a major contribution to age-dependent muscle wasting, also called sarcopenia. This age-acquired deficit in muscles contributes profoundly to reduced quality of life in elderly people and predisposes them to an increased risk of morbidity, disability, and mortality (Visser and Schaap, 2011). The etiology of sarcopenia is multifaceted and involves several intrinsic and extrinsic factors. Despite the clinical, social, and economic relevance of sarcopenia, the precise mechanisms for the age-related loss of muscle mass and function are not yet fully understood. Age-related changes in muscle are complex, with key features including myofiber atrophy, profound weakness that is partially independent of muscle mass loss, myofiber degeneration, accumulation of dysfunctional mitochondria, and increased oxidative stress. Until recently, it was thought that age-associated atrophy and weakness were secondary to motor-neuron loss in the brain or in the spinal cord. However, this hypothesis has been recently challenged. In fact, little neuronal death occurs in most areas of the aging nervous system, and there is no decline of lower motor neurons during aging (Chai et al., 2011; Morrison and Hof, 1997). Conversely, it is emerging that neuromuscular junctions (NMJs) and their interactions with myofibers are greatly altered during aging, resulting in a loss of muscle innervation (Chai et al., 2011; Valdez et al., 2010). Oxidative stress and decreased release of trophic factors are independent influences on NMJ integrity and contribute to denervation (Jang et al., 2010, 2012; Jang and Van Remmen, 2011). However, to date, it is not known whether myofiber denervation is due to deleterious changes in muscle cells themselves, in neurons, or both.
Two lifestyle adaptations, caloric restriction and exercise (Jang et al., 2012; Valdez et al., 2010), have been consistently demonstrated to extend lifespan and, in parallel, to mitigate age-related alterations of NMJ. Macroautophagy, hereafter named autophagy, is activated by both of these conditions in skeletal muscles and other tissues (Grumati et al., 2010, 2011a, 2011b; He et al., 2012; Rubinsztein et al., 2011; Wohlgemuth et al., 2010). Autophagy is a highly conserved homeostatic mechanism used for the degradation and recycling of bulk cytoplasm, long-lived proteins, and organelles through the lysosomal machinery (Mizushima and Komatsu, 2011). The autophagy machinery generates double membrane vesicles (autophagosomes) that engulf and sequester target cellular components (Mizushima and Komatsu, 2011). Autophagosomes are then delivered to and fuse with lysosomes to degrade their contents. Therefore, autophagy can be defined as a process of cytosolic renovation. In mammals, the relationship between autophagy inhibition and aging is still largely phenomenological and correlative. Conversely, robust genetic evidence in worms and flies clearly demonstrates this relationship. Deficient expression of ATG1, ATG7, ATG8, BECN1 (Beclin1), and SESTRIN1 (which is also required for basic autophagy) shortens the lifespan of the fruit fly D. melanogaster and of the nematode C. elegans (Lee et al., 2010b; Rubinsztein et al., 2011; Simonsen et al., 2008). On the other hand, increased autophagy contributes to longevity, and mutation of essential Atg genes prevents the gain of longevity (Meléndez et al., 2003). A recent report shows that aging can be controlled by a single tissue; overexpression of FOXO transcription factor or its target EIF4EBP1 in fly skeletal muscle abolishes the age-associated decline in autophagy and increases longevity (Demontis and Perrimon, 2010). We previously identified FOXO3A as a regulator of autophagy (Mammucari et al., 2007, 2008; Zhao et al., 2007), and genetic variations in the human FOXO3A have been linked to longevity in multiple population studies (Kenyon, 2010). The role of autophagy in sarcopenia in mammals is still controversial. Both impaired and excessive autophagy have been associated with aging sarcopenia (Wenz et al., 2009; Wohlgemuth et al., 2010). However, loss-of-function experiments that support the involvement of autophagy in aging sarcopenia are still lacking. Here, we show that specific inhibition of autophagy in skeletal muscle leads to abnormalities in motor neuron synapses that ultimately lead to denervation whereas accumulation of dysfunctional mitochondria triggers oxidation of contractile elements, causing a decrease in force generation.
Results
Autophagy Declines during Aging
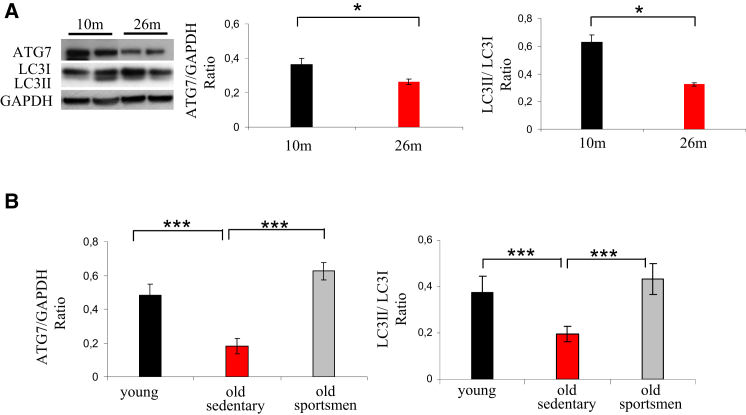
Age-related muscle loss is believed to result from a general decline of protein synthesis or an enhancement of protein breakdown. To study autophagy in aging, we monitored the expression of autophagy markers, such as LC3 (MAP1LC3A) and the critical E1-like enzyme, ATG7, in mice and humans. Aged mice showed a decline of ATG7 protein and, concurrently, of LC3 lipidation when compared to young animals (Figure 1A). Then we tested whether this decrease of the autophagy system occurs in humans. An important decrease of ATG7 protein and LC3II was found in muscle biopsies of elderly sedentary (sarcopenic) subjects (Figure 1B). Because exercise activates autophagy and counteracts most age-related characteristics, including the decline of muscle mass (Zampieri et al., 2014), we tested whether long-life regular exercise in humans was able to prevent the reduction of these autophagy markers. ATG7 protein and LC3II were maintained in muscle biopsies of senior sportsmen that had been previously shown to have better muscle mass and strength than elderly sedentary subjects (Zampieri et al., 2014).
Figure 1.
Autophagy Is Impaired during Aging
(A) Representative immunoblotting for the critical E1-like enzyme, ATG7, LC3II, and LC3I on muscle extracts from gastrocnemius of 10- and 26-month-old mice. The graphs show the quantification of ATG7 protein and the LC3II/LC3I ratio. Values are mean ± SEM; n = 4 of each condition; ∗p < 0.05.
(B) Aging reduces whereas exercise maintains expression of ATG7 and LC3 lipidation in humans. The graphs show the quantification of ATG7 protein and LC3II/LC3I ratio revealed by immunoblotting on muscle biopsies. Values are mean ± SEM; n = 6 young; n = 10 elderly, sedentary men; and n = 4 senior sportsmen; ∗∗∗p < 0.0001.
Autophagy Inhibition Affects Neuromuscular Synaptic Morphology and Function
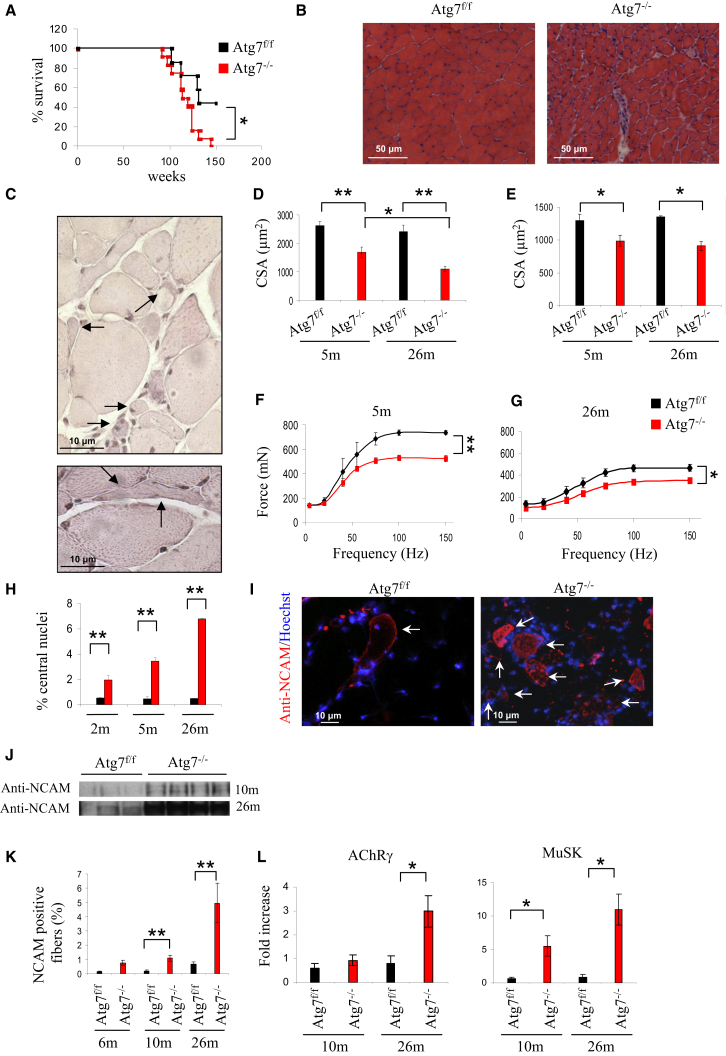
To mimic the decrease of ATG7 in aging, we characterized muscle-specific autophagy-deficient (Atg7−/−) mice that we have generated (Masiero et al., 2009). The absence of autophagy caused a significant reduction of animal survival (Figure 2A). Morphological analyses of aged mice showed higher levels of atrophy, center-nucleated fibers, and inflammation in Atg7−/− muscles compared to age-matched controls (Figures 2B and S1A). Fiber size was affected in Atg7−/−, and fibers displayed great variability in dimension. Some fibers atrophied to a level that made them hardly detectable (Figure 2C). These small fibers did not express the typical markers of regeneration and, therefore, are adult atrophic myofibers (data not shown). Quantification of the cross-sectional area (CSA) of Atg7−/− revealed that, although muscle atrophy was present in both oxidative and glycolytic fibers, it was more pronounced in the latter (Figures 2D and 2E).
Figure 2.
Morphological and Functional Changes in Muscles of Atg7−/− during Aging
(A) Kaplan-Meier curve of Atg7f/f and Atg7−/− mice. Atg7−/− mice die earlier than their Atg7f/f counterparts. Comparison of Atg7f/f and Atg7−/− survival curves was performed by both Mantel-Cox and Gehan-Breslow-Wilcoxon tests. Atg7−/− mice show a significant reduction in the lifespan compared to controls (∗p < 0.05).
(B) Morphological analyses of aged Atg7f/f and Atg7−/− tibialis anterior (TA) muscles. H&E staining shows that Atg7−/− muscles are atrophic with interstitial inflammation and center-nucleated fibers.
(C) Higher magnification of atrophic fibers of aged Atg7−/− mice showing angular, flat fibers and extremely small fibers (arrows).
(D) Quantification of the cross-sectional area (CSA) of glycolitic myofibers in TA muscles of adult and aged Atg7f/f and Atg7−/− mice. Values are mean ± SEM. At least four muscles of each group were analyzed; ∗∗p < 0.01; ∗p < 0.05.
(E) Quantification of CSA of oxidative myofibers of TA. Values are mean ± SEM; n > 4 for each group; ∗p < 0.05.
(F and G) Force measurements performed in vivo. (F) Atg7−/− mice show a profound decrease in maximal force generation in adult animals. Values are mean ± SEM (n = 5); ∗∗p < 0.01. (G) Aging reduces force production in both Atg7f/f and Atg7−/− mice, therefore aggravating the already profound weakness of Atg7−/− mice. Values are mean ± SEM; n = 4; ∗p < 0.05.
(H) Quantification of center-nucleated myofibers in TA (n > 4 for each group; ∗∗p < 0.01). Values are mean ± SEM.
(I) Representative images of immunostaining for NCAM expression in aged mice. Localization of NCAM along the entire fibers indicates the loss of muscle-nerve interaction.
(J) Immunoblotting for NCAM protein on muscle extracts from adult and aged gastrocnemius muscles. Atg7−/− muscles express higher levels of NCAM than age-matched Atg7f/f.
(K) Quantification of NCAM-positive fibers. Values were normalized for the total number of myofibers in muscle section (at least four muscles of each group were analyzed; ∗∗p < 0.01). Atg7−/− mice are characterized by much-higher age-dependent increase in NCAM-positive fibers than age-matched Atg7f/f. Values are mean ± SEM.
(L) Expression levels of acetylcholine receptor AChR γ-subunit (CHRNG) (left) and MuSK (right). MuSK and AChR are upregulated in adult and aged Atg7−/− muscles (n > 5; ∗p < 0.05). Values are mean ± SEM.
The deterioration of Atg7−/− muscles was also confirmed by the increase of center-nucleated fibers (Figure 2H). Correspondingly, Atg7−/− muscles were weaker than controls both in adult and elderly mice (Figures 2F and 2G). Given that the features of atrophic fibers resemble denervated fibers, and a decline in innervation and loss of motor units are known to be important endogenous causes of sarcopenia, we tested whether denervation plays a role in the phenotype of Atg7−/− mice. We first examined the expression pattern of the neural cell adhesion molecule (NCAM), a molecule that is enriched in the postsynaptic membrane of the NMJ but largely absent in adult myofibers. However, NCAM is re-expressed along the entire muscle fiber after the loss of innervation. Immunohistochemistry revealed that, during aging, there is a progressive increase of NCAM-positive fibers in controls. Importantly, Atg7−/− muscles showed 5- to 7-fold more NCAM-positive fibers when compared to age-matched controls both in adult and elderly mice (Figures 2I–2K and S1B). The expression of two other markers of denervation, MuSK (MUSK) and the acetylcholine receptor (AChR) γ-subunit (CHRNG) were significantly more upregulated in Atg7−/− muscles (Figure 2L). To confirm these findings, we used a recently described in vivo imaging approach where two distinct AChR pools are differently color labeled (Röder et al., 2008, 2010) to monitor NMJ morphology and stability.
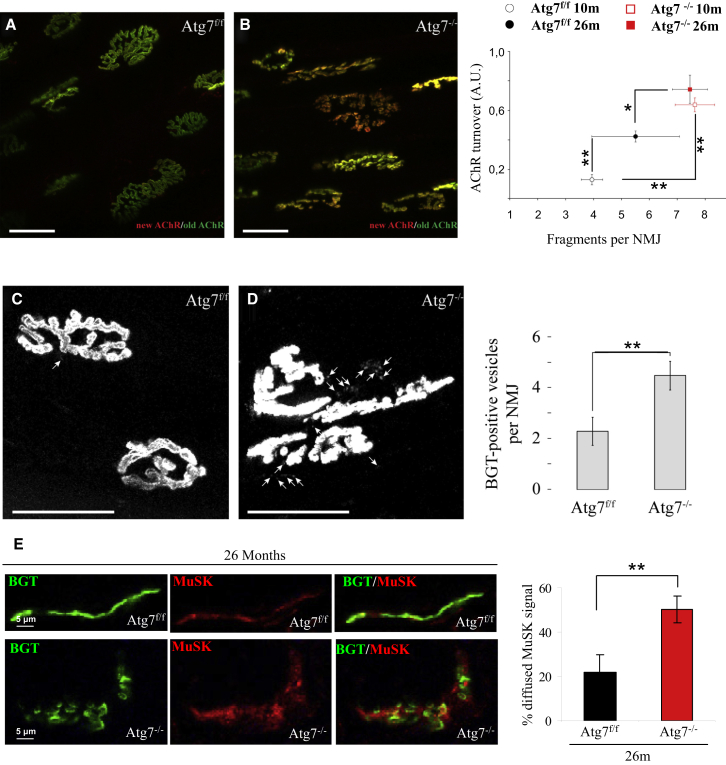
Automated image analysis revealed that, in contrast to control muscles, NMJs of adult Atg7 knockout mice showed fragmented morphology (Figures 3A and 3B) and instability (Figures 3A and 3B). Whereas in controls, the changes in morphology and instability progressively increased with age, this was not the case in Atg7 knockout mice (Figure 3B). NMJs of both adult and aged Atg7-deficient animals showed significantly more fragmentation and AChR turnover than 26-month-old (aged) control animals (Figure 3B, right panel).
Figure 3.
Neuromuscular Junctions of Atg7−/− Myofibers Are Unstable and Fragmented
(A and B) Representative images of Atg7f/f and Atg7−/− neuromuscular junctions (NMJ) obtained using confocal in vivo microscopy. Muscles were in vivo pulse labeled with bungarotoxins (BGTs) conjugated with different fluorophores. BGT-Alexa Fluor 647 (shown in green) was injected 10 days before microscopy, thus identifying stable AChR, whereas BGT-Alexa Fluor 555 (shown in red), injected 1 hr prior to microscopy, identifies newly incorporated AChR. Micrographs show representative maximum z projections of confocal in vivo images of NMJs from Atg7f/f (A) and Atg7−/− (B). The panel on the right shows the quantification of AChR turnover as a function of NMJ fragmentation of adult and aged Atg7f/f versus Atg7−/−. Data are from at least four muscles of each group; ∗∗p < 0.01; ∗p < 0.05. A.U., arbitrary units. In the right panel of (B), values are mean ± SEM.
(C and D) Muscles were labeled with BGT-Alexa Fluor 555 48 hr before microscopy. In vivo confocal imaging was used to determine the amount of structures containing endocytic AChR. Micrographs show representative maximum z projections of confocal in vivo images of NMJs from Atg7f/f (C) and Atg7−/− (D). The panel on the right shows the average number of vesicular BGT-Alexa Fluor 555-positive structures per optical section. Data from 13 and 27 NMJs for Atg7f/f and Atg7−/−, respectively; ∗∗p < 0.01. In the right panel of (D), values are mean ± SEM.
(E) Immunohistochemistry of MuSK expression on aged Atg7f/f and Atg7−/− myofibers. Upper panel shows normal pattern of NMJs in Atg7f/f mice, where MuSK (red) localizes to NMJ, revealed by BGT-Alexa Fluor 647 (green). Lower panel shows MuSK (red) that accumulates inside myofibers at the level of NMJ (green). Panel on the right shows that the amount of diffused MuSK staining normalized over the total number of MuSK-positive NMJs is higher in aged Atg7−/− than Atg7f/f; ∗∗p < 0.01. In the right panel, values are mean ± SEM.
Exocytic and endocytic processes mediate delivery of newly formed AChR and retrieval of old AChR, respectively. We monitored the localization and the number of the vesicles containing AChR. Inhibition of autophagy led to accumulation of vesicles close to the endplates that contain bungarotoxin (BGT) (Figures 3C and 3D), further corroborating an impairment of AChR turnover and of endosome trafficking. To determine whether alteration in endosome trafficking might also affect MuSK, a kinase that affects a plethora of signaling pathways that are important for NMJ stability, we monitored MuSK localization. Immunohistochemistry revealed that most MuSK colocalized with AChR at the plasma membrane in control fibers (Figure 3E). Importantly, Atg7−/− caused a significant loss of MuSK on the plasma membrane and a concomitant enrichment of internalized MuSK (Figure 3E). These findings confirm an alteration of AChR and MuSK recycling and localization in Atg7−/− mice. To determine whether the changes of NMJ are secondary to p62 (p62/SQSTM) accumulation into aggregates that clear signaling factors such as NRF2 or secondary to inhibition of the conjugation system, we knocked down p62 in Atg7−/−. For in vivo transfection experiments, we used bicistronic vectors that simultaneously encode small hairpin RNAs (shRNAs) and GFP. In this way, detection of GFP fluorescence allowed us to monitor the efficiency of transfection and the changes that occurred to p62-positive aggregates. After 14 days of transfection, Atg7−/− tibialis anterior (TA) muscle was cleared by p62-positive inclusions (Figure S2A). However, despite the absence of p62-positive aggregates, the percentage of denervated NCAM-positive fibers was not improved (Figure S2B).
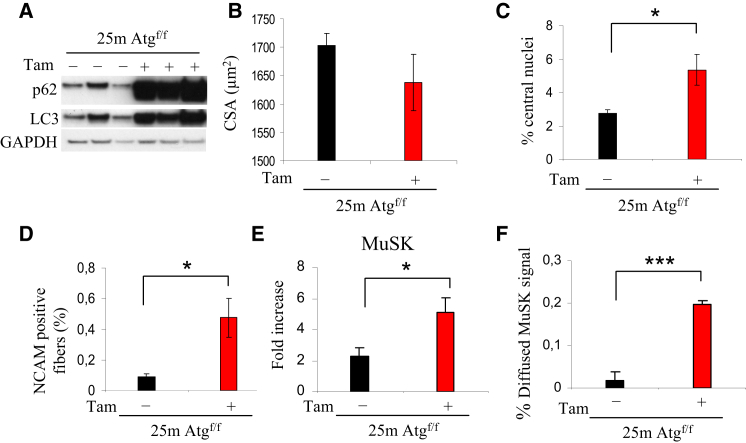
To understand the role of autophagy in age-related NMJ alterations, we used the inducible muscle-specific Atg7 knockout mice that we have recently generated (Masiero et al., 2009). The Atg7 gene was acutely deleted in 22-month-old mice, and mice were sacrificed 3 months later. Western blots for p62 and LC3 revealed that autophagy was successfully blocked in aged mice (Figure 4A). The inhibition of autophagy in old mice led to an exacerbation of muscle mass degeneration as revealed by the increase of centronucleated fibers and the decrease of CSA (Figures 4B and 4C). Fiber size distribution analysis revealed that acute Atg7 deletion led to an enrichment of small fibers when compared to controls (Figure S3), suggesting that denervation occurred in a discrete number of fibers. We then monitored NMJ to determine whether autophagy inhibition in old mice was sufficient to destabilize muscle-nerve interaction. Atg7 deletion led to a significant increase of denervated NCAM-positive fibers (Figure 4D). Denervation is also supported by the finding that the MuSK transcript is significantly induced in Atg7−/− (Figure 4E). Acute inhibition of autophagy in aged mice caused a significant loss of MuSK on the myofiber plasma membrane and a concomitant enrichment of internalized MuSK protein (Figure 4F).
Figure 4.
Acute Inhibition of Autophagy in Aged Mice Exacerbates Features of Sarcopenia and NMJ Degeneration
(A) Immunoblotting for LC3 and p62 proteins on muscle extracts from 25-month-old tamoxifen-inducible Atg7−/− female mice. Three months after the tamoxifen treatment, skeletal muscles were collected and analyzed.
(B) Acute inhibition of autophagy in old mice induces muscle degeneration. Quantification of the CSA of myofibers in TA muscles of aged tamoxifen-inducible Atg7−/− and Atg7f/f mice. Values are mean ± SEM; at least five muscles of each group were analyzed.
(C) Quantification of center-nucleated myofibers in TA of tamoxifen-inducible Atg7−/− (n > 5 for each group; ∗p < 0.05). Values are mean ± SEM.
(D) Acute inhibition of Atg7 in aged female mice increases the number of denervated NCAM-positive fibers when compared to age-matched controls. Values were normalized for the total number of myofibers in muscle section (at least five muscles of each group were analyzed; ∗p < 0.05). Values are mean ± SEM.
(E) Expression levels of MuSK after acute inhibition of Atg7 in old mice. MuSK is upregulated in aged tamoxifen-inducible Atg7−/− muscles (∗p < 0.05; n > 5). Values are mean ± SEM.
(F) Acute inhibition of autophagy in aged mice led to an increased amount of diffused MuSK staining; at least four muscles of each group were analyzed; ∗∗∗p < 0.001. Values are mean ± SEM.
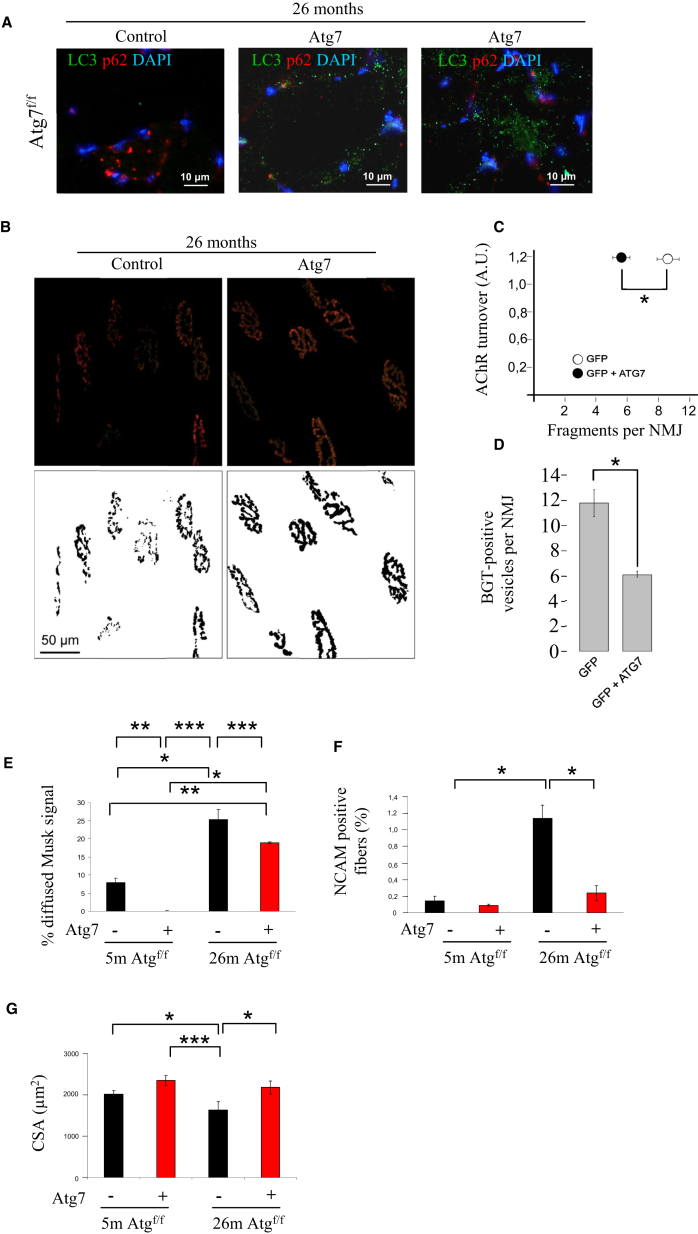
Rescue of Atg7 Expression in Aged Mice Improves Neuromuscular Synaptic Function
To determine the involvement of autophagy in NMJ maintenance during aging, we overexpressed ATG7 protein in 26-month-old Atg7f/f mice to restore normal autophagy flux in aged mice. ATG7 expression was sufficient to increase LC3-positive vesicles in sarcopenic muscles (Figure 5A). When we monitored the effect of ATG7 expression on NMJ of aged mice, we found a significant improvement in morphology; NMJ was significantly less fragmented than age-matched controls (Figures 5B and 5C). Correspondingly, restoring autophagy in aged mice led to a decrease of vesicles close to the endplates that contain BGT, further corroborating a reactivation of AChR turnover and of endosome trafficking (Figure 5D). This was confirmed by the fact that MuSK localization was improved by the expression of ATG7 (Figure 5E). Rescuing ATG7 protein and autophagy led to a reduction of denervated NCAM-positive fibers that returned to the values of adult mice (Figure 5F). Finally, improvement of autophagy flux and NMJ morphology resulted in an enhancement in muscle mass (Figure 5G).
Figure 5.
Rescue of Atg7 Expression in Aged Mice Improves Neuromuscular Synaptic Function
(A) Rescue of Atg7 expression restores autophagosome formation in 26-month-old mice. TA muscles were transfected with Atg7 expression plasmid, and 14 days later, TA were collected and stained for LC3 (green), p62 (red), and nuclei (blue).
(B) Confocal in vivo microscopy showing that rescue of ATG7 expression greatly reduces AChR fragmentation (data from at least four muscles per group; ∗p < 0.05) of aged Atg7f/f mice. Representative images are shown.
(C) Quantification of the AChR turnover as a function of NMJ fragmentation of aged Atg7f/f. Restoring Atg7 expression greatly improves NMJ fragmentation; n = 189 NMJs for Atg7f/f -GFP and 158 Atg7f/f GFP-ATG7; ∗∗p < 0.01. Values are mean ± SEM.
(D) Average number of vesicular BGT-Alexa Fluor 555-positive structures per optical section. Data from 189 NMJs for Atg7f/f -GFP and 158 Atg7f/f GFP-ATG7, respectively; ∗p < 0.05. Values are mean ± SEM.
(E) Rescue of Atg7 expression and autophagy in aged mice decreased the amount of diffused MuSK staining (at least three muscles of each group were analyzed; ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05). Values are mean ± SEM.
(F) Restoring Atg7 expression in aged mice improves the number of denervated NCAM-positive fibers when compared to age-matched controls and adult mice (three muscles of each group were analyzed; ∗p < 0.05). Values are mean ± SEM.
(G) Quantification of the CSA of myofibers in TA muscles of adult and aged Atg7f/f in presence or absence of Atg7 expression. Values are mean ± SEM; at least three muscles of each group were analyzed; ∗p < 0.05.
Autophagy Inhibition Enhances Mitochondrial Dysfunction and Oxidative Stress that Induces NMJ Instability
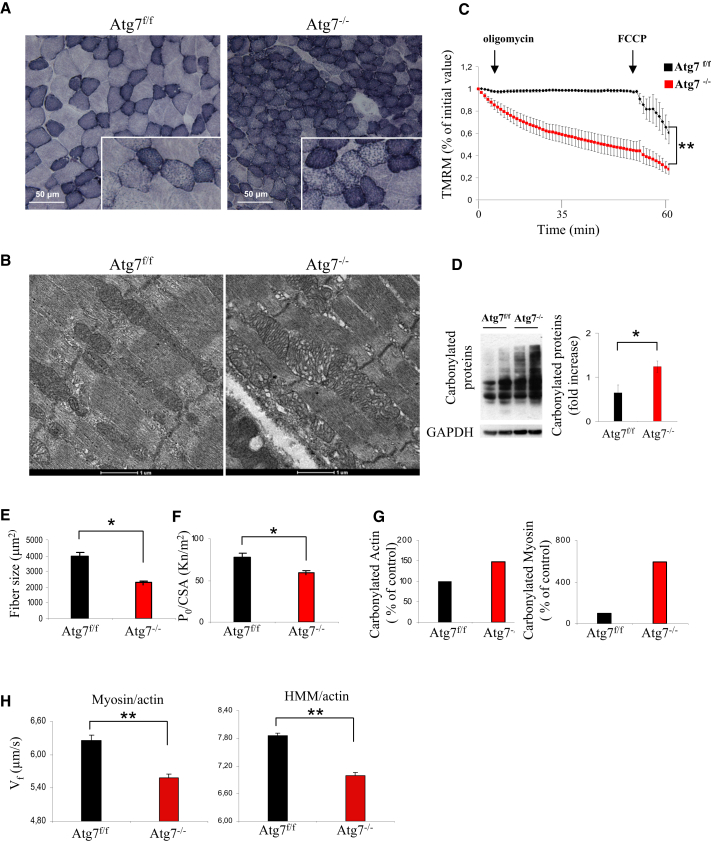
One potential explanation of age-related loss of muscle mass and innervation is due to accumulation of dysfunctional mitochondria that generate an enhancement of reactive oxygen species (ROS) production and oxidative stress. Over the past years, several researchers have reported that oxidative stress might be involved in etiology of weakness, NMJ degeneration, and sarcopenia (Jang et al., 2010, 2012). In fact, inhibition of autophagy induced an accumulation of mitochondria as revealed by succinate dehydrogenase (SDH) staining (Figure 6A). Electron microscopy analyses revealed the presence of giant mitochondria containing abnormal cristae (Figure 6B). The abnormal mitochondria are also dysfunctional. Atg7−/− flexor digitorum brevis (FDB) myofibers showed a significant increase of mitochondria that depolarized when the F1F0-ATPase was blocked by oligomycin (Grumati et al., 2010; Figure 6C). Similarly, FDB myofibers isolated from aged Atg7f/f showed a significant increase in depolarized mitochondria, which was corrected by restoring ATG7 expression (Figure S4A).
Figure 6.
Autophagy Inhibition Induces Oxidative Stress and Mitochondrial Dysfunction
(A) SDH staining on serial sections of aged Atg7f/f and Atg7−/− muscles (TA) shows an accumulation of abnormal mitochondria in Atg7−/− myofibers.
(B) Electron microscopy images of extensor digitorum longus muscles from aged Atg7f/f and Atg7−/− mice show accumulation of abnormal mitochondria displaying alterations in size, cristae morphology, and matrix density.
(C) Measurement of mitochondrial membrane potential. TMRM staining was monitored in at least 20 fibers per group (∗∗p < 0.001). Values are mean ± SEM.
(D) Overall protein carbonylation of aged Atg7f/f and Atg7−/− muscles, revealed by Oxyblot. A representative immunoblot for carbonylated proteins is depicted on the left, and densitometric quantification of the carbonylated proteins is in the graph on the right. Aged Atg7−/− mice show higher ongoing protein carbonylation than Atg7f/f (n = 5; ∗p < 0.05). Values are mean ± SEM.
(E and F) In vitro analysis of isolated skinned muscle fibers from gastrocnemius muscles of Atg7f/f and Atg7−/−. (E) Single Atg7−/− myofibers are more atrophic (E) and weaker (F) than Atg7f/f counterparts (at least 20 fibers for each condition; ∗p < 0.05). Values are mean ± SEM.
(G) Carbonylation of the pooled actin (on the left) and myosin (on the right) proteins, extracted from Atg7f/f and Atg7−/− GCN and used for in vitro motility assay. Atg7−/− show higher carbonylation than Atg7f/f.
(H) In vitro motility assay reveals reduced actin sliding velocity (Vf) on myosin (left panel) and HMM (right panel) extracted from adult Atg7−/− muscles in comparison with Atg7f/f. Reduced actin sliding velocity was statistically significant in Atg7−/− (n = 4 of each condition; ∗∗p < 0.01) on both myosin and HMM. Values are mean ± SEM.
The alteration of mitochondrial morphology and function is associated with increased oxidative stress as revealed by protein carbonylation (Figure 6D). Atg7−/− muscles showed a 2-fold increase of carbonylated proteins when compared to age-matched controls. We performed a proteomic approach on carbonylated proteins and found that mitochondrial and sarcomeric proteins are more oxidized in Atg7−/− than controls (Table S1; Figure S4B). Because oxidative stress is believed to affect force generation, its enhancement might contribute to age-dependent weakness. Morphological and functional analyses on isolated single-skinned fibers confirmed that Atg7−/− myofibers are more atrophic and generate less force (Figures 6E and S4C). When the absolute force was normalized for myofiber size, the resulting specific force was still significantly lower (Figure 6F). Thus, not only the muscles became smaller, but there was a general impairment in force transmission, which led to profound weakness. The reduction of specific force may be caused by posttranslational modifications of contractile proteins induced by ROS. We then purified actin and myosin from Atg7−/− muscles and confirmed that both proteins were more oxidized in Atg7−/− mice (Figure 6G; Table S1). Acto-myosin function was investigated using an in vitro motility assay approach (Canepari et al., 2012). Actin sliding velocity on both myosin and heavy meromyosin fraction (HMM) of Atg7−/− was slower compared to controls (Figure 6H), confirming that an alteration of the functional properties of contractile proteins occurs when autophagy is reduced.
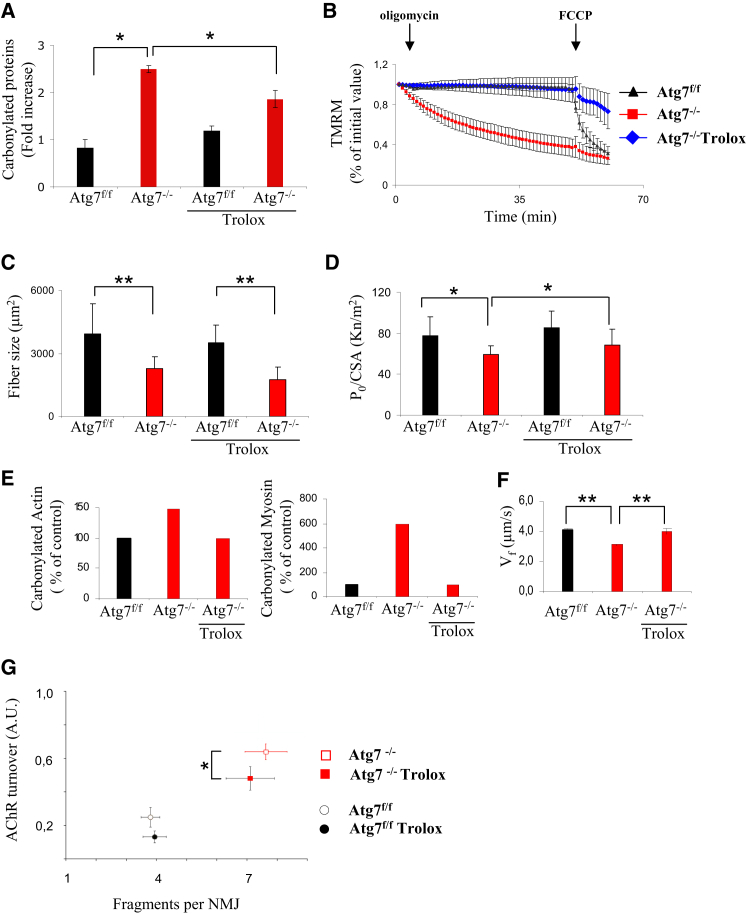
To test the role of oxidative stress in sarcopenia, we treated animals with Trolox, a potent antioxidant, for 4 weeks. The treatment successfully reduced the amount of total carbonylation on muscle protein extracts (Figures 7A and S4B; Table S1). Protein carbonylation of Trolox-treated Atg7−/− no longer differed from age-matched controls. The proteomic approach confirmed that Trolox abolished the oxidation of mitochondrial and sarcomeric proteins (Table S1; Figure S4B). Importantly, antioxidant treatment blocked the oligomycin-dependent mitochondrial depolarization of Atg7−/− mice (Figure 7B). We then addressed whether atrophy, weakness, and NMJ degeneration are affected by blunting oxidative stress. The treatment did not rescue myofiber size (Figures 7C and S5) but reduced the drop of specific force in isolated Atg7-deficient myofibers (Figure 7D). Trolox completely prevented carbonylation of purified myosin and actin (Figures 7E and S4B) and restored a normal acto-myosin function (Figure 7F). Looking at NMJ, we found that inhibition of ROS in adult Atg7−/− mice partially reduced NMJ instability whereas it did not give protection from NMJ fragmentation (Figure 7G). In conclusion, these findings strongly suggest that ROS production directly affects acto-myosin interaction and force generation but shows a limited involvement in the changes occurring at NMJ and no effect on atrophy.
Figure 7.
Inhibition of Oxidative Stress Restores the Ability of Mitochondria to Maintain Membrane Potential and the Functionality of Actin-Myosin Complex but Shows Limited Effect on NMJ
(A) Trolox treatment reduces the level of overall protein carbonylation in Atg7−/− muscles, thus abolishing the difference with Atg7f/f (n = 4 mice per condition; ∗p < 0.05). Values are mean ± SEM.
(B) Trolox treatment restores the ability of Atg7−/− mitochondria to maintain membrane potential (n > 20 fibers per group; ∗∗p < 0.001). Values are mean ± SEM.
(C) Trolox treatment does not affect fiber size in both Atg7f/f and Atg7−/− muscles (n = 4 mice per condition; ∗∗p < 0.01). Values are mean ± SEM.
(D) In vitro isolated skinned fiber analysis: Trolox treatment rescues the specific muscle force of isolated Atg7−/− myofibers; data re-expressed as mean ± SD; n = 4 mice per condition; ∗p < 0.05.
(E) Trolox treatment reduces the level of actin (left panel) and myosin (right panel) protein carbonylation in Atg7−/− muscles, thus abolishing the difference with Atg7f/f; n = 4 samples. Values are mean ± SEM.
(F) Trolox treatment rescues actin sliding velocity (Vf) in adult Atg7−/− muscles in in vitro motility assay; n = 4 mice per condition; ∗∗p < 0.01. Values are mean ± SEM.
(G) Confocal in vivo microscopy showing that Trolox treatment slightly reduces AChR turnover (data from at least four muscles per group; ∗p < 0.05) but does not have any effect on NMJ fragmentation of adult Atg7f/f and Atg7−/− mice. Values are mean ± SEM.
Discussion
The present study provides several important insights concerning the role of autophagy in NMJ preservation during aging. The most striking conclusion is that autophagy controls two important destabilizing factors that are (1) the removal of dysfunctional mitochondria that produce ROS and (2) the endosome recycling of AChR that affects MuSK localization and muscle-nerve interaction. The consequence of aging is the deterioration of tissue function mainly due to accumulation of damaged DNA, proteins, and organelles. Autophagy is a critical system to maintain the cell clear from dysfunctional organelles and mis- or unfolded proteins that are prone to aggregate and to preserve DNA stability (Sandri, 2010). Due to these actions and that autophagy declines with aging, there is consensus in considering autophagy as an antiaging system. Genetic evidence in flies and worms sustain this concept, but these data are still lacking in mammals. In fact, autophagy knockout mice die as newborns due to a general energy failure (Kuma et al., 2004), and most of the tissue-specific knockouts (Hara et al., 2006; Komatsu et al., 2006; Nakai et al., 2007) show severe organ dysfunction that precludes aging studies. Instead, our findings support the notion that autophagy failure contributes to aging.
Aging is a multisystemic disorder that affects multiple organs, leading to alterations of metabolism and tissue function. The postmitotic tissues such as brain, heart, and skeletal muscle are the most susceptible to age-related disease, and their dysfunction contributes importantly to precocious aging. The important role of muscle in healthy aging has been sustained by recent studies in Drosophila. Activation of autophagy by overexpression of FOXO transcription factor in the muscles of flies increases longevity (Demontis and Perrimon, 2010). The extension of lifespan is due to reduction of food intake and insulin release from neurosecretory cells, confirming that maintenance of a normal autophagy level in skeletal muscle, but not in adipose tissue, positively affects whole-body metabolism (Demontis and Perrimon, 2010). However, the role of autophagy in age-related muscle loss in mammals is still unclear, being either beneficial or detrimental (Gaugler et al., 2011; Wenz et al., 2009; Wohlgemuth et al., 2010). Our findings support the notion that autophagy is critical to prevent age-related denervation and weakness. Indeed, autophagy inhibition is sufficient to trigger a cascade of events that resemble those happening in elderly people.
Because mitochondria are the main producers of ROS, dysfunctional mitochondria are thought to play a key role in muscle function decline. In old age, a significant portion of mitochondria are abnormally enlarged, more rounded in shape, and display vacuolization in the matrix and shorter cristae (Peterson et al., 2012). Moreover, an increasing portion of them are also depolarized or nonfunctional. These features are present in autophagy-deficient muscles of adult animals, suggesting that defects in mitochondrial turnover are involved in age-dependent mitochondrial abnormalities. It is established that ROS production is increased in aged muscle in both the subsarcolemmal and intermyofibrillar pools of mitochondria (Chabi et al., 2008). This increase in ROS has been reported to induce oxidation of complex V, leading to decreased ATP production (Peterson et al., 2012). Accordingly, adult Atg7−/− muscles showed a significant increase of carbonylated ATP synthase (Table S1). Consistent with the ROS-mediated inhibition of ATP synthase, the energy-stress sensor of the cell, AMPK, is activated in Atg7-null muscles (not shown; Masiero et al., 2009). Our data show that mitochondrial dysfunction can be reversed by blocking ROS production. This finding is consistent with a recent report that showed improvement of age-related deficit, including mitochondrial function, by expressing a mitochondrial-targeted human catalase (Lee et al., 2010a).
Excessive ROS production does not only damage mitochondria but also cellular proteins. In muscle, oxidation of contractile proteins has been described, but the functional meaning of this condition is unclear. Our data establish that oxidative stress affects acto-myosin function and force generation. We showed by proteomic analyses that contractile proteins are carbonylated and that Trolox treatment reverts both protein oxidation and the myosin capacity of moving actin filaments, ultimately improving muscle force. It is well established that changes in muscle mass and strength tend to be dissociated in elderly persons, the decline of muscle strength being three times faster than the decrease of muscle mass (Peterson et al., 2012). This suggests that alteration in the quality of contractile proteins plays a critical role during age-related decrease of muscle force. Accordingly, Atg7−/− myofibers show a significant decrease of force compared to age-matched controls, and this decline is corrected by Trolox. However, whereas the antioxidant improves acto-myosin function, it does not protect from muscle atrophy, although we cannot exclude that extending Trolox treatment for longer periods may successfully inhibit the signaling pathways controlling muscle atrophy. Previous studies reported that mitochondrial ROS production may contribute to NMJ instability (Dobrowolny et al., 2008; Jang et al., 2010, 2012). Overexpression of uncoupling protein (UCP1), specifically in skeletal muscle, is sufficient to dismantle NMJ and to induce distal motor neuron degeneration (Dupuis et al., 2009). Our data suggest that ROS partially contribute to NMJ instability. In fact, whereas Trolox treatment slightly reduced the turnover of old and new AChR, it did not prevent NMJ fragmentation.
In conclusion, our data strongly support the concept that autophagy in a postmitotic tissue is required for the correct interplay between muscle and nerve and for the quality control of mitochondria. Our findings also sustain the concept that the beneficial effects of caloric restriction and exercise on aging are a consequence of autophagy reactivation.
Experimental Procedures
Animals, Human Biopsies, In Vivo Transfection, and RNAi Experiments
Animals were handled by specialized personnel under the control of inspectors of the Veterinary Service of the Local Sanitary Service (ASL 16—Padova), the local officers of the Ministry of Health. All procedures are specified in the projects approved by the Italian Ministero della Salute, Ufficio VI (authorization numbers C65). Subjects were male volunteers who received detailed information about the study and gave informed consent. Approval from the ethics committees of the City of Vienna and the Comenius University in Bratislava for medical ethics was obtained at the outset of the study. Muscle-specific Atg7-null (Atg7−/−) and tamoxifen-inducible muscle-specific Atg7−/− mice have been previously described (Masiero et al., 2009). Tamoxifen-induced Cre LoxP recombination was activated by oral administration of tamoxifen-containing chow (Tam400/Cre ER Harlan). In vivo transfection and RNAi experiments were performed as previously described (Sandri et al., 2004). Sequences and plasmids are described in the Supplemental Experimental Procedures. For in vitro validation of shRNA constructs, C2C12 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/10% fetal bovine serum and transfected with shRNA constructs using Lipofectamine 2000 (Life Technologies). Cells were lysed and processed for gene expression analyses or western blot.
Measurements of Muscle Force In Vivo
Muscle force was measured in living animals as previously described (Blaauw et al., 2008).
Single-Fiber Dissection, Solutions, and Experimental Setup
The method used for single-muscle-fiber dissection, the solutions, and the experimental setup have been previously described (Bottinelli et al., 1996).
Single-Fiber Analysis
Single-fiber analysis was performed as previously described (Bottinelli et al., 1996). The experiments were performed at a temperature of 12°C. Sarcomere length was determined and set at 2.5 μm. The CSA of the fibers was determined without correction for swelling. Absolute (Po) and specific forces (Po/CSA) of the fibers were determined. At the end of the mechanical experiment, fibers were put in 20 μl of Laemmli buffer and stored at −20°C for subsequent electrophoretic analysis of myosin heavy chain (MHC) isoform content.
Contractile Proteins for In Vitro Motility Assay
Myosin Extraction from Bulk Muscles
Myosin isoform 2B was extracted from bulk gastrocnemius muscles of mice according to a procedure previously described in detail (Canepari et al., 2000) and used to prepare the HMM.
HMM Preparation
HMM was obtained by a proteolytic digestion with α-chimotrypsin of myosin according to a modification of the method of Margossian and Lowey (1982) previously described in detail (Canepari et al., 2000).
Actin Preparation
G-actin was extracted as described by Pardee and Spudich (1982) from acetone powder prepared from the residues of mice muscles after myosin extraction. After polymerization, F-actin was labeled by incubation for several hours with rhodamine-phalloidine (Molecular Probes R415) as described by Kron et al. (1991).
IVMA
Myosin (or HMM) was diluted to 0.1 mg/ml in a high (or low) ionic strength buffer and infused in a flow cell treated with nitrocellulose and prepared according to Anson et al. (1995). The in vitro motility assay (IVMA) analysis was performed according to Canepari et al. (2000) and Bottinelli et al. (1999) at 25°C. The composition of the experimental buffer was 3-(N-morpholino)propanesulfonic acid 25 mM (pH = 7.4 at 25°C), KCl 25 mM, MgCl2 4 mM, EGTA 1 mM, dithiothreitol (DTT) 1 mM, glucose oxidase 200 μg/ml, catalase 36 μg/ml, glucose 5 mg/ml, and ATP 2 mM. Average velocities of actin filaments were determined. For each myosin and HMM sample, the velocities of at least 50 filaments were measured and their distribution characterized according to parametric statistics.
Gene-Expression Analyses
Total RNA was prepared from TA muscles using Trizol Reagent (Life Technologies). Complementary DNA generated with Life Technologies SuperScript III Reverse Transcriptase was analyzed by quantitative real-time PCR using Power SYBR Green Applied Biosystem. All data were normalized to glyceraldehyde 3-phosphate dehydrogenase. The oligonucleotide primers used are shown in Supplemental Experimental Procedures.
Immunoblotting
Cryosections of frozen gastrocnemius muscles were lysed in a buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 10 mM MgCl2, 0.5 mM DTT, 1 mM EDTA, 10% glycerol, 2% SDS, 1% Triton X-100, Roche Complete Protease Inhibitor Cocktail, and Sigma Protease Inhibitor Cocktail. The samples were immunoblotted as previously described (Sandri et al., 2004) and visualized with SuperSignal West Pico Chemiluminescent substrate (Pierce). A list of antibodies is shown in the Supplemental Experimental Procedures.
Protein Carbonyls Detection and Antioxidant Treatment
Carbonylation of muscle proteins was detected by using the OxyBlot Protein Oxidation Detection Kit from Millipore (Masiero et al., 2009). Mice were intraperitoneally injected with Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) 30 mg/kg daily for 4 weeks. For a detailed description of the detection of oxidized proteins, see the Supplemental Experimental Procedures.
2D OxyBlot Data Acquisition and Analysis
Spot detection and quantification was carried out using the Image Master 2D Platinum 7 software (GE Healthcare). For a detailed description of the procedure, see the Supplemental Experimental Procedures.
In-Gel Digestion, Mass Spectrometry Protein Identification, and Database Searches
For a detailed description of the procedure, see the Supplemental Experimental Procedures.
Histology, Fluorescence Microscopy, and Electron Microscopy
Cryosections of TA were stained for hematoxylin and eosin (H&E) and SDH. Immunofluorescence staining was performed on cryostatic sections as previously described (Masiero et al., 2009) and then monitored with a fluorescence microscope or confocal microscopy. Antibodies are described in the Supplemental Experimental Procedures. Fiber cross-sectional area was performed on TA or gastrocnemius (GCN) as described (Mammucari et al., 2007) and measured using ImageJ software. For electron microscopy, we used conventional fixation-embedding procedures based on glutaraldehyde-osmium fixation and Epon embedding.
Cell Culture and Transient Transfections
C2C12 myogenic cell lines were cultured in DMEM (GIBCO-Life Technologies) supplemented with 10% fetal bovine serum. Myoblasts were transfected using Lipofectamine 2000 (Life Technologies) according to the manufacturer’s instructions.
Single-Fiber Mitochondrial Membrane Potential Analyses
Mitochondrial membrane potential was measured in isolated transfected fibers from FDB muscles. Mitochondrial membrane potential was measured by epifluorescence microscopy based on the accumulation of tetramethylrhodamine, methyl ester (TMRM) fluorescence as previously described (Romanello et al., 2010).
MHC Identification
The MHC isoform composition of gastrocnemius muscles of mice was assessed by 8% SDS polyacrylammide gel electrophoresis as described by Pellegrino et al. (2003).
In Vivo Microscopy and Analysis of AChR Turnover and NMJ Fragmentation
In vivo microscopy of mice was performed under anesthesia using Zoletil and xylazine on a Leica SP2 confocal microscope equipped with a 63× 1.2 numerical aperture water immersion objective, essentially as described previously (Röder et al., 2008, 2010). Automated analysis of AChR turnover and NMJ fragmentation used algorithms described earlier (Röder et al., 2010).
Statistical Analysis and General Experimental Design
The sample size was calculated using size power analysis methods for a priori determination, based on the SD and effect size previously obtained using the experimental methods employed in the study. For animal studies, we estimated sample size from the expected number of knockout mice and littermate controls, which was based on Mendelian ratios. We calculated the minimal sample size for each group by at least four organisms. Considering a likely drop-off effect of 10%, we set the sample size of each group at five mice. To reduce the SD, we minimized physiological variation by using homogenous animals with the same sex and age. The exclusion criteria for animals were pre-established. In case of death, cannibalism, or sickness, the animal was excluded from analysis. Tissue samples were excluded in cases such as cryo-artifacts, histological artifacts, or failed RNA extraction. We included animals from different breeding cages by random allocation to the different experimental groups. Animal experiments were not blinded; however, when applicable, the experimenters were blinded to the nature of samples by using number codes until final data analysis was performed. Statistical tests were used as described in the figure legends and were applied upon verification of the test assumptions (e.g., normality). Generally, data were analyzed by Student’s t test. For all graphs, data are represented as means ± SEM.
Acknowledgments
This work was supported by Telethon-Italy (TCP04009), by the European Union (MYOAGE; contract: 223576 of FP7), by ERC (282310-MyoPHAGY), by the Italian Ministry of Education (MiUR) (PRIN 2010/2011), by the Leducq Foundation, and by CARIPARO to M.S. M.A.B. is currently the recipient of a postdoctoral fellowship from the Association Française contre les Myopathies (AFM). The authors also thank the 3P5 proteomics platform, Université Paris Descartes, Sorbonne Paris Cité, for the mass-spectrometry-based protein identifications.
Published: August 28, 2014
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information includes Supplemental Experimental Procedures, five figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.07.061.
Supplemental Information
References
- Anson M., Drummond D.R., Geeves M.A., Hennessey E.S., Ritchie M.D., Sparrow J.C. Actomyosin kinetics and in vitro motility of wild-type Drosophila actin and the effects of two mutations in the Act88F gene. Biophys. J. 1995;68:1991–2003. doi: 10.1016/S0006-3495(95)80376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauw B., Mammucari C., Toniolo L., Agatea L., Abraham R., Sandri M., Reggiani C., Schiaffino S. Akt activation prevents the force drop induced by eccentric contractions in dystrophin-deficient skeletal muscle. Hum. Mol. Genet. 2008;17:3686–3696. doi: 10.1093/hmg/ddn264. [DOI] [PubMed] [Google Scholar]
- Bottinelli R., Canepari M., Pellegrino M.A., Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J. Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R., Pellegrino M.A., Canepari M., Rossi R., Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J. Electromyogr. Kinesiol. 1999;9:87–95. doi: 10.1016/s1050-6411(98)00040-6. [DOI] [PubMed] [Google Scholar]
- Canepari M., Rossi R., Pellegrino M.A., Bottinelli R., Schiaffino S., Reggiani C. Functional diversity between orthologous myosins with minimal sequence diversity. J. Muscle Res. Cell Motil. 2000;21:375–382. doi: 10.1023/a:1005640004495. [DOI] [PubMed] [Google Scholar]
- Canepari M., Maffei M., Longa E., Geeves M., Bottinelli R. Actomyosin kinetics of pure fast and slow rat myosin isoforms studied by in vitro motility assay approach. Exp. Physiol. 2012;97:873–881. doi: 10.1113/expphysiol.2012.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabi B., Ljubicic V., Menzies K.J., Huang J.H., Saleem A., Hood D.A. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Chai R.J., Vukovic J., Dunlop S., Grounds M.D., Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS ONE. 2011;6:e28090. doi: 10.1371/journal.pone.0028090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F., Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolny G., Aucello M., Rizzuto E., Beccafico S., Mammucari C., Boncompagni S., Belia S., Wannenes F., Nicoletti C., Del Prete Z. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. doi: 10.1016/j.cmet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Dupuis L., Gonzalez de Aguilar J.L., Echaniz-Laguna A., Eschbach J., Rene F., Oudart H., Halter B., Huze C., Schaeffer L., Bouillaud F., Loeffler J.P. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS ONE. 2009;4:e5390. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler M., Brown A., Merrell E., DiSanto-Rose M., Rathmacher J.A., Reynolds T.H., 4th PKB signaling and atrogene expression in skeletal muscle of aged mice. J. Appl. Physiol. 2011;111:192–199. doi: 10.1152/japplphysiol.00175.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumati P., Coletto L., Sabatelli P., Cescon M., Angelin A., Bertaggia E., Blaauw B., Urciuolo A., Tiepolo T., Merlini L. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 2010;16:1313–1320. doi: 10.1038/nm.2247. [DOI] [PubMed] [Google Scholar]
- Grumati P., Coletto L., Sandri M., Bonaldo P. Autophagy induction rescues muscular dystrophy. Autophagy. 2011;7:426–428. doi: 10.4161/auto.7.4.14392. [DOI] [PubMed] [Google Scholar]
- Grumati P., Coletto L., Schiavinato A., Castagnaro S., Bertaggia E., Sandri M., Bonaldo P. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy. 2011;7:1415–1423. doi: 10.4161/auto.7.12.17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- He C., Bassik M.C., Moresi V., Sun K., Wei Y., Zou Z., An Z., Loh J., Fisher J., Sun Q. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.C., Van Remmen H. Age-associated alterations of the neuromuscular junction. Exp. Gerontol. 2011;46:193–198. doi: 10.1016/j.exger.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.C., Lustgarten M.S., Liu Y., Muller F.L., Bhattacharya A., Liang H., Salmon A.B., Brooks S.V., Larkin L., Hayworth C.R. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.C., Liu Y., Hayworth C.R., Bhattacharya A., Lustgarten M.S., Muller F.L., Chaudhuri A., Qi W., Li Y., Huang J.Y. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell. 2012;11:770–782. doi: 10.1111/j.1474-9726.2012.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C.J. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kron S.J., Toyoshima Y.Y., Uyeda T.Q., Spudich J.A. Assays for actin sliding movement over myosin-coated surfaces. Methods Enzymol. 1991;196:399–416. doi: 10.1016/0076-6879(91)96035-p. [DOI] [PubMed] [Google Scholar]
- Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Choi C.S., Birkenfeld A.L., Alves T.C., Jornayvaz F.R., Jurczak M.J., Zhang D., Woo D.K., Shadel G.S., Ladiges W. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Budanov A.V., Park E.J., Birse R., Kim T.E., Perkins G.A., Ocorr K., Ellisman M.H., Bodmer R., Bier E., Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C., Milan G., Romanello V., Masiero E., Rudolf R., Del Piccolo P., Burden S.J., Di Lisi R., Sandri C., Zhao J. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Mammucari C., Schiaffino S., Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4:524–526. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- Margossian S.S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M., Metzger D., Reggiani C., Schiaffino S., Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Meléndez A., Tallóczy Z., Seaman M., Eskelinen E.L., Hall D.H., Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Morrison J.H., Hof P.R. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Nakai A., Yamaguchi O., Takeda T., Higuchi Y., Hikoso S., Taniike M., Omiya S., Mizote I., Matsumura Y., Asahi M. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- Pardee J.D., Spudich J.A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Pellegrino M.A., Canepari M., Rossi R., D’Antona G., Reggiani C., Bottinelli R. Orthologous myosin isoforms and scaling of shortening velocity with body size in mouse, rat, rabbit and human muscles. J. Physiol. 2003;546:677–689. doi: 10.1113/jphysiol.2002.027375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C.M., Johannsen D.L., Ravussin E. Skeletal muscle mitochondria and aging: a review. J. Aging Res. 2012;2012:194821. doi: 10.1155/2012/194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder I.V., Petersen Y., Choi K.R., Witzemann V., Hammer J.A., 3rd, Rudolf R. Role of Myosin Va in the plasticity of the vertebrate neuromuscular junction in vivo. PLoS ONE. 2008;3:e3871. doi: 10.1371/journal.pone.0003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder I.V., Choi K.R., Reischl M., Petersen Y., Diefenbacher M.E., Zaccolo M., Pozzan T., Rudolf R. Myosin Va cooperates with PKA RIalpha to mediate maintenance of the endplate in vivo. Proc. Natl. Acad. Sci. USA. 2010;107:2031–2036. doi: 10.1073/pnas.0914087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanello V., Guadagnin E., Gomes L., Roder I., Sandri C., Petersen Y., Milan G., Masiero E., Del Piccolo P., Foretz M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29:1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D.C., Mariño G., Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Sandri M. Autophagy in skeletal muscle. FEBS Lett. 2010;584:1411–1416. doi: 10.1016/j.febslet.2010.01.056. [DOI] [PubMed] [Google Scholar]
- Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S.H., Goldberg A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Cumming R.C., Brech A., Isakson P., Schubert D.R., Finley K.D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- Valdez G., Tapia J.C., Kang H., Clemenson G.D., Jr., Gage F.H., Lichtman J.W., Sanes J.R. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. USA. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M., Schaap L.A. Consequences of sarcopenia. Clin. Geriatr. Med. 2011;27:387–399. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Wenz T., Rossi S.G., Rotundo R.L., Spiegelman B.M., Moraes C.T. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. USA. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wohlgemuth S.E., Seo A.Y., Marzetti E., Lees H.A., Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp. Gerontol. 2010;45:138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri S., Pietrangelo L., Loefler S., Fruhmann H., Vogelauer M., Burggraf S., Pond A., Grim-Stieger M., Cvecka J., Sedliak M. Lifelong Physical Exercise Delays Age-Associated Skeletal Muscle Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2014 doi: 10.1093/gerona/glu006. [DOI] [PubMed] [Google Scholar]
- Zhao J., Brault J.J., Schild A., Cao P., Sandri M., Schiaffino S., Lecker S.H., Goldberg A.L. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.