Abstract
Ylang-ylang (Cananga odorata Hook. F. & Thomson) is one of the plants that are exploited at a large scale for its essential oil which is an important raw material for the fragrance industry. The essential oils extracted via steam distillation from the plant have been used mainly in cosmetic industry but also in food industry. Traditionally, C. odorata is used to treat malaria, stomach ailments, asthma, gout, and rheumatism. The essential oils or ylang-ylang oil is used in aromatherapy and is believed to be effective in treating depression, high blood pressure, and anxiety. Many phytochemical studies have identified the constituents present in the essential oils of C. odorata. A wide range of chemical compounds including monoterpene, sesquiterpenes, and phenylpropanoids have been isolated from this plant. Recent studies have shown a wide variety of bioactivities exhibited by the essential oils and the extracts of C. odorata including antimicrobial, antibiofilm, anti-inflammatory, antivector, insect-repellent, antidiabetic, antifertility and antimelanogenesis activities. Thus, the present review summarizes the information concerning the traditional uses, phytochemistry, and biological activities of C. odorata. This review is aimed at demonstrating that C. odorata not only is an important raw material for perfume industry but also considered as a prospective useful plant to agriculture and medicine.
1. Introduction
Cananga odorata Hook. F. & Thomson, which is commonly called ylang-ylang, is a fast growing tree and can found natively in tropical Asia such as Philippines, Malaysia, Indonesia, and some other islands of Indian Ocean, mainly the Comoro, Nossi Be, and Madagascar islands. This plant has been well-known for its fragrant flower and has been introduced to China, India, Africa, and America. Ylang-ylang essential oils have already been widely utilized in the food industry as well as in the perfume industry and aromatherapy. Primarily, the ylang-ylang essential oil is derived from the flower of the C. odorata plant via water or water and steam distillation. Ylang-ylang oil has been described to possess medium to strong initial aroma with fresh, floral, slightly fruity fragrant yet delicate. Furthermore, the flower is also described to produce intensely sweet scent which is similar to jasmine [1]. Ylang-ylang oil has been approved to be generally recognized as safe by Flavor and Extract Manufacturers Association (FEMA) and is widely used as flavouring agent and adjuvant. Currently, ylang-ylang oil can be found in various cosmetic and households products such as the massage oils, moisturizing creams, perfumes, and even scented candles. It is also believed that the medicinal properties exhibited by ylang-ylang oil are one of the main factors that contribute to its increasing popularity in the field of aromatherapy.
Although the uses of ylang-ylang oil and its safety as food ingredient have been also reviewed previously [2], during that time period the studies on the pharmacological activities of the Cananga odorata plant were still very limited. Basically, a very brief review was done covering the antibacterial, antifungal, amebicidal, and cytotoxic activities of the ylang-ylang essential oil [2]. Perhaps, it is due to the improvement in different biological assays and accessibility of chemical purification and identification techniques, and it has seemed greatly impacted by the research activities carried out by researchers. In particular, an apparent increase in the differential biological activities investigation on medicinal plants has enabled diversified applications of existing known natural products. For instance, the recent extensive explorations of differential pharmacological properties of ylang-ylang and their active compounds have significantly opened up its new commercial avenues for agriculture [3]. Insightfully there is a great increase in number of pharmacological studies done on C. odorata in recent years, particularly surrounding its biological properties and chemical components [4–8]. Therefore, the current review aimed to compile or summarize these important findings and further highlight the importance of C. odorata as a potential promising drug discovery candidate for future.
2. Taxonomic Classification and Nomenclature of Cananga odorata
Taxonomic classification and nomenclature of Cananga odorata are as follows:
-
kingdom: Plantae, plants,
-
subkingdom: Tracheobionta, vascular plants,
-
superdivision: Spermatophyta, seed plants,
-
division: Magnoliophyta, flowering plants,
-
class: Magnoliopsida, dicotyledons,
-
subclass: Magnoliidae,
-
order: Magnoliales,
-
family: Annonaceae, custard-apple family,
-
genus: Cananga (DC.) Hook. f. & Thomson, ylang-ylang,
-
species: Cananga odorata (Lam.) Hook. f. & Thomson, ylang-ylang.
3. Botany
3.1. Botanical Name
3.1.1. Common Names
C. odorata is commonly known as ylang-ylang. The English names of C. odorata are ylang-ylang, perfume tree, Cananga, and cadmia. Meanwhile, the other common names for C. odorata are listed in Table 1.
Table 1.
The common names of C. odorata from different regions.
| Regions | Common names |
|---|---|
| General | Ylang-ylang, perfume tree, Cananga, and cadmia (English) |
|
| |
| Oceania | Canang odorant (French) Chiráng, irang (Palau) Derangerang, derangirang (Nauru) Ilahnglahng, ilanlang (Kosrae) Ilang-ilang, alang-ilang (Guam) Ilangilang, lengileng, alangilang, pur-n-wai, pwurenwai, seir en wai (Pohnpei) Ilanilan (Marshall Islands) Lanalana (Hawai‘i) Makosoi, mokohoi, makasui, mokosoi (Fiji) Mohokoi (Tonga) Moso‘oi (Samoa) Moto‘i (French Polynesia) Moto‘oi, mata‘oi, mato‘oi (Cook Islands, Niue, Tahiti) Motoi (Marquesas-Nuku Hiva, Niue) Mutui (Marquesas-Fatu Hiva) Pwalang (Puluwat atoll) Pwanang, pwuur, pwalang (Chuuk) Sa‘o (Solomon islands: Kwara‘ae) |
|
| |
| South East Asia | Ilang-ilang, alang-ilang (Philippines) Sagasein, kedatngan, kadatnyan (Myanmar) Kernanga (Indonesia) Fereng, kradang naga (Thailand) Kenanga, chenanga, ylang-ylang (Malaysia) |
|
| |
| India | Apurvachampaka, chettu sampangi, karumugai (India) |
Adapted from [9] with slight modifications.
3.2. Synonyms
According to the plant list, there are more than twenty synonyms which have been recorded for C. odorata. For instance, Cananga mitrastigma (F. Muell.) Domin, Canangium mitrastigma (F. Muell.) Domin, Cananga odorata var. odorata, Cananga odoratum (Lam.) Baill. ex King, Canangium odoratum (Lam.) Baill. ex King, Canangium odoratum var. velutinum Koord. & Valeton, Cananga scortechinii King, Canangium scortechinii King, Fitzgeraldia mitrastigma F. Muell., Unona cananga Spreng., Unona leptopetala DC., Unona odorata (Lam.) Dunal, Unona odorata (Lam.) Baill., Unona odoratissima Blanco, Unona ossea Blanco, Uvaria axillaris Roxb., Uvaria cananga Banks, Uvaria odorata Lam., Uvaria ossea (Blanco) Blanco, and Uvaria trifoliata Gaertn. [50].
4. Botanical Description and Distribution
C. odorata belong to the Annonaceae family, with 125 genera and 2050 species. To date, the Cananga genus consists of two species of plant, namely, C. odorata and C. latifolia. C. odorata is a perennial tropical tree which grows natively in South-East Asia countries such as Philippines and Malaysia, and it also occurs naturally in several Pacific islands including Australia. After that, it has been introduced into China, India, Africa, and America due to its economic importance [51].
The morphological features of the C. odorata plant are briefly described in Table 2 and illustrated in Figure 1. Basically, C. odorata is a medium-sized evergreen tree whichgenerally grows up to 15 meters height with long drooping branches [9].
Table 2.
The morphological features of C. odorata leaves, stems, flowers, fruits, and seeds.
| Part | Descriptions |
|---|---|
| Leaves | Colour: dark shiny green (above), duller and lighter green (beneath) Arrangement: alternate, single plane along twigs Length: 9–21 cm; width: 4–9 cm Shape: ovate-oblong to broadly elliptic with wavy margin; rounded and unequal base; acuminate apex |
|
| |
| Twigs/petiole | Petiole colour: light green; twig colour: light green (young), brown (old) Petiole length: 6–15 mm |
|
| |
| Flowers | Odor: highly fragrant Length: 7.5 cm Arrangement: hanging axillary in a group of 4–12 flowers with umbellate arrangement; scattering around the older parts of twigs Pedicels: short, 1–2.5 cm long Calyx: three, broad, pointed, and hairy Petals: six, slightly thicken, twisted, pointed, hairy, 4–6 cm long, green (young), yellow to yellowish-brown (mature) |
|
| |
| Fruits | Colour: dark green to black (ripe) Shape: ovoid Length: 1.5–2.3 cm long |
|
| |
| Seeds | Shape: hard, flattened, ovoid, and pitted Size: 6 mm diameter Colour: pale brown |
Summarized from [9] with slight modifications.
Figure 1.
Morphology of C. odorata. (a) Mature C. odorata flower with yellow petals, (b) young yellowish-green C. odorata flower, (c) young C. odorata plant in Rimba Ilmu Botanic Garden, University of Malaya, and (d) leaves of C. odorata plant (images are obtained from Dr. Sugumaran (a) and Mr. Cheah ((b)–(d)) from University of Malaya).
5. Ethnomedicinal Uses
C. odorata has a variety of medicinal properties and traditional uses. The strongly fragrant yellow flower of C. odorata has been reported to be used to enhance the scent of coconut oil before being used for massage by Polynesians live in South Pacific islands [52]. In Java, the dried flowers of C. odorata are used to treat malaria and malaria-like symptoms. Similarly, it is also recognized as medicinal plants used against malaria traditionally in Vietnam [27]. Meanwhile, it has been also reported that the pounded fresh flowers paste being used to treat asthma. The flowers and bark of C. odorata are used to treat pneumonia and stomach ache by the local communities and traditional healers from Northern Mariana Islands [53]. In Indonesia, ylang-ylang oil is used to enhance euphoria feel during sex and also reduce sexual anxiety [54]. In line with the above mentioned traditional usage, ylang-ylang has been reported to be used as antidepressant to treat depression and nervousness. It has been also reported to have blood pressure lowering effect suggesting its potential use in managing hypertension [51].
According to both of the folks from India and islanders of the Indian Ocean, the leaves of C. odorata is believed to relieve itchiness by direct topical application and also to treat dandruff [55]. Indian has also used ylang-ylang oil to treat headaches, eye inflammation, and gout [52]. Apart from that, the traditional healers from Papuan New Guinea believe that by consuming the decoction of the heated inner bark of C. odorata is ableto treat gout [56]. Besides that, the bark of the plant is believed to be effective in treating stomach ailments. It is also being used as laxative by communities in Tonga and Samoa. Meanwhile, the Indian used the decoction of the bark of the plant to treat rheumatism, phlegm, ophthalmia, ulcers, and fevers [57].
6. Phytochemistry
The phytochemistry of C. odorata is well documented. C. odorata is well known for its essential oil. Essential oils are referred as the natural, complex, and volatile compounds which exhibit distinctive scent that are produced by aromatic plants as secondary metabolites [58]. Generally, the essential oils can be extracted from the aromatic plants by steam or hydrodistillation. However, various combinations of extraction methods are necessary to extract all the volatile phytochemicals present in the C. odorata. Besides the steam and hydrodistillation extraction methods, simultaneous steam distillation-solvent extraction and supercritical fluid extraction (SFE) were also developed to completely isolate most of the volatile secondary metabolites of ylang-ylang flower [13]. More advanced methods have been employed to analyze the volatile components of C. odorata due to several disadvantages presented by using distillation method such as time consuming and thermal degradation. For instance, Headspace-Solid Microextraction method coupled with Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS) was used to characterize all the volatile compounds of C. odorata flower at different stages of development [59].
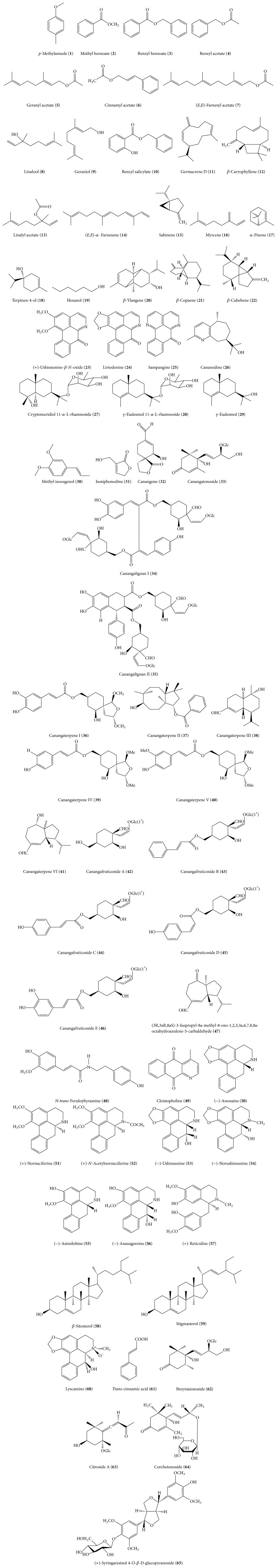
Numerous chemical composition studies have been conducted on the essential oil of different parts of C. odorata. In one of the earliest reports, ylang-ylang essential oil was shown to contain monoterpene hydrocarbons, oxygen-containing monoterpenes, sesquiterpene hydrocarbons, oxygen-containing sesquiterpenes, benzenoids, acetates, benzoates, and phenols. To date, many compounds have been identified from the essential oil of ylang-ylang. Essentially, most of the compounds identified from the essential oil from different part of C. odorata plant are listed in Table 3. In 1986, a total of 52 compounds from the volatile, oxygenated, and hydrocarbon fractions of first grade ylang-ylang essential oil from Madagascar were identified by combined gas chromatography-mass spectrometry (GC-MS) and proton nuclear magnetic resonance (1H NMR). The study revealed that the main components identified from the oxygenated fraction of ylang-ylang essential oil were p-methylanisole (1), methyl benzoate (2) and benzyl benzoate (3), benzyl acetate (4), geranyl acetate (5), cinnamyl acetate (6) and (E,E)-farnesyl acetate (7), linalool (8), geraniol (9), and benzyl salicylate (10) and their molecular structures are shown in (Figure 2). Linalool (8) was shown to be main component present in oxygenated fraction (28%) that is responsible for the floral smell of ylang-ylang. Meanwhile, the hydrocarbon fraction of ylang-ylang oil consisted of mainly sesquiterpenes and monoterpenes whereby both germacrene D (11) and β-caryophyllene (12) represented 63% of the total hydrocarbon fraction of ylang-ylang oil [60]. γ-Muurolene (13) and (E,E)-farnesyl acetate (7) were both sesquiterpenes identified for the first time in ylang-ylang oil in [60]. In 2012, Benini and colleagues [15] demonstrated a total of 32 compounds which were not previously reported in ylang-ylang oil were detected from the C. odorata flower samples obtained from Grande Comore, Mayotte, Nossi Be, and Ambanja (Table 3). Furthermore, the characterization of ylang-ylang essential oils was further improved by the use of comprehensive two-dimensional GC coupled to time-of-flight MS (GC×GC-TOFMS) by the similar group of researchers. Brokl and colleagues [16] demonstrated that GC×GC-TOFMS was able to reveal more chemical components present in ylang-ylang flower (Table 3), suggesting that this technology is capable of providing better insight on chemical polymorphism as well as of studying the different parameters of “terroir effect” on phytoconstituents.
Table 3.
The constituents identified from the essential oil of C. odorata.
| Class | Constituents | Plant parts | Reference |
|---|---|---|---|
| Monoterpenes | (E)-β-Ocimene | Leaf, fruit | [10–12] |
| (Z)-β-Ocimene | Leaf, fruit | [10–12] | |
| 1,8-Cineole | Leaf, flower, fruit | [10, 12–14] | |
| Bornyl acetate | Leaf | [11] | |
| Camphene | Leaf, flower | [11] | |
| Geraniol | Leaf, flower | [11] | |
| Geranyl acetate | Flower | [11, 13] | |
| Limonene | Leaf, flower, fruit | [10–14] | |
| Linalool | Leaf, flower | [10–13] | |
| Linalyl acetate | Leaf | [11] | |
| Myrcene | Leaf, fruit | [10–12] | |
| Neral | Flower | [15] | |
| Nerol | Flower | [14] | |
| Neryl acetate | Flower | [15] | |
| p-Cymene | Leaf, fruit | [11, 12] | |
| Plinol a | Flower | [16] | |
| Plinol d | Flower | [16] | |
| Sabinene | Leaf, fruit | [10–12] | |
| Terpinen-4-ol | Leaf, fruit | [10–12] | |
| Terpinolene | Leaf, fruit | [10–12] | |
| Thujanol | Fruit | [12] | |
| trans-Linalool oxide acetate | Flower | [15] | |
| trans-β-Ocimene | Flower | [13, 14] | |
| α-Phellandrene | Leaf, fruit | [10–12] | |
| α-Pinene | Leaf, flower, fruit | [10–14] | |
| α-Pyronene | Fruit | [16] | |
| α-Terpinene | Leaf, fruit | [10–12] | |
| α-Terpineol | Leaf, fruit | [10, 12–14] | |
| α-Thujene | Leaf, fruit | [11, 12] | |
| β-Myrcene | Flower | [13, 14] | |
| β-Phellandrene | Leaf | [11] | |
| β-Pinene | Leaf, flower, fruit | [10–14] | |
| γ-Terpinene | Leaf, fruit | [10–12] | |
|
| |||
| Sesquiterpenes | (E,E)-Farnesal | Leaf | [11] |
| (E,E)-Farnesol | Leaf, flower | [11] | |
| (E,E)-α-Farnesene | Flower | [11, 13–15] | |
| (E,Z)-Farnesal | Leaf | [11] | |
| (2E,2Z)-Farnesal | Flower | [15] | |
| (2Z,6E)-Farnesyl acetate | Flower | [15] | |
| 1,10-diepi-Cubenol | Flower | [15] | |
| 1H-Indole | Flower | [16] | |
| 1-epi-Cubenol | Flower | [15] | |
| 5-Indanol | Flower | [15] | |
| Aromadendrene | Leaf | [11] | |
| Bicycloelemene | Flower | [15] | |
| Bicyclogermacrene | Leaf | [10–12] | |
| Calamene | Flower | [13] | |
| Caryophyllene epoxide | Leaf | [10] | |
| Caryophyllene oxide | Leaf, flower | [11, 12, 14] | |
| Cedrol | Flower | [13, 14] | |
| Copaborneol | Flower | [15] | |
| Cyperene | Flower | [15] | |
| Germacrene D | Leaf, flower, fruit | [10–14] | |
| Globulol | Leaf | [11] | |
| Guaiol | Flower | [15] | |
| Isogermacrene D | Flower | [15] | |
| Jejunol | Flower | [15] | |
| Levoglucosenone | Flower | [16] | |
| Selina-4(15),5-diene | Flower | [15] | |
| Spathulenol | Leaf | [11] | |
| t-Cadinol | Leaf | [10, 12] | |
| t-Muurolol | Flower | [13, 14] | |
| trans-Nerolidol | Flower | [13, 14] | |
| Viridiflorol | Leaf | [11] | |
| Zonarene | Flower | [15] | |
| α-Amorphene | Leaf, flower | [10, 12] | |
| α-Bisabolol | Flower | [13, 14] | |
| α-Bulnesene | Leaf | [11] | |
| α-Cadinol | Leaf | [10, 12] | |
| α-Cedrene | Flower | [13] | |
| α-Copaene | Leaf, flower | [10–12] | |
| α-Cubebene | Leaf | [11] | |
| α-Gurjunene | Leaf | [11, 12] | |
| α-Humulene | Leaf, flower, fruit | [10–14] | |
| α-Muurolene | Leaf | [10, 12] | |
| α-Ylangene | Leaf, flower | [10, 12–14] | |
| β-Bourbonene | Leaf, flower | [11, 14, 15] | |
| β-Caryophyllene | Leaf, flower, fruit | [10–12] | |
| β-Copaene | Leaf | [12] | |
| β-Cubebene | Leaf, flower | [10–12, 14] | |
| β-Elemene | Leaf | [11, 12] | |
| γ-Cadinene | Leaf | [10, 12] | |
| γ-Muurolene | Flower, fruit | [13] | |
| δ-Cadinene | Leaf, flower | [10–12] | |
| δ-Cadinol | Flower | [13, 14] | |
| δ-Elemene | Leaf | [10, 12] | |
| ε-Cadinene | Flower | [13] | |
| τ-Cadinene | Flower | [13] | |
| τ-Cadinol | Flower | [13, 14] | |
| τ-Muurolene | Flower | [13] | |
|
| |||
| Aliphatic compounds | (2E,6E)-Farnesyl acetate | Flower | [11, 13, 14, 17] |
| (E)-Hex-2-enal | Leaf | [10, 12] | |
| (E)-Hex-2-enol | Leaf, flower | [10, 12] | |
| (Z)-Hex-3-enol | Leaf, flower | [10, 12] | |
| 2-Hexenyl acetate | Flower | [13, 14] | |
| 2-Methyl-3-buten-2-ol | Flower | [13, 14] | |
| 3-Hexenyl acetate | Flower | [13, 14] | |
| 3-Methyl-2-buten-1-ol | Flower | [13, 14] | |
| 3-Methyl-2-buten-1-yl acetate (prenyl acetate) | Flower | [14, 17] | |
| Benzyl alcohol | Flower | [13, 14] | |
| Decane | Flower | [16] | |
| Diethyl 1,5-pentanedioate | Flower | [16] | |
| Dodecane | Flower | [16] | |
| Methyl 3-methylbutanoate | Flower | [16] | |
| Methyl caprylate | Flower | [16] | |
| n-Hexanol | Leaf, fruit | [10, 12] | |
| Heptanal | Flower | [15] | |
| Tetracosane | Flower | [15] | |
| Tricosane | Flower | [15] | |
| Undecane | Flower | [16] | |
|
| |||
| Phenylpropanoids | (E)-Cinnamyl acetate | Flower | [11, 13] |
| 1,4-Dimethylbenzene | Flower | [14] | |
| 1-Methoxy-1-propylbenzene | Flower | [16] | |
| 1-Phenyl-2-propen-1-ol | Flower | [16] | |
| 1-Phenylallyl acetate | Flower | [16] | |
| 2-Methoxy-4-methylphenol | Flower | [14] | |
| 2-Phenylethyl acetate | Flower | [13] | |
| 3,4-Dimethoxytoluene | Flower | [14] | |
| 3-Buten-2-ol benzoate | Flower | [14] | |
| 3-Hexen-1-ol benzoate | Flower | [14] | |
| 3-Methyl-2-buten-1-yl benzoate | Flower | [15] | |
| 4-(2-Propenyl)-phenol | Flower | [14] | |
| 4-Allyl-phenyl-acetate | Flower | [15] | |
| 4-Methoxy benzaldehyde | Flower | [16] | |
| 4-Methoxyphenyl acetate | Flower | [14] | |
| Anethol | Flower | [13, 14] | |
| Benzyl acetate | Flower | [11, 13, 14] | |
| Benzyl benzoate | Flower | [11, 13, 14] | |
| Benzyl salicylate | Flower | [11, 13, 14] | |
| Benzylaldehyde | Flower | [14] | |
| Benzyl-n-butyrate | Flower | [14] | |
| Butyl benzoate | Flower | [14] | |
| Cinnamyl alcohol | Flower | [14] | |
| Ethyl benzoate | Flower | [13, 14] | |
| Isoeugenol | Flower | [14] | |
| Methoxyphenol | Flower | [14] | |
| Methyl benzoate | Flower | [11, 13, 14] | |
| Methyl-2-methoxybenzoate | Flower | [14] | |
| Methyl-4-methoxybenzoate | Flower | [14] | |
| Methyleugenol | Flower | [13, 14] | |
| p-Cresyl methyl ether (p-methylanisole) | Flower | [13, 17, 18] | |
| p-Vinyl-guaiacol | Flower | [15] | |
| Vanillin | Flower | [15] | |
| Veratrole | Flower | [15] | |
|
| |||
| Nitrogen-bearing compounds | Phenylacetonitrile | Flower | [14] |
| 2-Phenyl-1-nitroethane | Flower | [14] | |
| Methyl anthranilate | Flower | [14] | |
Figure 2.
The molecular structures of the constituents isolated from different part of C. odorata.
There are various factors that can influence the chemical composition and quality of the volatile secondary metabolites being extracted from the aromatic plants and flowers, particularly the extraction method, extraction time, and the flower conditions [13]. The essential oil extracted from the flower of C. odorata is the important main raw material for perfume industry. To date, four grades of ylang-ylang oil have been developed and are commercially available: the Extra, First, Second, and Third which contain different chemical compositions that determine the quality and uses of the oil. The Extra quality of ylang-ylang oil is highly recommended to be used in production of high-grade perfumes. This is because the Extra grade oil is rich in strongly odoriferous molecules such as linalool (8),p-cresyl methyl ether (p-methylanisole) (1), methyl benzoate (2), benzyl acetate (4), and geranyl acetate (5) which are the volatile compounds that give the fragrance. Meanwhile the other grades contain increasing amount of sesquiterpene hydrocarbons which are less volatile such as β-caryophyllene (12), germacrene D (11), and (E,E)-α-farnesene (14). For instance, the First and Second grades are used in cosmetics. Lastly, the Third grade oil is being used for scenting of soaps. Besides depending on the fractionation based on distillation times, the chemical composition of ylang-ylang essential oils can be varied significantly depending on the stages of flower maturity [59, 61] and also geographical area which presents different environmental and agronomic conditions [15, 17]. Qin and colleagues [59] revealed high level of volatile polymorphism occurring along the 7 different flower development stages with only 52.45% of Bray-Curtis similarity value among all stages. The study showed that large amounts of volatile compounds including hydrocarbon, esters, and alcohols were detected in the full bloom stage of C. odorata which was the most suitable period for harvesting as those volatile compounds may have contributed to the aroma profile of C. odorata [59].
In term of geographical locations, by comparing the essential oil in the flower and fruits, the fruits of C. odorata from Cameroon were found to contain more abundant of monoterpenic essential oil such as sabinene (15), myrcene (16), α-pinene (17), and terpinen-4-ol (18) while the composition of essential oils present in the leaves of C. odorata from Cameroon was quite similar as compared to flower essential oil [10]. Similarly, another study [11] revealed that the composition of essential oil present in the leaves of C. odorata from Australia was relatively similar to the findings from Cameroon [10] but with larger amounts of hexanol (19) and absence of sabinene (15). More recently, a study focused on the variation in the chemical profiles of essential oils from C. odorata among the Western Indian Ocean islands such as Union of Comoros, Madagascar, and Mayotte as they are known to be the current main producers of ylang-ylang essential oils [15]. The study revealed that there is a significantly high variation in terms of the proportion of essential oils constituents from each area of origin throughout the Western Indian Ocean islands [15].
With the advancement of bioinformatics, a number of genes responsible for volatile compounds biosynthesis pathway were elucidated with use of high-throughput RNA sequencing technology. Jin and colleagues [61] successfully characterized the functionality of four full-length of terpene synthases (TPSs), CoTPS1, CoTPS2, CoTPS3, and CoTPS4, extracted from yellow flower of C. odorata. One of the TPSs specifically known as CoTPS2 was found to be novel and multifunctional in which it could catalyze the synthesis of sesquiterpenes including β-ylangene (20), β-copaene (21), and β-cubebene (22) [61].
Besides the extensive studies on the phytoconstituents in the essential oil of C. odorata, the medicinal properties of nonvolatile constituents from the plant part have been investigated and reported as well. Several new compounds were isolated from the methanolic extract of the seeds of C. odorata in 1999 [62]. The study revealed a new stereoisomer of ushinsunine-β-N-oxide (23) and another 10 newly discovered compounds from this species for the first time. The isolated compounds from this extract are listed in (Table 4). For instance, liriodenine (24), a cytotoxic oxoaporphine alkaloid, isolated from C, odorata was demonstrated to be a potent inhibitor of topoisomerase II in both in vitro and in vivo [62]. Besides the cytotoxic and antineoplastic activity of this compound, liriodenine (24) was also shown to be active against Gram-positive bacteria, yeast, and filamentous fungi. Sampangine (25) was another alkaloid isolated from the chloroform extract of the stem bark of C. odorata [19]. Literatures revealed that sampangine (25), a copyrine alkaloid, exhibited antifungal, antimycobacterial, antimalarial activities and demonstrated cytotoxic effects toward human malignant melanoma cells [63]. A more recent study isolated and characterized four compounds from the fruits of C. odorata including cananodine (26), new guaipyridine sesquiterpenes, cryptomeridiol 11-α-L-rhamnoside (27) and γ-eudesmol 11-α-L-rhamnoside (28), both being new eudesmane sesquiterpenes, and lastly the γ-eudesmol (29), a previously known eudesmane sesquiterpene [20]. The study also demonstrated that all the identified compounds displayed cytotoxicity against both hepatocarcinoma cancer cell lines, Hep G2, and Hep 2,2,15. Cryptomeridiol 11-α-L-rhamnoside (27) and γ-eudesmol (29) exhibited the most potent cytotoxic activity against Hep G2 and Hep 2,2,15 cell lines. Moreover, Ragasa and colleagues [33] revealed the isolation of methyl isoeugenol (30), benzyl benzoate (3), and farnesyl acetate (7) from dichloromethane extract of air dried flower of C. odorata. The study further showed that the compound methyl isoeugenol (30) exhibited antibacterial and antifungal activities [33].
Table 4.
The identified chemical constituents from different extracts of C. odorata.
| Extracts | Family | Name of constituents | References |
|---|---|---|---|
| Methanolic extract of C. odorata seed | Quinoline alkaloids | (+)-Ushinsunine-β-N-oxide | [18] |
| Cleistopholine | [18] | ||
| Liriodenine | [18] | ||
| (−)-Anonaine | [18] | ||
| (+)-Nornuciferine | [18] | ||
| (+)-N-Acetylnornuciferine | [18] | ||
| (−)-Ushinsunine | [18] | ||
| (−)-Norushinsunine | [18] | ||
| (−)-Asimilobine | [18] | ||
| (+)-Reticuline | [18] | ||
| Lyscamine | [18] | ||
| (−)-Anaxagoreine | [18] | ||
| Phytosterols | Stigmasterol | [18] | |
| β-Sitosterol | [18] | ||
| Phenylpropanoids | N-trans-Feruloyltyramine | [18] | |
| trans-Cinnamic acid | [18] | ||
|
| |||
| Chloroform extract of C. odorata stem bark | Quinoline alkaloids | Liriodenine | [19] |
| Sampangine | [19] | ||
|
| |||
| Methanolic extract of C. odorata fruit | Guaipyridine alkaloids | Cananodine | [20] |
| Cycloeudesmane sesquiterpenoids | Cryptomeridiol 11-α-L-rhamnoside | [20] | |
| γ-Eudesmol 11-α-L-rhamnoside | [20] | ||
| γ-Eudesmol | [20] | ||
| Quinoline alkaloids | Cleistopholine | [20] | |
| (+)-Ushinsunine-β-N-oxide | [20] | ||
| Lyscamine | [20] | ||
| Phenylpropanoids | N-trans-Feruloyltyramine | [20] | |
|
| |||
| Acetone extract of C. odorata stems and leaves | Lactones | Isosiphonodine | [21] |
| Canangone | [21] | ||
|
| |||
| Methanol extract of dried leaves of C. odorata | Megastigmane glycoside | Canangaionoside | [22] |
| Breyniaionoside A | [22] | ||
| Citroside A | [22] | ||
|
| |||
| Methanol extract of flower buds of C. odorata | Lignans | Canangalignan I | [8] |
| Canangalignan II | [8] | ||
| Canangaterpene I | [8] | ||
| Terpenoids | Canangaterpene II | [8] | |
| Canangaterpene III | [8] | ||
| Canangaterpene IV | [23] | ||
| Canangaterpene V | [23] | ||
| Canangaterpene VI | [23] | ||
| (3R,3aR,8aS)-3-Isopropyl-8a-methyl-8-oxo-1,2,3,3a,6,7,8,8a-octahydroazulene-5-carbaldehyde | [8] | ||
|
| |||
| Methanol extract of leaves of C. odorata var. fruticosa | Monoterpene glucosides | Canangafruticoside A | [24] |
| Canangafruticoside B | [24] | ||
| Canangafruticoside C | [24] | ||
| Canangafruticoside D | [24] | ||
| Canangafruticoside E | [24] | ||
| Ionone glucosides | Corchoionoside C | [24] | |
| Lignans | (+)-Syringaresinol 4-O-β-D-glucopyranoside | [24] | |
Furthermore, two lactone compounds have been isolated from the leaves and stems of C. odorata in conjunction with the searching for bioactive constituents from the C. odorata plant by a group of researchers [21]. Isosiphonodin (31) and a new spirolactone, named as canangone (32) were the two lactones isolated and identified from the acetone extract of dried leaves and stems of C. odorata [21]. Recently, a new megastigmane glucoside named as canangaionoside (33) was identified from the methanolic extract of the dried leaves of C. odorata [22]. Three new lignan dicarboxylates and six new terpenoid derivatives were also isolated by Matsumoto and colleagues from the methanolic extract of C. odorata flower buds [8, 23]. The new lignans isolated from the flower buds of C. odorata were named as canangalignans I (34) and II (35) [8], whereas canangaterpenes I, II, III, IV, V, and VI (36–41) were the six new terpenoid derivatives identified from the methanolic extract of C. odorata flower buds [8, 23]. They also indicated that one of the newly discovered terpenoids, canangaterpene I (36), exhibited potent antimelanogenesis activity [8]. Lastly, five usual monoterpene glucosides were also isolated and named as canangafruticosides A-E (42–46) by Nagashima and colleagues [24]. The chemical structures of both nonvolatile and volatile chemical compounds mentioned above are illustrated in Figure 2.
7. Bioactivities of C. odorata
Various biological activities of C. odorata have been extensively studied over the past decades. The detailed information of respective biological activities of C. odorata is being discussed as below. A summarized form of biological activities of C. odorata is then provided in Table 6.
Table 6.
Bioactivities of C. odorata essential oils and extracts.
| Bioactivities | Part used | Type of extracts | Dosage/Results | Suggested constituents with respective activities | References |
|---|---|---|---|---|---|
| Antimicrobial (i) Antibacterial (ii) Antifungal (iii) Antiprotozoal |
(i) Whole plant (ii) Bark (iii) Leaf |
(i) Essential oil (ii) n-Hexane (iii) Ethyl acetate (iv) Ethanolic (v) Methanolic (vi) Cyclohexane (vii) Petroleum ether (viii) Chloroform |
(i) Well diffusion assay: 100–400 µg/well tested against variety of Gram-positive, Gram-negative bacteria and fungi | (i) Linalool (ii) Linalyl acetate (iii) Liriodenine (iv) O-Methylmoschatoline (v) 3,4-Dihydroxybenzoic acid (vi) Methyl eugenol (vii) n/a (viii) n/a |
[26] |
| (ii) Disc diffusion assay: 200–400 μg/disc tested against variety of Gram-positive, Gram-negative bacteria and fungi | [28] | ||||
| (iii) 0.23 mg/mL (MIC90%) against S. aureus | [32] | ||||
| (iv) 12.5 ± 3.9 µg/mL (IC50) against P. falciparum FcB1 strain | [33] | ||||
|
| |||||
| Antibiofilm | Flower | Essential oil | (i) 0.01% (v/v) showed 80% inhibition against biofilm for S. aureus ATCC 6538 | (i) cis-Nerolidol (ii) trans-Nerolidol |
[5] |
| (ii) Inhibit adherence phase of both clinical strains of S. aureus and K. pneumonia (2 logs reduction) | |||||
|
| |||||
| Antioxidant | (i) Bark (ii) Leaf (iii) Flower |
(i) Ethyl acetate (ii) Methanolic (iii) Essential oil |
(i) 79% DPPH inhibition tested at 50 ppm | (i) n/a | [26] |
| (ii) 290.0 ± 13.1% of ferric reducing power at 0.5 µg/mL | (ii) n/a | [34] | |||
| (iii) 63.8 ± 0.45% of DPPH inhibition | (iii) n/a | [30] | |||
| (iv) 75.5 ± 0.51% inhibition in the β-carotene bleaching test (v) DPPH radical scavenging activity (80.06 ± 0.02%) |
|||||
|
| |||||
| Insecticidal (i) A. aegypti (ii) C. quinquefasciatus (iii) An. Dirus (mosquitoes) |
Flower | Essential oil | (i) Tested 1%, 5%, and 10% (w/v) on A. aegypti, C. quinquefasciatus, and An. Dirus, LC50 values of 9.77%, 8.82%, and 4.99%, respectively | (i) n/a | [35] |
| (ii) 10% in soybean oil exhibited oviposition-deterrent and ovicidal activities | (ii) n/a | [36] | |||
| (iii) 0.1 mg/mL showed larvicidal activity against A. aegypti | (iii) n/a | [37] | |||
| (iv) LD50 at 52.96 ppm against immature stage of A. aegypti | (iv) n/a | [38] | |||
| (iv) Musa domestica (housefly) | (v) Prepared in ethyl alcohol, LT50 of 52.08 hours, and LC50 of 29.36% towards Musa domestica | (v) n/a | [39] | ||
| (v) R. speratus (termite) | (vi) 2 mg/filter showed 18.0 ± 5.8% and 94.0 ± 4.0% mortalities after 2 and 7 days of exposure | (vi) n/a | [40] | ||
| (vi) S. zeamais (agriculture pest) | (vii) LD50 value of 33.14 μg/adult | (vii) Linalool | [41] | ||
| (viii) LD50 value of 14.77 mg/L (vapour phase toxicity bioassay) | |||||
|
| |||||
| Insect repellent (i) A. aegypti (ii) C. quinquefasciatus (iii) An. dirus (mosquitoes) (iv) T. castaneum (beetle) |
(i) Flower (ii) Leaf |
Essential oil | (i) Prepared in soybean oil, ED50 of 0.045, 2.149, and <0.003 mg/cm2against A. aegypti, A. dirus,and C. quinquefasciatus,respectively | (i) Linalool | [42] |
| (ii) Protection time towards A. aegypti, A. dirus, and C. quinquefasciatus (8.4, 24.0, and 60.0 minutes, resp.) | |||||
| (iii) Prepared in ethyl alcohol, protection time against A. aegypti, and C. quinquefasciatus (86.67 ± 10.40 and 126.0 ± 15.77 minutes) at 0.33 µL/cm2 | (ii) n/a | [43] | |||
| (iv) Strongest repellent effect at 5 μL/g of oats | (iii) n/a | [3] | |||
| (v) 98% repellency after 2 and 4 hours exposure | |||||
|
| |||||
| Antimelanogenesis | (i) Flower bud | Methanolic | (i) Inhibition on melanin production in B16 melanoma 4A5 cells | (i) Canangaterpenes I (ii) (3R,3aR,8aS)-3-Isopropyl-8a-methyl-8-oxo-1,2,3,3a,6,7,8,8a-octahydroazulene-5-carbaldehyde |
[8] |
| (ii) Terpenoid derivatives, canangaterpenes I (IC50 = 3.6 µM), and (3R,3aR,8aS)-3-isopropyl-8a-methyl-8-oxo-1,2,3,3a,6,7,8,8a-octahydroazulene-5-carbaldehyde (IC50 = 2.5 µM) | |||||
| (ii) Seed | (iii) Inhibition on tyrosinase protein expression in mouse B16 melanoma cells | (iii) N-trans-Feruloyltyramine | [18] | ||
|
| |||||
| Anti-inflammatory | (i) n/a (ii) Leaf (iii) Fruit |
(i) Essential oil (ii) Methanolic (iii) Ethanolic |
(i) Strong lipoxygenase inhibitory effect (~80%) at 0.5 μg/mL | (i) Linalool | [6] |
| (ii) Inhibition on nitric oxide release in RAW264.7 (97.9 ± 14.6%) at 50 µg/mL | (ii) Linalyl acetate | [34] | |||
| (iii) In carrageenan induced paw edema model, paw volume inhibition of 62.9% at 100 mg/kg | (iii) n/a | [44] | |||
|
| |||||
| Sedative, relaxing, and harmonizing effect | n/a | Essential oil | (i) Reduced systolic and diastolic BP through sniffing | (i) n/a | [45] |
| (ii) Decreased pulse rate and stress level | |||||
| (iii) Increased alertness | |||||
| (iv) Transdermal administration resulted decrease in both physiological and behavioural level | (ii) n/a | [46] | |||
|
| |||||
| Effect on mood and cognitive performance | n/a | Essential oil | Reduced alertness mood and calmness but without increased cognitive performance | n/a | [47] |
|
| |||||
| Spermatotoxic | Root bark | Ethanolic | (i) Immobilized rat's sperm within seconds | (i) A 52 kd protein | [48] |
| (ii) 50 mg/100 g body weight/day reduced sperm motility | (ii) n/a | [49] | |||
| (iii) 100 mg/100 g body weight/day caused 94% abnormal sperm morphology | |||||
|
| |||||
| Antihyperglycemic | (i) Leaf and stem (ii) Flower buds |
(i) Dichloromethane (ii) Methanolic |
(i) Alpha-amylase inhibitory effect with 22.6 ± 1.3% (leaf) and 25.3 ± 3.3% (stem) inhibition at 7.8 μg/mL | (i) n/a | [7] |
| (ii) Aldose reductase inhibitory effect, IC50 at 1.2, 1.5, and 0.8 μM by canangaterpene I, (E)-[(1R,3R,5S,6S,8S)-6-hydroxy-1,3-dimethoxy-2-oxaspiro[4,5]decan-8-yl]methyl] caffeate, and canangafruiticoside E respectively | (ii) Canangaterpene I (iii) (E)-[(1R,3R,5S,6S,8S)-6-Hydroxy-1,3-dimethoxy-2-oxaspiro[4,5]decan-8-yl]methyl] caffeate (iv) Canangafruiticoside E |
[23] | |||
IC50: half maximal inhibitory concentration.
LD50: median lethal dose.
LT50: median lethal time.
ED50: median effective dose.
n/a: not available.
8. Antimicrobial Activity
In the last decade, the emergence of multidrug resistance pathogens and strains with reduced susceptibility due to indiscriminate use of antibiotics has become a global concern [64] as the clinical efficacy of many existing antibiotics has been compromised. As a consequence, the therapy of the infections inflicted by the multidrug resistant pathogen is complicated and has led to substantial increased hospitalizations and greater risk for morbidity and mortality [65]. This issue has necessitated the scientist to screen for novel antimicrobial substances from various medicinal plant sources including the essential oils or the extracts from aromatic plants which have been reported to possess phytochemicals with antimicrobial activities [66]. The antimicrobial properties of the essential oils and extracts of C. odorata have been tested against various Gram-positive and Gram-negative pathogens as well as pathogenic fungi (Table 5).
Table 5.
The antimicrobial activities screening of different C. odorata extracts.
| Plant part | Extracts | Pathogens tested | Screening assay | Reference |
|---|---|---|---|---|
| Bark | n/a | Gram-positive bacteria | Disc diffusion assay | [25] |
| Bacillus subtilis | ||||
| Bacillus megaterium | ||||
| Staphylococcus aureus | ||||
| Sarcina lutea | ||||
| Streptococcus-β-haemolyticus | ||||
| Gram-negative bacteria | ||||
| Escherichia coli | ||||
| Pseudomonas aeruginosa | ||||
| Shigella flexneri | ||||
| Shigella shiga | ||||
| Shigella boydii | ||||
| Shigella dysenteriae | ||||
| Shigella sonnei | ||||
| Salmonella typhi | ||||
| Klebsiella | ||||
| Fungi | ||||
| Aspergillus flavus | ||||
| Aspergillus niger | ||||
| Aspergillus versicolor | ||||
| Candida albicans | ||||
| n-hexane | Gram-positive bacteria | Well diffusion assay | [26] | |
| Propionibacterium acnes | ||||
| Fungi | ||||
| Candida albicans | ||||
| Ethyl acetate | Gram-positive bacteria | [26] | ||
| Propionibacterium acnes | ||||
| Fungi | ||||
| Candida albicans | ||||
| Ethanolic | Gram-positive bacteria | [26] | ||
| Propionibacterium acnes | ||||
| Fungi | ||||
| Candida albicans | ||||
| Ethanolic | Protozoan parasite | In vitro bioassay | [27] | |
| Cyclohexane | Plasmodium falciparum FcB1 strain | |||
| Methylene chloride | ||||
| Methanolic | ||||
|
| ||||
| Whole plant | Essential oils | Gram-positive bacteria | Disc diffusion assay | [28] |
| Methicillin-resistant Staphylococcus aureus ATCC 700699 | ||||
| Gram-positive bacteria | [29] | |||
| Bacillus cereus | ||||
| Bacillus subtilis | ||||
| Bacillus megaterium | ||||
| Bacillus polymyxa | ||||
| Streptococcus-β-haemolyticus | ||||
| Streptococcus aureus | ||||
| Streptococcus lutea | ||||
| Gram-negative bacteria | ||||
| Escherichia coli | ||||
| Shigella dysenteriae | ||||
| Shigella flexneri | ||||
| Shigella sonnei | ||||
| Pseudomonas aeruginosa | ||||
| Salmonella typhi B | ||||
| Salmonella paratyphi A | ||||
| Salmonella paratyphi B | ||||
| Fungi | [30] | |||
| Rhizopus oryzae | ||||
| Aspergillus niger | ||||
| Aspergillus fumigatus | ||||
| Aspergillus krusli | ||||
| Candida albicans | ||||
| Saccharomyces cerevisiae | ||||
| Fungi | ||||
| Candida albicans ATCC 48274 | ||||
| Rhodotorula glutinis ATCC 16740 | ||||
| Schizosaccharomyces pombe ATCC 60232 | ||||
| Saccharomyces cerevisiae ATCC 2365 | ||||
| Yarrowia lipolytica ATCC 16617 | ||||
|
| ||||
| Leaf | Methanolic | Gram-positive bacteria | Well diffusion assay | [31] |
| Staphylococcus aureus | ||||
| Gram-negative bacteria | ||||
| Salmonella typhi | ||||
| Escherichia coli | ||||
| Vibrio cholera | ||||
| Fungi | ||||
| Epidermophyton floccosum | ||||
| Microsporum gypseum | ||||
| Trichophyton mentagrophytes | ||||
| Protozoan parasite | In vitro bioassay | [27] | ||
| Plasmodium falciparum FcB1 strain | ||||
| Petroleum ether | Gram-positive bacteria | Well diffusion assay | [31] | |
| Staphylococcus aureus | ||||
| Gram-negative bacteria | ||||
| Salmonella typhi | ||||
| Escherichia coli | ||||
| Vibrio cholera | ||||
| Fungi | ||||
| Epidermophyton floccosum | ||||
| Microsporum gypseum | ||||
| Trichophyton mentagrophytes | ||||
| Chloroform | Gram-positive bacteria | Well diffusion assay | [31] | |
| Staphylococcus aureus | ||||
| Gram-negative bacteria | ||||
| Salmonella typhi | ||||
| Escherichia coli | ||||
| Vibrio cholera | ||||
| Fungi | ||||
| Epidermophyton floccosum | ||||
| Microsporum gypseum | ||||
| Trichophyton mentagrophytes | ||||
| Ethanolic | Protozoan parasite | In vitro bioassay | [27] | |
| Cyclohexane | Plasmodium falciparum FcB1 strain | |||
| Methylene chloride | ||||
n/a: not available.
Recently, the stem bark extracts of C. odorata obtained from Indonesia were shown to exhibit potent antimicrobial activities using the agar well disc diffusion assay. The study has demonstrated that n-hexane, ethyl acetate, and ethanolic extracts of C. odorata stem bark possessed good activity against Propionibacterium acnes and Candida albicans. The ethanolic extract of C. odorata at the dose of 400 μg/well exhibited an inhibition zone of 19 ± 1.58 mm when tested against P. acnes. In fact, the activity index stands at 0.63 when being relative to the standard drug which is known as chloramphenicol [26]. Among the three extracts of C. odorata tested, the n-hexane extracts of C. odorata stem bark showed the highest inhibitory effect on C. albicans growth (17 ± 1.58 mm) at the dose of 100 μg/well. It represents the activity index of 0.56 when being relative to the standard drug known as nystatin [26]. Meanwhile, in another study the researchers have taken the research to another next level of assessment. The purified constituents from the bark of plant were used to evaluate the antimicrobial activity on different species of bacteria. Besides, they have also examined the antifungi activities of these purified compounds [28]. In that particular research, those three tested compounds which are known as O-methylmoschatoline, liriodenine (24), and 3,4-dihydroxybenzoic acid were showing a significant antibacterial and antifungal activities at their respective dose at 200 μg/disc and 400 μg/disc. Among the three purified compounds, liriodenine (24) emerged as the strongest compound in exerting its antibacterial and antifungal activities against Klebsiella sp. and C. albicans, respectively [25].
On the other hand, a study was conducted specifically on the antimicrobial activity of the C. odorata leaf extracts. Three different extracts of C. odorata leaf were prepared and tested against selected Gram-positive and Gram-negative bacteria as well as different fungal strains [26]. The methanolic extract of C. odorata exhibited the highest antimicrobial activities as compared to petroleum ether and chloroform extracts. Moreover, the study also suggested that the Gram-negative bacteria demonstrated higher resistance than the Gram-positive bacteria against all the extracts of C. odorata leaf [31]. Similarly, the antimicrobial activity of the essential oil of C. odorata showed high inhibitory effect with MIC90% values at 0.23 mg/mL against S. aureus ATCC 25923 and clinical strains S. aureus [32]. However, both E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 showed high resistance towards the essential oils of C. odorata which did not show inhibitory capacity up to the maximum concentration (27.12 mg/mL) tested in the study [32]. The study also has characterized the essential oil of C. odorata using GC-MS and revealed that the essential oil of C. odorata contained trans-β-caryophyllene (12.92%), linalool (8) (11.38%), germacrene D (11) (11.21%), benzyl acetate (4) (10.34%), and geranyl acetate (5) (9.87%) [32].
Meanwhile, the essential oil of C. odorata obtained from steam distillation was shown to exhibit weak antibacterial activity against P. acnes strains [67]. The inhibition zones of C. odorata essential oils against 5 strains of P. acnes were only ranging from 8.8 ± 0.7 mm to 9.5 ± 0.7 mm [67] which were relatively smaller as compared to ethanolic extract of C. odorata described in [26]. In effort of discovering the potential usage of ylang-ylang oil as alternative treatment for irritable bowel syndrome, three different antibacterial assays, namely, disc diffusion assay, turbidometric assay, and zone of clearance assay were conducted against E. coli [68]. However, essential oil of C. odorata demonstrated relatively low antibacterial activity against E. coli where the essential oil of C. odorata did not inhibit the growth of E. coli in either the agar plate or liquid culture and also did not show any killing ability against E. coli from the zone clearance assay [68]. The essential oil of C. odorata has also showed to exhibit no inhibitory effect against Malassezia furfur, which is a fungal pathogen associated with seborrheic dermatitis [69]. In contrast, another study demonstrated that the essential oils of C. odorata which contained germacrene D (11) (20%) and β-caryophyllene (12) (17%) exhibited slight fungicidal activity (12 ± 2 mm) against Trichophyton mentagrophytes TIMM2789 using agar diffusion assay [70].
The synergistic effects of ylang-ylang oil with different combinations of essential oils for treatment of microbial infections have also been reported. For an example, a study has proven that the combinations of ylang-ylang oil and thyme oil were significantly more effective against S. aureus ATCC 25923 and its synergistic effect was observed between both of the essential oils in which the inhibition zone was increased by 38.4% as compared to thyme oil alone [71]. However, a slight antagonism effect was then observed when ylang-ylang oil was used together with thyme oil against Escherichia coli ATCC 25922, the inhibition zone was reduced by 48.9% when compared to thyme oil alone [71]. Similarly, another study revealed that blended essential oil preparation which is comprised of lavender, clary sage, and ylang-ylang oils in the ratio 3 : 4 : 3 displayed a strong antibacterial and antifungal activities against Staphylococcus aureus ATCC 6538, Staphylococcus epidermidis, Escherichia coli ATCC 25923, Pseudomonas aeruginosa ATCC 9027, and Candida albicans ATCC 10231 [72]. The results also revealed that the preparation showed a synergistic antimicrobial effect against all the tested microorganisms. The increased antimicrobial activities displayed from the blended essential oil preparation as compared to the single or pure essential oil were believed to be contributed by the increased active components such as linalool (8) and linalyl acetate (13) present in the blended preparation [72].
Besides that, antiplasmodial activity of C. odorata was also evaluated by a group of researchers from Vietnam [27]. Nyugen-Pouplin and colleague revealed that the cyclohexane extract of C. odorata leaves at 10 μg/mL exerted moderate antiplasmodial activity (75% inhibition) against Plasmodium falciparum FcB1 strain with IC50 value of 12.5 ± 3.9 μg/mL [27]. The result of present study somehow ascertains the folkloric claim on C. odorata used as medicinal plant to treat malaria and malaria-like symptoms in Indonesia and Vietnam.
Overall, ylang-ylang oil and different extracts of C. odorata showed better antibacterial activities against Gram-positive bacteria than Gram-negative bacteria. For instance, S. aureus showed high susceptibility to the essential oils and extracts of C. odorata as compared to other tested Gram-negative bacteria. Studies also showed that C. odorata exhibited a remarkable antifungal activity. Disc and well diffusion assay were the most common tests being employed to evaluate the antimicrobial activity of the essential oil and extracts of C. odorata. Although the antimicrobial activity of C. odorata tested was not as potent as other essential oils and extracts of other plants, studies have demonstrated that the synergistic effects observed from the combinations of different medicinal plants and herbs may potentiate the antimicrobial activities against pathogens.
9. Antibiofilm Properties
Many bacteria possess the ability to form biofilm, which is a slimy layer comprised of bacterial cells that protected by self-synthesized matrices of polysaccharides and proteins that allows attachment to various surfaces such as polystyrene, glass, and stainless steel in different environments [73]. The formation of microbial biofilms poses a significant challenge to current clinical and industrial settings as microbial biofilms are associated with dramatically enhanced tolerance towards most antimicrobial agents and disinfectant chemicals as well as the body's immune system. Hence, the increased resistance developed by the formation of biofilm contributes to the chronicity of microbial infections and leading to therapy failure [74]. Although many approaches have been implemented in controlling biofilms, the discovery for novel, natural, and effective antibiofilm agents is still undergoing in order to cope with the increased biofilm-associated health problems. The plant-derived essential oils have been explored extensively to combat biofilm formation. For instance, oregano oil [75], eucalyptus oil [76], tea tree oil [77], cinnamon oil [73], and lemon grass [78] have been demonstrated to exhibited potent antibiofilm activities against wide range of bacteria. Recently, the antibiofilm activity of cananga oil also has been evaluated in several studies [5]. A study revealed that ylang-ylang oil exhibited strong antibiofilm activity at dose-dependent manner against biofilm formation of Staphylococcus aureus ATCC 6538 [5]. The study utilized a static biofilm formation assay, confocal laser microscopy, and also scanning electron microscopy to examine the effect of cananga oil on biofilm formation of S. aureus [5]. It was found that 0.01% (v/v) of ylang-ylang oil showed more than 80% inhibition against biofilm formation of S. aureus as compared to the control group but did not inhibit the growth of S. aureus. Furthermore, the study also suggested that both cis-nerolidol and trans-nerolidol were the constituents in ylang-ylang oil that is responsible for the inhibition of biofilm formation [5]. Furthermore, another study combined the unique properties of magnetic nanoparticles which have been reported to be effective delivery systems with the ylang-ylang oil as a coating agent for surfaces of implantations with the intention to reduce the development of biofilm [79]. The study has shown the incorporation of ylang-ylang oil with iron oxide@C14 nanoparticles effective in inhibiting the initial adherence phase of clinical strains of both S. aureus and Klebsiella pneumonia with more than 2-log reduction to the coated catheter specimens [79]. The results of the study have suggested the potential use of ylang-ylang oil in nanobiosystems with antibiofilm activity [79].
10. Antioxidant Properties
The generation of free-radical intermediates through oxidative stress has been known to cause disturbances in metabolic processes. They are known to be responsible for cellular injuries and disease formation due to the destruction of unsaturated lipids, proteins, and DNA. The implications of oxidative damage have been linked to many human diseases such as cancer, cardiovascular diseases, inflammatory processes, cataracts, and even the normal ageing process [80]. Recently, natural occurring antioxidants have been of great interest because of people's concerns over the use of synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate, and tert-butylhydroquinone (TBHQ) which may have adverse effects on human health [81]. The antioxidant activity of C. odorata extracts was evaluated using DPPH assay to determine the free radical scavenging abilities of the extracts. The result of the study revealed that the ethyl acetate extract of the stem bark of C. odorata exhibited the highest percentage of DPPH inhibition (79%) as compared to other tested plant extracts [26]. Besides the DPPH assay, the antioxidant activity of methanolic extract of C. odorata leaves was also determined by ferric ion reducing power assay. The extract showed a total of 290.0 ± 13.1% of ferric reducing power at 0.5 μg/mL [34].
Normally, a series of antioxidant assays will be utilized to examine different aspects of antioxidant property of plant extract. In a particular study, antioxidant activity of the C. odorata essential oils was assessed using free radical-scavenging, β-carotene bleaching, and the luminol-photochemiluminescence assays [30]. The results of the study revealed that all the tests indicated that essential oil of C. odorata was a decent source of antioxidant. In detail, the free radical-scavenging activity of C. odorata was 63.8 ± 0.45% of DPPH inhibition and the value was twice higher than that of trolox, one of the reference oils with potent antioxidant activity. Furthermore, the results were further supported by the lipid peroxidation inhibitory activity displayed by the essential oil of C. odorata (75.5 ± 0.51% inhibition) in the β-carotene bleaching test. The luminol-photochemiluminescence assay also showed that the essential oil of C. odorata exhibited effective superoxide radical scavenging activity [30]. Consistently, the essential oils extracted from the flower of C. odorata originated from Madagascar also exhibited good DPPH radical scavenging activity (80.06 ± 0.02%) [82].
11. Antivector/Insecticidal/Antipest Properties
Dengue disease, which is a tropical and subtropical mosquito-borne viral illness, has become a public health concern worldwide. According to World Health Organization [83], statistics showed that approximately 2.5 billion people live in countries that are endemic for dengue and estimated that 50–100 million infections occur annually. There was dramatic increase in the number of reported cases of dengue disease in Malaysia, particularly in 2013 where incidences of dengue fever (143.27 per 100,000 population) were doubled as compared to 2012 (72.2 per 100,000 population) [84, 85]. However, the prevention of dengue fever is only restricted to managing the vector Aedes aegypti due to absence of effective prophylactics or vaccine against the infection. Generally, synthetic insecticides such as DDT and other chlorinated hydrocarbons are used to control the mosquitoes. However, the continuous application of these synthetic compounds has resulted in the development of resistant strains of mosquito vectors particularly A. aegypti. Hence, considerable study has shifted the interest towards natural products which may be effective in controlling the vector population. Larvicidal, ovicidal and repellent properties of essential oils and extracts from several plant species against mosquito vector have been evaluated including Cananga odorata. Studies have demonstrated that the essential oil of Cananga odorata possessed repellent properties as well as oviposition-deterrent and ovicidal activities against several mosquito species. In 2011, the insecticidal activity of the essential oils of Cananga odorata prepared in soybean oil was evaluated using standard WHO susceptibility testing protocols. It was found that the essential oil extracted from ylang-ylang flower at doses of 1%, 5%, and 10% (w/v) exhibited low insecticidal activity and knockout rate against all three types of adult mosquito species including the Aedes aegypti, Culex quinquefasciatus, and Anopheles dirus, with LC50 values of 9.77%, 8.82%, and 4.99%, respectively [35]. Targeting the breeding sites of mosquitoes is one of the effective strategies to control and eradicate the population density of the mosquito vectors. Furthermore, the mosquito life cycle can be disrupted by preventing them from undergoing oviposition which is an important event shaping both individual fitness and vectorial capacity in life history of mosquito [86]. Study has revealed that the essential oil of C. odorata may serve as a potential mosquito egg control agent against the species of Aedes aegypti, Anopheles dirus, and Culex quinquefasciatus. It was found that 10% C. odorata in soybean oil exhibited significantly high oviposition-deterrent and ovicidal activities against all three tested mosquito species. However, further study was suggested by the author as most of the results obtained from previous studies related to oviposition-deterrent and ovicidal were not promising and most of the mosquito eggs were shown to be tolerant to the action of insecticides [36]. Besides that, larvicidal and pupicidal activities of the essential oil of Cananga odorata against three immature stages of Aedes aegypti, Anopheles dirus, and Culex quinquefasciatus were evaluated [87]. Although the essential oil of C. odorata was not as effective as the essential oil of Syzygium aromaticum which was the most effective against all immature stages of the three tested mosquito species in the study, higher larvicidal and pupicidal activities were demonstrated by C. odorata essential oils against all immature stages of both C. quinquefasciatus and Anopheles dirus as compared to A. aegypti [87]. Similar results were also demonstrated in [37] whereby the essential oils of C. odorata exhibited low larvicidal activity against A. aegypti with only 40.0 ± 4.1% mortality observed at dose of 0.1 mg/mL. In a more recent study on the larvicidal activity of C. odorata, the chemical composition of the essential oils was determined with GC-MS and was evaluated together with the insecticidal activity of the plants against the third and fourth instar stage of A. aegypti [38]. The study revealed that the essential oils of C. odorata demonstrated moderate insecticidal activity with LD50 at 52.96 ppm against the immature stage of A. aegypti among the plants evaluated [38]. Benzyl acetate (4), linalool (8), and benzyl benzoate (3) were the three major compounds identified from the essential oils of C. odorata with the percentage of 18.2%, 14.1%, and 12.3%, respectively [38].
Moreover, recent study also showed that C. odorata oil prepared in ethyl alcohol possessed larvicidal effect and oviposition-deterrent activity as well against house fly, Musca domestica. The control of house fly is also essential as it is known to be a serious disease causing pest which can transmit pathogenic organisms such as protozoa cysts, parasites, enteropathogenic bacteria, and enterovirus to human and livestock. The study demonstrated that C. odorata oil exhibited larvicidal effect against the 3rd instar larvae of house fly with median lethal time (LT50) value of 52.08 hours and LC50 value of 29.36% as compared to cypermethrin (10% w/v), a common chemical insecticide, with LT50 and LC50 of 31.63 hours and 11.45%, respectively [39]. Furthermore, excellent oviposition-deterrent activity was also demonstrated by C. odorata oil with 100% effective repellency value against the female house fly from undergoing oviposition at both concentrations of 1.65 μL/cm2 and 3.30 μL/cm2 [39].
Seo and colleague [40] also assessed the insecticidal activities of the essential oil from C. odorata flower against Japanese termite, Reticulitermes speratus Kolbe. The fumigation bioassay employed by the study [40] found that the essential oil C. odorata at 2 mg/filter paper resulted in cumulative mortalities of 18.0 ± 5.8% and 94.0 ± 4.0% of the termites after 2 and 7 days of exposure, respectively.
Besides that, the essential oil of C. odorata leaves has been demonstrated to possess antipest properties as well and could be considered to have the potential to be developed as possible natural fumigant or insecticide for control of insect associated with storage products [41]. The study showed that topical application of essential oil of C. odorata leaves exhibited toxicity against Sitophilus zeamais, which is a pest associated with corn storage, with a LD50 value of 33.14 μg/adult [41]. Furthermore, fumigant activity of C. odorata essential oil against S. zeamais was also evaluated using vapour phase toxicity bioassay. The results showed that the essential oil of C. odorata leaves exhibited fumigant toxicity against S. zeamais with a LD50 value of 14.77 mg/L. The study also suggested that linalool (8), which is a competitive inhibitor of acetylcholinesterase, might be the active component that accounted for the insecticidal activity of C. odorata essential oil [41].
12. Insect-Repellent Properties
Insect repellent is known to be one of the most effective ways to reduce the transmission of vector-borne diseases especially from mosquito [88]. With the fact that no effective vaccine against dengue is available, protection from mosquito bites could be only achieved by preventing physical contact with mosquitoes using repellents. Studies have indicated that the essential oil of Cananga odorata prepared in soybean oil possessed certain degree of repellent activity against the adult mosquito of A. aegypti, A. dirus, and C. quinquefasciatus with the ED50 of 0.045, 2.149, and <0.003 mg/cm2. The essential oil of Cananga odorata also demonstrated a moderate time of protection against A. aegypti, A. dirus, and C. quinquefasciatus at a duration of 8.4, 24.0, and 60.0 minutes, respectively [42] even though the protection time of DEET-based repellent which remains the gold standard of protection, in which 23.8% DEET showed a complete 5 hours protection against A. aegypyi bites [89]. Meanwhile, another study revealed that the protection time was improved by C. odorata oil prepared in ethyl alcohol at 0.33 μL/cm2 against A. aegypti and C. quinquefasciatus with 86.67 ± 10.40 and 126.0 ± 15.77 minutes, respectively [43]. Similarly, a more recent study revealed that essential oil of C. odorata prepared in coconut oil at 0.33 μL/cm2 showed a better activity with 98.9% protection from bites of A. aegypti with an improved protection time for 88.7 ± 10.4 minutes among the three tested diluents [4]. The discrepancy between the studies may be due to many factors that might affect the efficacy of the repellent such as the species and density of mosquito, the age, gender, and biochemical attractiveness of the subject as well as the experimental conditions [43]. Most of the studies indicated above have shown that indeed the essential oils of C. odorata demonstrated good mosquito-repelling properties against different species of mosquitoes.
Besides the repellent activity against mosquito, the essential oil of C. odorata leaves has been shown to exhibit repellent activity against Tribolium castaneum, a red flour beetle which is known to be the pest associated with stored products, hence protecting the stored products from insect damage [3, 90]. The essential oil of C. odorata leaves was shown to have the strongest repellent effect against T. castaneum at concentration of 5 μL per gram of oats as compared to other tested essential oils in the study [90]. Caballero-Gallardo and colleagues [3] also demonstrated that essential oil from C. odorata exhibited the highest percentage of repellency of 98% at 0.2 μg/cm2 after both exposure times of 2 and 4 hours against T. castaneum, suggesting that it can be considered excellent candidates as natural repellents.
13. Antimelanogenesis
Melanin production or melanogenesis determines the skin color of animals and humans. Although melanogenesis is a major protective mechanism against UV-induced skin injury, the excessive production of melanin due to extensive UV exposure can lead to dermatological disorders. There has been increasing interest towards the findings of alternative herbal for treatment of hyperpigmentation because of the increased reports of potential mutagenicity and cases of ochronosis due the use of tyrosinase inhibitor such as hydroquinone, which is one of the most widely prescribed compounds found in nowadays cosmetic products and depigmenting agents [91]. Recently, the methanolic extract of the flower buds of C. odorata was found to exhibit inhibitory effect against melanogenesis [8]. The inhibitory effect of the constituents extracted from the flower buds of C. odorata was demonstrated by the detection of the melanin content in theophylline-stimulated B16 melanoma 4A5 cells via photometric method at 405 nm [8]. The study indicated that several compounds isolated from the methanolic extract of the flower buds of C. odorata displayed the inhibitory effect on melanogenesis and without induced any cytotoxicity to B16 melanoma 4A5 cells. Furthermore, there were two terpenoid derivatives (compound 5, canangaterpenes I (36) (IC50 = 3.6 μM) and compound 12, (3R,3aR,8aS)-3-isopropyl-8a-methyl-8-oxo-1,2,3,3a,6,7,8,8a-octahydroazulene-5-carbaldehyde (47) (IC50 = 2.5 μM)) exhibited stronger activity in inhibiting the production of melanin than the positive control, arbutin (IC50 = 174 μM) [8]. Also, the study found that lignans with a catechol moiety and without the glucosyl moieties are essential for the inhibitory activity of melanogenesis [8]. Therefore, the study showed that the flower buds of C. odorata contain terpenoid derivatives which may have high potential for the treatment of skin disorder or cosmetic industry. Besides that, N-trans-feruloyltyramine (48), which was a phenylpropanoid isolated from the methanolic extract of the seeds of C. odorata [18], may be another constituent that is responsible for the suppression of melanogenesis as this compound has been reported to show more potent inhibitory activity on the expression of tyrosinase protein (an important enzyme in melanin biosynthesis) in mouse B16 melanoma cells than the kojic acid (a tyrosinase inhibitor) [92]. In contrast, a study showed that the aqueous extract of C. odorata did not inhibit dopachrome formation (−19.8 ± 0.7%) which indicated that no antityrosinase activity exhibited by the aqueous extract of C. odorata [93]. These observations deduced that the inhibitory effects on melanogenesis of C. odorata extracts are involving the regulation of tyrosinase gene expression rather than the direct inhibition of tyrosinase activity.
14. Anti-Inflammatory Properties
Inflammatory diseases such as rheumatism, arthritis, and pelvic inflammatory disease continue to be one of the major health concerns worldwide. Traditional remedies have been known to be one of the most common ways to treat inflammatory diseases. For instance, the folkloric practice of treating joint pain with Willow (Salix alba) bark has led to the discovery of aspirin as the most commonly used pain reliever for 100 years [94]. Despite that, many steroidal and nonsteroidal anti-inflammatory drugs (NSAIDs) have been introduced to treat various inflammatory disorders. However, adverse side effects including renal problems, gastrointestinal irritation, and even myocardial infarction and strokes have been reported due to the prolonged use of steroidal and NSAIDs [94]. Hence, researchers have becoming more interested in evaluating the anti-inflammatory potential of plants traditionally used for relieving aches, asthma, and pains for the discovery and development of potent anti-inflammatory drugs. Traditionally, different parts of C. odorata plants have been exploited and used to treat fever, asthma, and pains. Several scientific evaluations were also conducted on the anti-inflammatory activities of C. odorata.
Wei and Shibamoto [6] demonstrated that the essential oil of C. odorata displayed anti-inflammatory properties using 15-lipoxygenase inhibitor screening assay. Lipoxygenases are enzymes that catalyze the metabolism of arachidonic acid in producing metabolites that regulate inflammatory response in mammals. The essential oil of C. odorata showed strong lipoxygenase inhibitory effect (~80%) at a concentration of 0.5 μg/mL and also exhibited lipoxygenase inhibitory activity that appeared similar to nordihydroguaiaretic acid, a standard lipoxygenase inhibitory chemical, in a reverse dose-response manner [6]. The study also suggested that the lipoxygenase inhibitory effect was accounted by the major constituents present in the essential oils such as linalool (8), linalyl acetate (13), and other volatile constituents [6]. These chemical constituents are normally found to possess anti-inflammatory activities in previous experimentations [95]. Furthermore, the methanolic extract of C. odorata leaves was shown to possess moderate inhibitory effect (97.9 ± 14.6%) on nitric oxide release in macrophage RAW264.7 cells with low cytotoxicity (cell viability: 89.7 ± 0.5%) at 50 μg/mL [34]. Although nitric oxide is produced to act as a defense and regulatory molecule during inflammatory reactions, it may damage normal tissue when it is excessively produced [34, 96]. Overall, the findings indicated that the methanolic extract of C. odorata leaves may be a potential anti-inflammatory agent as the release of nitric oxide by macrophages has long been associated with inflammation.
Besides the in vitro studies mentioned above, the anti-inflammatory activity of C. odorata also had been evaluated in experimental animals recently. The ethanolic extract of C. odorata fruit was shown to exhibit significant anti-inflammatory activity in the carrageenan induced paw edema model of Wistar albino rats with LD50 > 2000 mg/kg [44]. The acute oral toxicity study indicated that the ethanolic extract C. odorata fruit was more effective in inhibition of paw volume (62.9%) at dose of 100 mg/kg than aspirin with inhibition of 60.14% at dose of 300 mg/kg. Furthermore, the author of the study suggested that anti-inflammatory effect of the extract might be due to the presence of flavonoids and tannins which is responsible for inhibiting both cyclooxygenase and lipoxygenase pathway [44].
15. Sedative, Relaxing, and Harmonizing Effects
The essential oil obtained from the leaves of C. odorata using hydrodistillation method extraction was shown to possess sedative effect and certain degree of physiological influence on human [45]. The study indicated that sniffing C. odorata oil decreased the systolic and diastolic blood pressure of human from 106.43 ± 11.51 mmHg to 105.20 ± 10.72 mmHg and 70.60 ± 10.53 mmHg to 69.20 ± 11.71 mmHg, respectively, demonstrating that the oil exhibited sedative effect. The results were further supported by the decreased pulse rate after sniffing C. odorata (73.40 ± 7.38 bpm) as compared to the control (75.33 ± 7.55 bpm). On top of that, the study also found that C. odorata essential oil exhibited relaxing effect on the volunteers after sniffing the oil, reducing the stress index from high level (73.33 KU/L) to medium level (49.50 KU/L). The stress level was also determined by measuring the alpha brain wave of the volunteers and the results showed that sniffing the essential oil of C. odorata increased an individual's alpha brain wave or also decreased one's stress level [45]. Similar results were also evidenced by Hongratanaworakit and Buchbauer [46] whereby the inhalation of ylang-ylang oil significantly decreased both systolic and diastolic blood pressure and pulse rate, indicating that inhalation of ylang-ylang oil decreased autonomic nervous system arousal. Besides that, the similar study [46] evaluated the effect of inhalation of ylang-ylang on the behavioural level of subjects in the aspect of alertness and attentiveness. The study demonstrated that the subjects felt more attentive and more alert after inhaling the oil, suggesting that the effect of inhalation of ylang-ylang oil is characterized as “harmonization” which resulted in uncoupling of physiological (reduced ANS arousal) and behavioural arousal process (increased behavioural activation) [46]. Meanwhile, the similar group of researchers found that transdermal administration of ylang-ylang oil to healthy subjects resulted in both decreased physiological arousal and deactivation of behavioural level whereby the subjects experienced more calm and relaxed after transdermal administration [97]. The findings of these studies indicated that the differential effects of essential oils depend on the route of administration whereby inhalation and percutaneous administration of the essential oils give different pharmacological and psychological effects either with or without involving the olfactory processing [97]. Moreover, the most recent study evaluated the sedative effects of ylang-ylang oil with the use of sphygmomanometer and electrocardiogram (EKG) to determine the blood pressure and heart rate, respectively, of the subjects after the inhalation of the fragrance of the oil [98]. Similarly, this study also indicated that ylang-ylang oil showed sedative effectiveness where declination of 12-lead EKG demonstrating decreased heart rate was observed in the group treated with ylang-ylang oil [98]. Overall, the available studies have shown that the essential oil of C. odorata indeed possess sedative, relaxing, and also harmonization effects on human and also explained its usefulness in aromatherapy and medicine such as reduction of blood pressure or relief of depression and stress in human.
16. Effects on Mood and Cognitive Performance
Studies have shown that the mood and cognitive performance of a healthy individual can be modulated by aromas of essential oils. A study revealed that ylang-ylang aroma acted significantly different on the cognitive performance of the healthy volunteers as compared to the control group and the peppermint aroma [47]. Ylang-ylang aroma produced a reduced alertness mood and increased calmness of the healthy volunteers but absence in the enhancement of cognitive performance and also lengthened processing speed [47]. Ishiguchi and colleagues evaluated the effect of inhalation of ylang-ylang essential oil by detecting the electroencephalography background activity of the volunteers [99]. They revealed that alpha 1 (8–9.9 Hz) brain waves which present in deep relaxation was increased significantly during inhalation of ylang-ylang essential oil and also reduced alertness mood of the volunteers [99]. Thus, Ishiguchi and colleagues suggested that the lowering effect of alertness and increased alpha 1 brain waves may be the physiological basis for relaxation effect of aromatherapy with ylang-ylang [99]. Furthermore, reduction of the amplitude of auditory P300 which is associated with the higher cognitive processing was observed in healthy volunteers during inhalation of ylang-ylang aroma, suggesting a relaxing effect of aroma on cognitive function. Watanabe and colleagues [100] elucidated the effect of ylang-ylang aroma on the auditory P300 of healthy individual and patient with temporal lobe epilepsy (TLE) who have impaired odor identification. The study demonstrated exposure to ylang-ylang aroma prolonged latencies of P300 in both control and TLE groups while only significant reduction of P300 amplitudes in healthy volunteers was observed. The absence of P300 amplitudes reduction in TLE patients suggested that their information processing was not altered during the exposure to ylang-ylang aroma or the fact that TLE patients had lower P300 amplitudes under odourless condition as compared to the controls [100].
17. Spermatotoxic Properties
Overpopulation is known to be a global issue and public health concern. The ever-increasing human population causes various detrimental effects including environmental degradation, poverty, and rise in unemployment. Therefore, many studies have been focusing on the discovery and development of novel and more potent contraceptive. Currently, medicinal plants have also received huge attention for its use as contraceptives due to their little side effects. C. odorata was also found to possess spermicidal activity in both in vitro and in vivo study [48]. In the in vitro study, the sperms obtained from healthy male rats were immobilized by 50% ethanolic extract of root bark of C. odorata within seconds. In the in vivo study, the administration of crude extract at 50 mg/100 g body weight/day reduced the motility of sperm of the rat significantly (5 ± 0.38 seconds) as compared to the control rat (30 ± 1.98 seconds). Furthermore, reduced sperm count and 94% abnormal sperm morphology were also observed when 100 mg/100 g body weight/day of crude extract was administrated into rats. The biochemical findings of the study indicated that the crude extract of C. odorata root bark reduced the production of testosterone, altered the metabolism of stored spermatozoa in the testes, and led to the deficiency in nutrients for proper sperm maturation [48]. Comparison between the antifertility effects of extract of C. odorata bark and gossypol which is a well-studied antifertility agent has been conducted as well [49]. The study suggested that C. odorata bark extract may be a better antifertility agent than gossypol as reversibility in the motility of sperm was observed in the C. odorata treated group after withdrawal of the extract. The study also managed to isolate the active component of the extract and was determined as a 52 kd protein which immobilized the sperm in vitro within seconds [49].
18. Antihyperglycemic Effects and Antidiabetic Complications Properties
Diabetes mellitus is a common metabolic disorder characterized by chronic hyperglycemia, as a result from defects in insulin production and insulin action. Currently, there is a need to develop safe and treatment for diabetes as most of the available medications have several adverse effects. According to [101], it was found that approximately 1200 species of plants were used as traditional medicine in treating diabetes globally. The leaves and stem extracts of C. odorata were found to exhibit alpha-amylase inhibitory effects [7]. Both leaves and stem extracts of C. odorata demonstrated 22.6 ± 1.3% and 25.3 ± 3.3% inhibition, respectively, at 7.8 μg/mL on porcine pancreatic α-amylase enzyme. However, both leaves and stem extracts of C. odorata did not exhibit any inhibitory effect on α-glucosidase enzyme [7]. The results of the study suggested that the extracts of C. odorata may have the potential to be used as α-amylase inhibitor in managing postprandial hyperglycemia. Another study revealed that several terpenoid derivatives and flavonoids isolated from the flower buds of C. odorata possessed inhibitory effects on aldose reductase [23]. Aldose reductase is an enzyme in the polyol pathway that reduces glucose to sorbitol with the use of NADPH. The accumulation of intracellular sorbitol as a result of abnormal activation of polyol pathway may lead to chronic complications of diabetes such as diabetic neuropathy, retinopathy, nephropathy, and cataract [102]. Hence, the inhibition of aldose reductase activity may help in preventing diabetic complications. The study showed that canangaterpene I, (E)-[(1R,3R,5S,6S,8S)-6-hydroxy-1,3-dimethoxy-2- oxaspiro[4.5]decan-8-yl]methyl caffeate, and canangafruticoside E exhibited potent inhibitory effects on aldose reductase with IC50 at 1.2, 1.5, and 0.8 μM, respectively, with comparison to a reference compound, chlorogenic acid with IC50 at 0.7 μM [23].
19. Commercial Uses
Many patents exist which describe the commercial application of ylang-ylang oil. Of the 866 references to “ylang-ylang” that were located by “Scifinder,” 533 of these (61.5%) were patents. At the time of writing, recent patents involving ylang-ylang oil showed that a majority of the inventions have focused on field of health products and cosmetic uses. Ylang-ylang oil has been reported to be ingredients for many cosmetic products such as skin care products [103, 104], hair protecting products [105], hair growth promoter [106], and sunscreen compositions [107]. Besides that, the essential oils of C. odorata also present applications in agriculture and food industry. The essential oil of C. odorata has also been reported to be one of the ingredients for an invention used as repellent against insects, arachnids, and other arthropods [108]. In addition, C. odorata oil has also been incorporated as one of ingredients into a beverage formulations for the use as nutritional supplement [109]. All these patents demonstrate a strong commercial value and wide range of uses of C. odorata essential oil.
20. Conclusion
Extensive literature survey demonstrated that C. odorata is a medicinal and aromatic plant with a vast spectrum of pharmacological activities having considerable importance in agricultural and consumer products industries. The constituents such as O-methylmoschatoline, liriodenine (24), 3,4-dihydroxybenzoic acid, germacrene D (11), and β-caryophyllene (12) have been recognized as the bioactive molecules that possess antimicrobial activities. Linalool (8) is another compound that has been shown to exhibit insecticidal and anti-inflammatory activities. Besides that, it has been experimentally proven that C. odorata also possess antibiofilm, antioxidant, antidiabetic, antifertility, antimelanogenesis, insect-repellent, antihyperglycemic, sedative, and relaxing properties. And overall, this review emphasizes the potential of C. odorata to be used as new therapeutic drugs and also provides sufficient baseline information for future works and commercial exploitation.
Acknowledgments
This work was supported by the Monash University Malaysia ECR Grant (5140077-000-00), MOSTI eScience Fund (02-02-10-SF0215), and University of Malaya for High Impact Research Grant (Grant no. H-50001-A000027 and no. A000001-50001). The authors are thankful to Dr. Sugumaran Manickam and Mr. Cheah Yih Horng for providing the images of the plant taken from Rimba Ilmu Botanic Garden, University of Malaya.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Goodrich K. R. Floral scent in Annonaceae. Botanical Journal of the Linnean Society. 2012;169(1):262–279. doi: 10.1111/j.1095-8339.2012.01220.x. [DOI] [Google Scholar]
- 2.Burdock G. A., Carabin I. G. Safety assessment of Ylang-Ylang (Cananga spp.) as a food ingredient. Food and Chemical Toxicology. 2008;46(2):433–445. doi: 10.1016/j.fct.2007.09.105. [DOI] [PubMed] [Google Scholar]
- 3.Caballero-Gallardo K., Olivero-Verbel J., Stashenko E. E. Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum herbst. Journal of Agricultural and Food Chemistry. 2011;59(5):1690–1696. doi: 10.1021/jf103937p. [DOI] [PubMed] [Google Scholar]
- 4.Soonwera M., Phasomkusolsil S. Efficacy of Thai herbal essential oils as green repellent against mosquito vectors. Acta Tropica. 2015;142:127–130. doi: 10.1016/j.actatropica.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Lee K., Lee J.-H., Kim S.-I., Cho M. H., Lee J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus . Applied Microbiology and Biotechnology. 2014;98(22):9447–9457. doi: 10.1007/s00253-014-5903-4. [DOI] [PubMed] [Google Scholar]
- 6.Wei A., Shibamoto T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. Journal of Agricultural and Food Chemistry. 2010;58(12):7218–7225. doi: 10.1021/jf101077s. [DOI] [PubMed] [Google Scholar]
- 7.Jemain M. M., Nik Musa'adah M. N., Rohaya A., Rashid L. A., Nor Hadiani I. In vitro antihyperglycaemic effects of some Malaysian plants. Journal of Tropical Forest Science. 2011;23(4):467–472. [Google Scholar]
- 8.Matsumoto T., Nakamura S., Nakashima S., et al. Lignan dicarboxylates and terpenoids from the flower buds of Cananga odorata and their inhibitory effects on melanogenesis. Journal of Natural Products. 2014;77(4):990–999. doi: 10.1021/np401091f. [DOI] [PubMed] [Google Scholar]
- 9.Manner H. I., Elevitch C. R. Cananga odorata (ylang-ylang) Species Profiles for Pacific Island Agroforestry; 2006. [Google Scholar]
- 10.Zollo P., Biyiti L., Tchoumbougnang F., Menut C., Lamaty G., Bouchet P. Aromatic plants of tropical Central Africa. Part XXXII. Chemical composition and antifungal activity of thirteen essential oils from aromatic plants of Cameroon. Flavour and Fragrance Journal. 1998;13(2):107–114. [Google Scholar]
- 11.Brophy J., Goldsack R., Forster P. Essential oils from the leaves of some Queensland Annonaceae. Journal of Essential Oil Research. 2004;16(2):95–100. doi: 10.1080/10412905.2004.9698660. [DOI] [Google Scholar]
- 12.Chalchat J.-C., Garry R.-P., Menut C., Lamaty G., Malhuret R., Chopineau J. Correlation between chemical composition and antimicrobial activity. VI. Activity of some African essential oils. Journal of Essential Oil Research. 1997;9(1):67–75. doi: 10.1080/10412905.1997.9700717. [DOI] [Google Scholar]
- 13.Stashenko E., Martinez J. R., MacKu C., Shibamoto T. HRGC and GC-MS analysis of essential oil from Colombian ylang-ylang (Cananga odorata Hook fil. Et Thomson, forma genuina) Journal of High Resolution Chromatography. 1993;16(7):441–444. [Google Scholar]
- 14.Stashenko E. E., Torres W., Morales J. R. M. A study of the compositional variation of the essential oil of ylang-ylang (Cananga odorata Hook Fil. et Thomson, forma genuina) during flower development. Journal of High Resolution Chromatography. 1995;18(2):101–104. doi: 10.1002/jhrc.1240180206. [DOI] [Google Scholar]
- 15.Benini C., Ringuet M., Wathelet J.-P., Lognay G., du Jardin P., Fauconnier M.-L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour and Fragrance Journal. 2012;27(5):356–366. doi: 10.1002/ffj.3106. [DOI] [Google Scholar]
- 16.Brokl M., Fauconnier M.-L., Benini C., Lognay G., du Jardin P., Focant J.-F. Improvement of ylang-ylang essential oil characterization by GC×GC-TOFMS. Molecules. 2013;18(2):1783–1797. doi: 10.3390/molecules18021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benini C., Mahy G., Bizoux J. P., et al. Comparative chemical and molecular variability of Cananga odorata (Lam.) Hook. F. & Thomson forma genuina (ylang-ylang) in the Western Indian Ocean Islands: implication for valorization. Chemistry & Biodiversity. 2012;9(7):1389–1402. doi: 10.1002/cbdv.201100306. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh T.-J., Chang F.-R., Wu Y.-C. The constituents of Cananga odorata . Journal of the Chinese Chemical Society. 1999;46(4):607–611. doi: 10.1002/jccs.199900084. [DOI] [Google Scholar]
- 19.Rao J. U. M., Giri G. S., Hanumaiah T., Rao K. V. J. Sampangine, a new alkaloid from Cananga odorata . Journal of Natural Products. 1986;49(2):346–347. doi: 10.1021/np50044a029. [DOI] [Google Scholar]
- 20.Hsieh T.-J., Chang F.-R., Chia Y.-C., Chen C.-Y., Chiu H.-F., Wu Y.-C. Cytotoxic constituents of the fruits of Cananga odorata . Journal of Natural Products. 2001;64(5):616–619. doi: 10.1021/np0005208. [DOI] [PubMed] [Google Scholar]
- 21.Caloprisco E., Fourneron J.-D., Faure R., Demarne F.-E. Unusual lactones from Cananga odorata (Annonaceae) Journal of Agricultural and Food Chemistry. 2002;50(1):78–80. doi: 10.1021/jf0105079. [DOI] [PubMed] [Google Scholar]
- 22.Matsunami K., Nagashima J., Sugimoto S., et al. Megastigmane glucosides and an unusual monoterpene from the leaves of Cananga odorata var. odorata, and absolute structures of megastigmane glucosides isolated from C. odorata var. odorata and Breynia officinalis . Journal of Natural Medicines. 2010;64(4):460–467. doi: 10.1007/s11418-010-0434-5. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto T., Nakamura S., Fujimoto K., et al. Structure of constituents isolated from the flower buds of Cananga odorata and their inhibitory effects on aldose reductase. Journal of Natural Medicines. 2014;68(4):709–716. doi: 10.1007/s11418-014-0843-y. [DOI] [PubMed] [Google Scholar]
- 24.Nagashima J., Matsunami K., Otsuka H., Lhieochaiphant D., Lhieochaiphant S. The unusual canangafruticosides A-E: five monoterpene glucosides, two monoterpenes and a monoterpene glucoside diester of the aryldihydronaphthalene lignan dicarboxylic acid from leaves of Cananga odorata var. fruticosa . Phytochemistry. 2010;71(13):1564–1572. doi: 10.1016/j.phytochem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Rahman M. M., Lopa S. S., Sadik G., et al. Antibacterial and cytotoxic compounds from the bark of Cananga odorata . Fitoterapia. 2005;76(7-8):758–761. doi: 10.1016/j.fitote.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Kusuma I. W., Murdiyanto, Arung E. T., Syafrizal, Kim Y. Antimicrobial and antioxidant properties of medicinal plants used by the Bentian tribe from Indonesia. Food Science and Human Wellness. 2014;3(3-4):191–196. doi: 10.1016/j.fshw.2014.12.004. [DOI] [Google Scholar]
- 27.Nguyen-Pouplin J., Tran H., Tran H., et al. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. Journal of Ethnopharmacology. 2007;109(3):417–427. doi: 10.1016/j.jep.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Chao S., Young G., Oberg C., Nakaoka K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. Flavour and Fragrance Journal. 2008;23(6):444–449. doi: 10.1002/ffj.1904. [DOI] [Google Scholar]
- 29.Kaisa M. A. A review on phytochemicals from some medicinal plants of Bangladesh. Journal of Pharmacy and Nutrition Sciences. 2011;1(1) doi: 10.6000/1927-5951.2011.01.01.14. [DOI] [Google Scholar]
- 30.Sacchetti G., Maietti S., Muzzoli M., et al. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chemistry. 2005;91(4):621–632. doi: 10.1016/j.foodchem.2004.06.031. [DOI] [Google Scholar]
- 31.Indrakumar I., Selvi V., Gomathi R., Karpagam S. Evaluation of antimicrobial activity of Cananga odorata (Lam.) Hook. F. & Thomson leaf extract: an in vitro study. Mintage Journal of Pharmaceutical and Medical Sciences. 2012;1(1):21–22. [Google Scholar]
- 32.Andrade B. F. M. T., Barbosa L. N., da Silva Probst I., Júnior A. F. Antimicrobial activity of essential oils. Journal of Essential Oil Research. 2014;26(1):34–40. doi: 10.1080/10412905.2013.860409. [DOI] [Google Scholar]
- 33.Ragasa C. Y., Tamboong B., Rideout J. Secondary metabolites from Jasminum sambac and Cananga odorata . ACGC Chemical Research Communications. 2003;16:40–47. [Google Scholar]
- 34.Choi E.-M., Hwang J.-K. Screening of Indonesian medicinal plants for inhibitor activity on nitric oxide production of RAW264.7 cells and antioxidant activity. Fitoterapia. 2005;76(2):194–203. doi: 10.1016/j.fitote.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Phasomkusolsil S., Soonwera M. Efficacy of herbal essential oils as insecticide against Aedes aegypti (Linn.), Culex quinquefasciatus(Say) and Anopheles Dirus (Peyton and Harrison) Southeast Asian Journal of Tropical Medicine and Public Health. 2011;42(5):1083–1092. [PubMed] [Google Scholar]
- 36.Phasomkusolsil S., Soonwera M. The effects of herbal essential oils on the ovipositiondeterrent and ovicidal activities of Aedes aegypti (Linn.), Anopheles dirus (Peyton and Harrison) and Culex quinquefasciatus (say) Tropical Biomedicine. 2012;29(1):138–150. [PubMed] [Google Scholar]
- 37.Seo S.-M., Park H.-M., Park I.-K. Larvicidal activity of ajowan (Trachyspermum ammi) and peru balsam (Myroxylon pereira) oils and blends of their constituents against mosquito, Aedes aegypti, acute toxicity on water flea, Daphnia magna, and aqueous residue. Journal of Agricultural and Food Chemistry. 2012;60(23):5909–5914. doi: 10.1021/jf301296d. [DOI] [PubMed] [Google Scholar]
- 38.Vera S. S., Zambrano D. F., Méndez-Sanchez S. C., Rodríguez-Sanabria F., Stashenko E. E., Duque Luna J. E. Essential oils with insecticidal activity against larvae of Aedes aegypti (Diptera: Culicidae) Parasitology Research. 2014;113(7):2647–2654. doi: 10.1007/s00436-014-3917-6. [DOI] [PubMed] [Google Scholar]
- 39.Soonwera M. Larvicidal and oviposition deterrent activities of essential oils against house fly (Musca domestica L.; Diptera: Muscidae) Journal of Agricultural Technology. 2015;11(3):657–667. [Google Scholar]
- 40.Seo S.-M., Kim J., Lee S.-G., Shin C.-H., Shin S.-C., Park I.-K. Fumigant antitermitic activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica), caraway (Carům carvi), dill (Anethum graveoiens), geranium (Pelargonium graveoiens), and litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratus kolbe) Journal of Agricultural and Food Chemistry. 2009;57(15):6596–6602. doi: 10.1021/jf9015416. [DOI] [PubMed] [Google Scholar]
- 41.Cheng J., Yang K., Zhao N. N., Wang X. G., Wang S. Y., Liu Z. L. Composition and insecticidal activity of the essential oil of Cananga odorata leaves against Sitophilus zeamais motschulsky (Coleoptera: Curculionidae) Journal of Medicinal Plants Research. 2012;6(19) doi: 10.5897/jmpr12.136. [DOI] [Google Scholar]
- 42.Phasomkusolsil S., Soonwera M. Comparative mosquito repellency of essential oils against Aedes aegypti (Linn.), Anopheles dirus (Peyton and Harrison) and Culex quinquefasciatus (Say) Asian Pacific Journal of Tropical Biomedicine. 2011;1(1):S113–S118. doi: 10.1016/s2221-1691(11)60136-6. [DOI] [PubMed] [Google Scholar]
- 43.Soonwera M., Phasomkusolsil S. Mosquito repellent from Thai essential oils against dengue fever mosquito (Aedes aegypti (L.)) and filarial mosquito vector (Culex quinquefasciatus (Say)) African Journal of Microbiology Research. 2014;8(17):1819–1824. doi: 10.5897/AJMR2014.6737. [DOI] [Google Scholar]
- 44.Maniyar Y. A., Devi C. H. J. Evaluation of anti-inflammatory activity of ethanolic extract of Cananga odorata Lam in experimental animals. International Journal of Basic & Clinical Pharmacology. 2015;4(2):354–357. doi: 10.5455/2319-2003.ijbcp20150439. [DOI] [Google Scholar]
- 45.Wang C. N. Effect of Melaleuca leucadendron, Cananga odorata and Pogostemon cablin oil odors on human physiological responses. Wood Research. 2012;3(2):p. 100. [Google Scholar]
- 46.Hongratanaworakit T., Buchbauer C. Evaluation of the harmonizing effect of ylang-ylang oil on humans after inhalation. Planta Medica. 2004;70(7):632–636. doi: 10.1055/s-2004-827186. [DOI] [PubMed] [Google Scholar]
- 47.Moss M., Hewitt S., Moss L., Wesnes K. Modulation of cognitive performance and mood by aromas of peppermint and ylang-ylang. International Journal of Neuroscience. 2008;118(1):59–77. doi: 10.1080/00207450601042094. [DOI] [PubMed] [Google Scholar]
- 48.Anitha P., Indira M. Impact of feeding ethanolic extract of root bark of Cananga odorata (Lam) on reproductive functions in male rats. Indian Journal of Experimental Biology. 2006;44(12):976–980. [PubMed] [Google Scholar]
- 49.Pankajakshy A., Madambath I. Spermatotoxic effects of Cananga odorata (Lam): a comparison with gossypol. Fertility and Sterility. 2009;91(5):2243–2246. doi: 10.1016/j.fertnstert.2008.05.075. [DOI] [PubMed] [Google Scholar]
- 50.The Plant List. Cananga odorata (Lam.) Hook. F. & Thomson. http://www.theplantlist.org/tpl1.1/record/kew-2695745.
- 51.Saedi N., Crawford G. H. Botanical briefs: ylang-ylang oil—extracts from the tree Cananga odorata . Cutis. 2006;77(3):149–150. [PubMed] [Google Scholar]
- 52.Holdsworth D. K. Traditional medicinal plants of Rarotonga, Cook Islands part I. Pharmaceutical Biology. 1990;28(3):209–218. doi: 10.3109/13880209009082815. [DOI] [Google Scholar]
- 53.Nandwani D., Calvo J. A., Tenorio J., Calvo F., Manglona L. Medicinal plants and traditional knowledge in the northern Mariana Islands. Journal of Applied Biosciences. 2008;8(2):323–330. [Google Scholar]
- 54.Holt S. Part 2: stimulants and dietary supplements. Alternative and Complementary Therapies. 1999;5(5):279–285. doi: 10.1089/act.1999.5.279. [DOI] [Google Scholar]
- 55.Jain S., Srivastava S. Traditional uses of some Indian plants among islanders of the Indian Ocean. Indian Journal of Traditional Knowledge. 2005;4(4):345–357. [Google Scholar]
- 56.Holdsworth D. K., Corbett L. Traditional medicinal plants of the north solomons province, Papua new guinea part II. Pharmaceutical Biology. 1988;26(1):45–50. doi: 10.3109/13880208809053887. [DOI] [Google Scholar]
- 57.Roloff A., Weisgerber H., Schütt P., Lang U. M., Stimm B. Enzyklopädie Der Holzgewächse: Handbuch Und Atlas Der Dendrologie. Wiley-VCH; 2012. [Google Scholar]
- 58.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—a review. Food and Chemical Toxicology. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 59.Qin X.-W., Hao C.-Y., He S.-Z., et al. Volatile organic compound emissions from different stages of Cananga odorata flower development. Molecules. 2014;19(7):8965–8980. doi: 10.3390/molecules19078965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaydou E. M., Randriamiharisoa R., Bianchini J.-P. Composition of the essential oil of ylang-ylang (Cananga odorata Hook Fil. et Thomson forma genuina) from Madagascar. Journal of Agricultural and Food Chemistry. 1986;34(3):481–487. doi: 10.1021/jf00069a028. [DOI] [Google Scholar]
- 61.Jin J., Kim M. J., Dhandapani S., et al. The floral transcriptome of ylang ylang (Cananga odorata var. fruticosa) uncovers biosynthetic pathways for volatile organic compounds and a multifunctional and novel sesquiterpene synthase. Journal of Experimental Botany. 2015;66(13):3959–3975. doi: 10.1093/jxb/erv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woo S. H., Reynolds M. C., Sun N. J., Cassady J. M., Snapka R. M. Inhibition of topoisomerase II by liriodenine. Biochemical Pharmacology. 1997;54(4):467–473. doi: 10.1016/S0006-2952(97)00198-6. [DOI] [PubMed] [Google Scholar]
- 63.Kluza J., Clark A. M., Bailly C. Apoptosis induced by the alkaloid sampangine in HL-60 leukemia cells: correlation between the effects on the cell cycle progression and changes of mitochondrial potential. Annals of the New York Academy of Sciences. 2003;1010(1):331–334. doi: 10.1196/annals.1299.059. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad I., Beg A. Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Journal of Ethnopharmacology. 2001;74(2):113–123. doi: 10.1016/S0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 65.Dahiya P., Purkayastha S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian Journal of Pharmaceutical Sciences. 2012;74(5):p. 443. doi: 10.4103/0250-474X.108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christaki E., Bonos E., Giannenas I., Florou-Paneri P. Aromatic plants as a source of bioactive compounds. Agriculture. 2012;2(3):228–243. doi: 10.3390/agriculture2030228. [DOI] [Google Scholar]
- 67.Luangnarumitchai S., Lamlertthon S., Tiyaboonchai W. Antimicrobial activity of essential oils against five strains of Propionibacterium acnes . Mahidol University Journal of Pharmaceutical Sciences. 2007;34(1–4):60–64. [Google Scholar]
- 68.Thompson A., Meah D., Ahmed N., et al. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome. BMC Complementary and Alternative Medicine. 2013;13, article 338 doi: 10.1186/1472-6882-13-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee J.-H., Lee J.-S. Chemical composition and antifungal activity of plant essential oils against Malassezia furfur . Korean Journal of Microbiology and Biotechnology. 2010;38(3):315–321. [Google Scholar]
- 70.Inouye S., Uchida K., Abe S. Vapor activity of 72 essential oils against a Trichophyton mentagrophytes . Journal of Infection and Chemotherapy. 2006;12(4):210–216. doi: 10.1007/s10156-006-0449-8. [DOI] [PubMed] [Google Scholar]
- 71.Kon K., Rai M. Antibacterial activity of Thymus vulgaris essential oil alone and in combination with other essential oils. Nusantara Bioscience. 2012;4(2):50–56. doi: 10.13057/nusbiosci/n040202. [DOI] [Google Scholar]
- 72.Tadtong S., Suppawat S., Tintawee A., Saramas P., Jareonvong S., Hongratanaworakit T. Antimicrobial activity of blended essential oil preparation. Natural Product Communications. 2012;7(10):1401–1404. [PubMed] [Google Scholar]
- 73.Kim Y.-G., Lee J.-H., Kim S.-I., Baek K.-H., Lee J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. International Journal of Food Microbiology. 2015;195:30–39. doi: 10.1016/j.ijfoodmicro.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 74.Stewart P. S., Costerton J. W. Antibiotic resistance of bacteria in biofilms. The Lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 75.Nostro A., Roccaro A. S., Bisignano G., et al. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Journal of Medical Microbiology. 2007;56(4):519–523. doi: 10.1099/jmm.0.46804-0. [DOI] [PubMed] [Google Scholar]
- 76.Goldbeck J. C., do Nascimento J. E., Jacob R. G., Fiorentini Â. M., da Silva W. P. Bioactivity of essential oils from Eucalyptus globulus and Eucalyptus urograndis against planktonic cells and biofilms of Streptococcus mutans . Industrial Crops and Products. 2014;60:304–309. doi: 10.1016/j.indcrop.2014.05.030. [DOI] [Google Scholar]
- 77.Sandasi M., Leonard C. M., Viljoen A. M. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes . Letters in Applied Microbiology. 2010;50(1):30–35. doi: 10.1111/j.1472-765x.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 78.Taweechaisupapong S., Ngaonee P., Patsuk P., Pitiphat W., Khunkitti W. Antibiofilm activity and post antifungal effect of lemongrass oil on clinical Candida dubliniensis isolate. South African Journal of Botany. 2012;78:37–43. doi: 10.1016/j.sajb.2011.04.003. [DOI] [Google Scholar]
- 79.Bilcu M., Grumezescu A. M., Oprea A. E., et al. Efficiency of vanilla, patchouli and ylang ylang essential oils stabilized by iron oxide@C14 nanostructures against bacterial adherence and biofilms formed by Staphylococcus aureus and Klebsiella pneumoniae clinical strains. Molecules. 2014;19(11):17943–17956. doi: 10.3390/molecules191117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miguel M. G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour and Fragrance Journal. 2010;25(5):291–312. doi: 10.1002/ffj.1961. [DOI] [Google Scholar]
- 81.Shahidi F. Antioxidants in food and food antioxidants. Food/Nahrung. 2000;44(3):158–163. doi: 10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 82.Wang H.-F., Wang Y.-K., Yih K.-H. DPPH free-radical scavenging ability, total phenolic content, and chemical composition analysis of forty-five kinds of essential oils. Journal of Cosmetic Science. 2008;59(6):509–522. [PubMed] [Google Scholar]
- 83.World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 84.Ministry of Health Malaysia. Health Facts 2014. Ministry of Health Malaysia; 2014. http://www.moh.gov.my/images/gallery/publications/HEALTH%20FACTS%202014.pdf. [Google Scholar]
- 85.Ministry of Health Malaysia. Health facts 2013. 2013, http://www.moh.gov.my/images/gallery/publications/HEALTH%20FACTS%202013.pdf.
- 86.Cardo M. V., Vezzani D., Carbajo A. E. Oviposition strategies of temporary pool mosquitoes in relation to weather, tidal regime and land use in a temperate wetland. Bulletin of Entomological Research. 2012;102(6):651–662. doi: 10.1017/s0007485312000259. [DOI] [PubMed] [Google Scholar]
- 87.Phasomkusolsil S., Soonwera M. Efficacy of Thai herbal essential oils against three immature stages of Aedes aegypti (Linn), Anopheles dirus (Peyton and Harrison) and Culex quinquefasciatus (say) Topclass Journal of Herbal Medicine. 2013;2:25–35. [Google Scholar]
- 88.Gupta R. K., Rutledge L. C. Role of repellents in vector control and disease prevention. The American Journal of Tropical Medicine and Hygiene. 1994;50(6, supplement):82–86. doi: 10.4269/ajtmh.1994.50.82. [DOI] [PubMed] [Google Scholar]
- 89.Fradin M. S., Day J. F. Comparative efficacy of insect repellents against mosquito bites. The New England Journal of Medicine. 2002;347(1):13–18. doi: 10.1056/nejmoa011699. [DOI] [PubMed] [Google Scholar]
- 90.Olivero-Verbel J., Tirado-Ballestas I., Caballero-Gallardo K., Stashenko E. E. Essential oils applied to the food act as repellents toward Tribolium castaneum . Journal of Stored Products Research. 2013;55:145–147. doi: 10.1016/j.jspr.2013.09.003. [DOI] [Google Scholar]
- 91.Parvez S., Kang M., Chung H.-S., et al. Survey and mechanism of skin depigmenting and lightening agents. Phytotherapy Research. 2006;20(11):921–934. doi: 10.1002/ptr.1954. [DOI] [PubMed] [Google Scholar]
- 92.Efdi M., Ohguchi K., Akao Y., Nozawa Y., Koketsu M., Ishihara H. N-trans-feruloyltyramine as a melanin biosynthesis inhibitor. Biological and Pharmaceutical Bulletin. 2007;30(10):1972–1974. doi: 10.1248/bpb.30.1972. [DOI] [PubMed] [Google Scholar]
- 93.Lin C.-C., Yang C.-H., Wu P.-S., Kwan C.-C., Chen Y.-S. Antimicrobial, anti-tyrosinase and antioxidant activities of aqueous aromatic extracts from forty-eight selected herbs. Journal of Medicinal Plant Research. 2011;5(26):6203–6209. doi: 10.5897/jmpr11.1134. [DOI] [Google Scholar]
- 94.Basu S., Hazra B. Evaluation of nitric oxide scavenging activity, in vitro and ex vivo, of selected medicinal plants traditionally used in inflammatory diseases. Phytotherapy Research. 2006;20(10):896–900. doi: 10.1002/ptr.1971. [DOI] [PubMed] [Google Scholar]
- 95.Peana A. T., D'Aquila P. S., Panin F., Serra G., Pippia P., Moretti M. D. L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9(8):721–726. doi: 10.1078/094471102321621322. [DOI] [PubMed] [Google Scholar]
- 96.Moncada S., Palmer R. M. J., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 97.Hongratanaworakit T., Buchbauer G. Relaxing effect of ylang ylang oil on humans after transdermal absorption. Phytotherapy Research. 2006;20(9):758–763. doi: 10.1002/ptr.1950. [DOI] [PubMed] [Google Scholar]
- 98.Jung D. j., Cha J. Y., Kim S. E., Ko I. G., Jee Y. S. Effects of Ylang-Ylang aroma on blood pressure and heart rate in healthy men. Journal of Exercise Rehabilitation. 2013;9(2):250–255. doi: 10.12965/jer.130007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ishiguchi A., Saitou A., Suenaga K., Ohta K., Matsuura M. 14. Effects of odors on electroencephalogram and subjective alterations. Clinical Neurophysiology. 2008;119(6, article e78) doi: 10.1016/j.clinph.2008.01.039. [DOI] [Google Scholar]
- 100.Watanabe S., Hara K., Ohta K., et al. Aroma helps to preserve information processing resources of the brain in healthy subjects but not in temporal lobe epilepsy. Seizure. 2013;22(1):59–63. doi: 10.1016/j.seizure.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 101.Hsu Y.-J., Lee T.-H., Chang C. L.-T., Huang Y.-T., Yang W.-C. Anti-hyperglycemic effects and mechanism of Bidens pilosa water extract. Journal of Ethnopharmacology. 2009;122(2):379–383. doi: 10.1016/j.jep.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 102.Saraswat M., Muthenna P., Suryanarayana P., Petrash J. M., Reddy G. B. Dietary sources of aldose reductase inhibitors: prospects for alleviating diabetic complications. Asia Pacific Journal of Clinical Nutrition. 2008;17(4):558–565. [PubMed] [Google Scholar]
- 103.Florence T., Hines M., Gan D. Cosmetic compositions and uses thereof. Google Patents, 2014.
- 104.Drapeau C., Kukulcan S., Jensen G. S. Skin care compositions containing combinations of natural ingredients. Google Patents, 2014.
- 105.Humphreys J., Sherman J. Compositions and methods for treating keratin based fibers. Google Patents, 2014.
- 106.Darsale C. S. Nourishing oil composition, pomade, composition for promoting hair growth, shampoo, conditioner, hair root stimulator, and methods for making and using the same. Google Patents, 2014.
- 107.Thaggard H. E. Reduced-alcohol sunscreen compositions and methods. Google Patents, 2014.
- 108.Hoag G. E., Anderson D. K. Natural volatile plant oils to repel arthropods. Google Patents, 2014.
- 109.West B. J., Jensen C. J., Palu A. K., Deng S., Wasden J. A. Morinda citrifolia and iridoid based formulations. Google Patents, 2014.