Abstract
Intravaginal infection with Chlamydia muridarum in mice can ascend to the upper genital tract, resulting in hydrosalpinx, a pathological hallmark for tubal infertility in women infected with C. trachomatis. Here, we utilized in vivo imaging of C. muridarum infection in mice following an intravaginal inoculation and confirmed the rapid ascent of the chlamydial organisms from the lower to upper genital tracts. Unexpectedly, the C. muridarum-derived signal was still detectable in the abdominal area 100 days after inoculation. Ex vivo imaging of the mouse organs revealed that the long-lasting presence of the chlamydial signal was restricted to the gastrointestinal (GI) tract, which was validated by directly measuring the chlamydial live organisms and genomes in the same organs. The C. muridarum organisms spreading from the genital to the GI tracts were detected in different mouse strains and appeared to be independent of oral or rectal routes. Mice prevented from orally taking up excretions also developed the long-lasting GI tract infection. Inoculation of C. muridarum directly into the upper genital tract, which resulted in a delayed vaginal shedding of live organisms, accelerated the chlamydial spreading to the GI tract. Thus, we have demonstrated that the genital tract chlamydial organisms may use a systemic route to spread to and establish a long-lasting infection in the GI tract. The significance of the chlamydial spreading from the genital to GI tracts is discussed.
INTRODUCTION
Chlamydia muridarum has been extensively used for studying the mechanisms of Chlamydia trachomatis pathogenesis and immunity (1–4) because intravaginal infection of mice with the C. muridarum organisms results in hydrosalpinges and infertility (1, 5). It is now known that both adequate ascension of C. muridarum to the upper genital tract and activation of an appropriate tubal inflammatory response are necessary for C. muridarum induction of hydrosalpinx (6–9). However, the precise pathogenic mechanisms remain unknown.
It has been shown that C. muridarum organisms spread to extragenital tract organs, including the gastrointestinal (GI) tract when examined within 14 days after inoculation (10, 11). The spreading becomes more obvious in mice deficient in genes coding for important host defense molecules such as interleukin-12 (IL-12) (12), gamma interferon (IFN-γ) (10, 13, 14), or the immunoglobulin mu chain (15). When the fate of C. muridarum organisms was monitored in the corresponding organs/systems in which the organisms were initially inoculated, the orally inoculated C. muridarum organisms established a long-lasting infection in the GI tract (lasting up to 260 days) while the intranasally, conjunctivally, or intravaginally inoculated organisms were cleared from the corresponding organs/systems within a month (16). Interestingly, the long-lasting GI tract infection did not cause any significant inflammatory pathology. Recently, Yeruva et al. (17) reported that C. muridarum organisms introduced into the mouse GI tract via an intragastric inoculation not only persisted in the GI tract for more than 100 days but were also more resistant to antibiotic treatment (17). These observations have led to the hypothesis that long-lasting chlamydial infection in the GI tract may serve as a reservoir for reinfecting the genital tract (18). Consistent with this hypothesis are the findings that GI tract infection with Chlamydia trachomatis has been detected not only in men having sex with men (MSM) (19, 20) but also in women (21). These women may acquire the GI tract infection orally (22). The oral transmission hypothesis is further supported by the observation that chlamydial DNA has been detected in pharyngeal samples (23). Chlamydia trachomatis antigens have been detected in enteroendocrine cells and macrophages of the small bowel in patients with severe irritable bowel syndrome (24), although the association of chlamydial infection in the GI tract with intestinal inflammatory diseases remains unclear (25, 26). The high prevalence of C. trachomatis infection in the GI tract (21, 27, 28) has prompted screening for chlamydial infection in both cervicovaginal and rectal swabs from both sexually active men and women (29, 30). Large-scale screenings of both cervicovaginal and rectal swabs not only will provide information for treatment and follow-up but may also aid in our understanding of the exact relationships between the vaginal and rectal C. trachomatis organisms in humans. The frequent detection of Chlamydia abortus in multiple organs of large animals (31) indicates that the C. abortus organisms may also be able to spread systemically in large animals just as the C. muridarum organisms do in mice.
The previous studies summarized above have shown that the genital C. muridarum can spread to the rest of the body, including the GI tract during the first few weeks of the infection (11–15), and that the orally or intragastrically inoculated C. muridarum can persist in the GI tract for long periods of time (16–18, 32). It remains unknown whether the genital C. muridarum spreading to the GI tract can also lead to the long-lasting infection in the GI tract. Addressing this question will allow us to identify a significant source of the long-lasting chlamydial infection in the GI tract, which may lay a foundation for further understanding the roles of the chlamydial GI tract infection in chlamydial pathogenicity.
To investigate chlamydial pathogenic mechanisms, we have recently engineered a luciferase-expressing C. muridarum strain (33). In cells infected with luciferase-expressing C. muridarum, luciferase gene expression and enzymatic activity (measured as bioluminescence intensity) correlated well with C. muridarum reticulate body (RB) proliferation. Since C. muridarum mature elementary bodies (EBs) display minimal levels of bioluminescence and the bioluminescence signals from the replicating RBs have a short half-life, the bioluminescence signal can be used for monitoring active chlamydial replication. More importantly, following an intravaginal inoculation with the luciferase-expressing C. muridarum, the bioluminescence signal is detected using an in vivo whole-body imaging technology in the areas where the lower and upper genital tracts are expected to be located. In the current study, we engineered a new version of the luciferase-expressing C. muridarum strain and used the same in vivo imaging technology for monitoring the traffic of the luciferase-expressing C. muridarum in real time. We found a rapid ascending of C. muridarum from the lower to the upper genital tracts following an intravaginal inoculation, which is consistent with what we previously reported (33). However, when we monitored the C. muridarum infection beyond the usual 1-month period, we found significant bioluminescent signal detectable in the mouse abdominal area for >100 days. Ex vivo imaging of the mouse organs revealed that the long-lasting presence of C. muridarum was restricted to the GI tract only. These imaging results were validated by directly measuring the chlamydial live organisms and genomes in the same organs. Thus, we have provided the first experimental evidence that the genital tract chlamydial organisms can spread to and establish a long-lasting infection in the GI tract. This observation warrants further investigation of the significance of the genital tract chlamydial spreading to the GI tract.
MATERIALS AND METHODS
Chlamydial organism growth.
Chlamydia muridarum strain Nigg organisms (initially acquired from Robert C. Brunham's lab at the University of Manitoba in 1999) were propagated and purified in HeLa cells (human cervical carcinoma epithelial cells, ATCC catalog number CCL2) as described previously (34). The full-genome sequence of this C. muridarum strain, designated Nigg3 or CMG0, is available under GenBank accession number CP009760.1. A clone was isolated from the Nigg3 or CMG0 stock using a plaque assay (35), and the clone designated Nigg3G0.1.1 or G0.1.1 for short (36) was used in the current study. The full genome sequence of Nigg3G0.1.1 is available under GenBank accession number CP009608.1. The Nigg3 stock was also used to cure of plasmid and two plasmid-free clones were established (designated Nigg3-CMUT3G5 or CMUT3G5 and Nigg3-CMUT3G42.2.1 or CMUT3G42.2.1). The full genome sequence of CMUT3G5 is available under GenBank accession number CP006974.1, while sequencing of the genome of CMUT3G42.2.1 is under way. The clone CMUT3G42.2.1 was previously used for transformation with a luciferase expression plasmid designated pGFP-luci-CM (33). For the current study, the CMUT3G5 clone was used for transformation with the same pGFP-luci-CM plasmid (33) as described previously (37, 38). Thus, the luciferase-expressing C. muridarum clone used in the current study was designated Nigg3-CMUT3G5-pGFP-Luci or G5-pGFP-Luci for short. The genome sequences of G0.1.1 and G5-pGFP-Luci are nearly identical with the only exception in the gene coding for TC0412. The G0.1.1 clone carries an insertion of the nucleotide “T” after the 84th position, while the G5-pGFP-Luci has the same nucleotide insertion after the 435th position. Both resulted in downstream frame shifts and premature determination codons in the gene coding for TC0412. Although different mutations in this gene from C. trachomatis were found to affect the infectivity of C. trachomatis in the mouse lower genital tract (39), mutations in this gene were shown to have no significant effect on either the infectivity or the pathogenicity of C. muridarum in the mouse genital tract (36). Both the G0.1.1 and G5-pGFP-Luci C. muridarum organisms were propagated in HeLa cells and purified as elementary bodies as mentioned above. Aliquots of the purified EBs were stored at −80°C until use.
Mouse infection.
Purified C. muridarum EBs (clone G0.1.1 or G5-pGFP-Luci) were used to infect 6- to 7-week-old female mice (Jackson Laboratories, Inc., Bar Harbor, ME) intravaginally, intrauterinally, or intrabursally with 2 × 105 inclusion-forming units (IFUs) as described previously (8, 9). The following three mouse strains were used in the current study: CBA/J (Jackson Laboratories stock number 000656), C57BL/6J (stock number 000664), and C3H/HeJ (stock number 000659). Five days prior to infection, each mouse was injected with 2.5 mg medroxyprogesterone (Depo-Provera; Pharmacia Upjohn, Kalamazoo, MI) subcutaneously to increase mouse susceptibility to infection. For some experiments, mice were fitted with 3-cm-diameter Elizabethan collars (Kent Scientific, Torrington, CT) to prevent autoinoculation with genital or rectal secretion. The presence of the collar on the mice's necks was monitored daily, and mice that lost their collar were immediately excluded from the experiment. After infection, mice were subjected to in vivo imaging, monitored for vaginal and rectal live-organism shedding, and sacrificed on different days postinfection for ex vivo imaging and quantitating C. muridarum live organisms or genome copies in different organs or tissue.
All animal experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (56). The protocol was approved by the Committee on the Ethics of Laboratory Animal Experiments of The University of Texas Health Science Center at San Antonio.
In vivo and ex vivo imaging.
Mice infected with the luciferase-expressing C. muridarum clone G5-pGFP-Luci were imaged using the Xenogen IVIS imaging system (PerkinElmer, Hopkinton, MA) on different days after infection. Prior to imaging, 500 μl of d-luciferin (40 mg/ml in sterile phosphate-buffered saline [PBS]) was intraperitoneally injected into each mouse. Twenty-five minutes after the injection, mice were anesthetized with 2% isoflurane. Bioluminescence images of the whole mouse bodies were captured as descried previously (33). Immediately after the whole-body imaging, mice were sacrificed by using an overdose of isoflurane followed by cervical dislocation. The mouse organs were taken out and placed in 100-mm petri dishes for ex vivo imaging. The intensity of bioluminescence was analyzed by using Living Image software from PerkinElmer.
Titrating live chlamydial organisms recovered from swabs and tissue homogenates.
For monitoring live-organism shedding from swab samples, cervicovaginal and anorectal swabs were taken every 3 to 4 days for the first week and weekly thereafter. To quantitate live chlamydial organisms, each swab was soaked in 0.5 ml of sucrose-phosphate-glutamic acid (SPG) and vortexed with glass beads, and the chlamydial organisms released into the supernatants were titrated on HeLa cell monolayers in duplicate. The infected cultures were processed for immunofluorescence assay as described below. Inclusions were counted in five random fields per coverslip under a fluorescence microscope. For coverslips with less than one IFU per field, entire coverslips were counted. Coverslips showing obvious cytotoxicity of HeLa cells were excluded. The total number of IFUs per swab was calculated based on the mean IFUs per view, the ratio of the view area to that of the well, the dilution factor, and inoculation volumes. When possible, a mean IFU/swab was derived from the serially diluted and duplicate samples for any given swab. The total number of IFUs/swab was converted into log10 and used to calculate the mean and standard deviation for mice of the same group at each time point.
For quantitating live organisms from mouse organs and tissue segments, immediately after ex vivo imaging, each organ or tissue segment was transferred to a tube containing 0.5 ml SPG. Each genital tract was cut into 5 segments/portions, including vagina/cervix (CV), left uterine horn (L-uh), right uterine horn (R-uh), left oviduct/ovary (L-ov), and right oviduct/ovary (R-ov). Each GI tract was divided into 5 segments, including stomach, small intestine (SI), cecum, colon, and anorectum (rectum). The organs and tissue segments were homogenized in cold SPG using a 2-ml tissue grinder (catalog number K885300-0002; Fisher Scientific, Pittsburgh, PA) or an automatic homogenizer (Omni Tissue Homogenizer, TH115; Omni International, Kennesaw, GA). The homogenates were further briefly sonicated and spun at 3,000 rpm for 5 min to pellet the remaining large debris. The supernatants were titrated for live C. muridarum organisms on HeLa cells as described above. The results were expressed as log10 IFUs per organ or tissue segment.
The immunofluorescence assays used for titrating live organisms were carried out as described previously (8, 36, 40). For titrating the live organisms recovered from a given sample, the mean number of inclusions per view was derived from counting five random views. The total number of live organisms in a given sample was calculated based on the mean inclusions per view, the ratio of the view area to that of the well, the dilution factor, and the inoculum volume and expressed as log10 IFUs per sample.
Titrating the number of C. muridarum genomes in the mouse samples using qPCR.
To quantitate the genome copies of C. muridarum, a portion of each tissue homogenate was transferred to the lysis buffer provided with Quick-gDNA miniPrep kit (catalog number 11-317C; Genesee Scientific, San Diego, CA) and subjected to DNA extraction according to the manufacturer's instructions. Each DNA preparation was eluted in 100 μl elution buffer, and 2 μl was used for quantitative PCR (qPCR). The following primers derived from the Chlamydia 16S rRNA coding region were used: forward primer (5′-CGCCTGAGGAGTACACTCGC-3AGGA), reverse primer (5′-CCAACACCTCACGGCACGAG-3′), and double-quenched probe (5′-CACAAGCAGTGGAGCATGTGGTTTAA-3′) (Integrated DNA Technologies, Coralville, IA). PCR was carried out in a total volume of 10 μl in a CFX96 Touch Deep Well Real-Time PCR detection system with iQ Supermix real-time PCR reagent (Bio-Rad, Hercules, CA). Genome copy numbers for a given sample in triplicate were calculated based on a standard plasmid DNA prep in the corresponding samples. The qPCR conditions included an initial denaturation step at 95°C for 3 min, followed by 40 cycles of amplification at 95°C for 15 s and 60°C for 1 min.
Statistical analyses.
Quantitative data, including the number of live organisms (IFUs) and genome copies, were analyzed using the Kruskal-Wallis test. Qualitative data, including incidence rates, were analyzed using Fisher's exact test. Semiquantitative data were analyzed using the Wilcoxon rank sum test.
RESULTS
Intravaginal inoculation with C. muridarum leads to a long-lasting infection in the GI tract of CBA/J mice.
We used a whole-body in vivo imaging technology for monitoring the distribution of the luciferase-expressing C. muridarum organisms in CBA/J mice following an intravaginal inoculation (Fig. 1). The luciferase-generated bioluminescence signal was detected as early as day 3 after the intravaginal inoculation, and the signal ascended to the lower abdominal area by day 7 in most mice. However, the signal persisted in the abdominal area 100 days after the inoculation, while the same mice cleared infection from the lower genital tract by day 35. We then used an ex vivo imaging for identifying the source organs of the bioluminescence signals (Fig. 2). The organs, including lungs, heart, spleen, liver, stomach-small intestine-large intestine-anorectum (gastrointestinal [GI] tract), kidneys, and ovary-oviduct-uterine horns-uterine-cervix-vagina (genital tract), from each mouse were arrayed in a petri dish for the ex vivo imaging. The bioluminescence signal was detected in both the lower (day 3) and upper (days 7 and 28) genital tract tissues during the first 4 weeks after the intravaginal inoculation. However, starting on day 28, most signals were localized to the GI tract tissues, including the rectum, cecum, colon, and stomach. The signals remained exclusively in the GI tract as time progressed. Since the bioluminescence signal correlates with active replication of chlamydial organisms (33), these findings suggest that C. muridarum spreads from the genital to the GI tracts and establishes a long-lasting infection in the GI tract.
FIG 1.
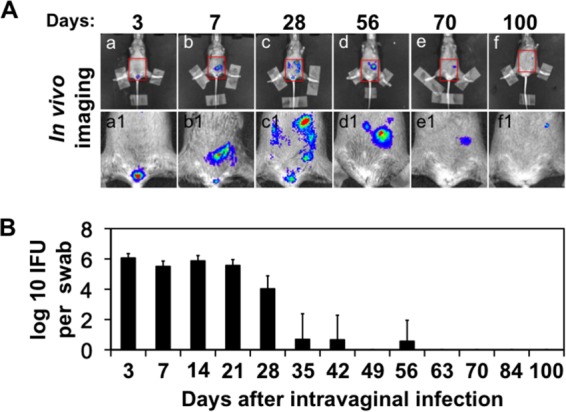
In vivo imaging of mice intravaginally infected with a C. muridarum strain that expresses a luciferase gene. CBA/J mice (n = 5) were intravaginally infected with luciferase-expressing C. muridarum. (A) On different days after infection as indicated at the top, a whole-body in vivo imaging technology was used to detect the luciferase-generated bioluminescence signals as displayed in red/green/blue colors (in the order of decreasing intensity, subpanels a to f). The mouse pelvic/abdominal areas marked with red squares were enlarged and are shown in subpanels a1 to f1 beneath the corresponding whole-mouse body images. Images taken from 1 of the 5 mice were shown. All mice displayed similar distribution patterns of the bioluminescence signals, which remained detectable in all 5 mice on day 100. Note that the bioluminescence signal was detected as early as day 3 in the vagina/cervix area after the intravaginal inoculation and the signal ascended to the lower abdominal area by day 7 and persisted there for >100 days. (B) The cervicovaginal swabs were taken from the same mice on different days after the intravaginal inoculation (as indicated along the x axis at the bottom of the figure) for titrating live organisms (displayed along the y axis in log10 scale). Note that live organisms were recovered as early as day 3 and maintained significantly high levels up to day 28. However, by day 35, most mice were cleared of genital tract infection.
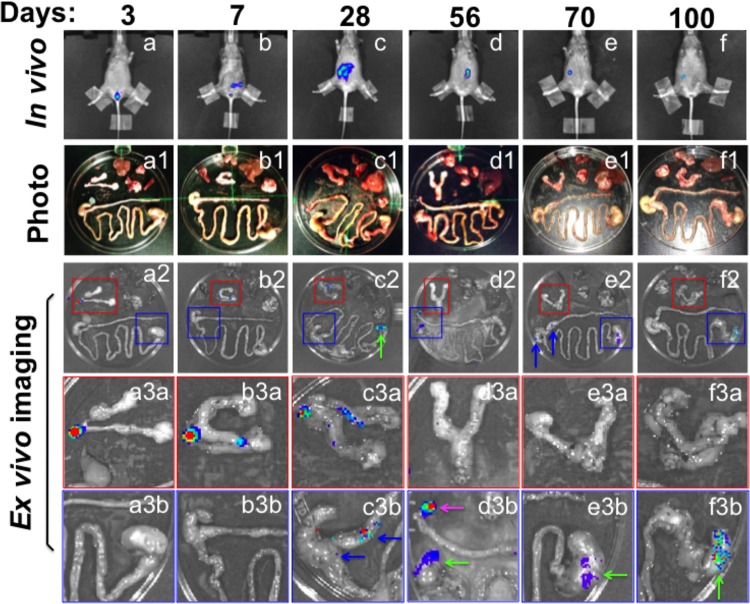
FIG 2.
Ex vivo imaging of organs from mice intravaginally infected with luciferase-expressing C. muridarum. CBA/J mice were intravaginally infected with luciferase-expressing C. muridarum organisms, and on different days after infection as indicated at the top, a whole-body in vivo imaging was taken (a to f) as described in Fig. 1 legend. At the same time, a group of 4 or 5 mice were sacrificed at each time point for harvesting the internal organs, including lungs, heart, spleen, liver, stomach-small intestine-cecum-large intestine-anorectum (gastrointestinal tract, or GI), kidneys, and ovary-oviduct-uterine horns-uterine-cervix-vagina (genital tract). Organs from the same mouse were arrayed in a petri dish as shown in panels a1 to f1. Ex vivo imaging was immediately taken to acquire bioluminescence images as shown in panels a2 to f2. Areas marked with red (genital tract) or blue (GI tract) squares were enlarged and are presented in panels a3a to f3a or a3b to f3b, respectively. Blue arrows point to cecum or colon, green arrows to stomach, and pink arrows to anorectum. At each time point, only one representative mouse-derived in vivo whole-body image and one ex vivo imaging of the organs from the same mouse are shown. Similar distribution patterns of the bioluminescence signals were found among all mice sacrificed at each time point. Note that although the bioluminescence signals were detectable only in the genital tract within the first 28 days after the intravaginal inoculation, the signals continued to be detected in the GI tract (and only in the GI tract) at the other time points.
The above-described imaging observations were further validated by detecting the C. muridarum organisms in the same mouse organs (Fig. 3). The homogenates of different segments of the genital and GI tracts as well as kidney, liver, spleen, heart, and lung from each mouse were carefully titrated for chlamydial live organisms and genomes. Both C. muridarum live organisms and genomes were detected in all organs on day 7 after intravaginal infection. By day 28, live organisms were no longer detectable in the lung, heart, or spleen tissues but displayed high titers in the genital and GI tract tissues. However, by day 56, live organisms were detected only in the GI tract tissues. Although chlamydial genomes were still detectable in the genital tract tissues, these genomes did not represent viable organisms. By day 70, chlamydial genomes were no longer detectable in the genital tract tissues. However, significant levels of both chlamydial live organisms and genomes were detected in the GI tract at all times. These titration results have largely confirmed the observations obtained with the imaging technology. Thus, we can conclude that the genital C. muridarum can establish a long-lasting infection exclusively in the GI tract. A closer examination of the GI tract chlamydial organism/genome recovery (Fig. 3) and ex vivo imaging (Fig. 2) seemed to reveal a discrepancy between the stomach and lower GI tract. Our organism/genome recovery data were consistent with what Yeruva et al. reported, i.e., that the lower GI tract, primarily the cecum/colon, was the major source of the GI tract chlamydial organisms (17, 32). However, the ex vivo imaging revealed dominant signals in the stomach but minimal signals in the lower GI tract (Fig. 2). This discrepancy was likely caused by the different accessibilities of the C. muridarum-expressed luciferase by the intraperitoneally delivered substrate d-luciferin. We speculate that d-luciferin may access the luciferase expressed by chlamydial organisms in the stomach much more easily than that expressed in the lower GI tract due to anatomic differences between the upper and lower GI tracts. For example, the submucosal blood vessels in the stomach may be more extensive and closer to the epithelial cells than those in the lower GI tract, which may promote the access of d-luciferin to Chlamydia-expressed luciferase.
FIG 3.
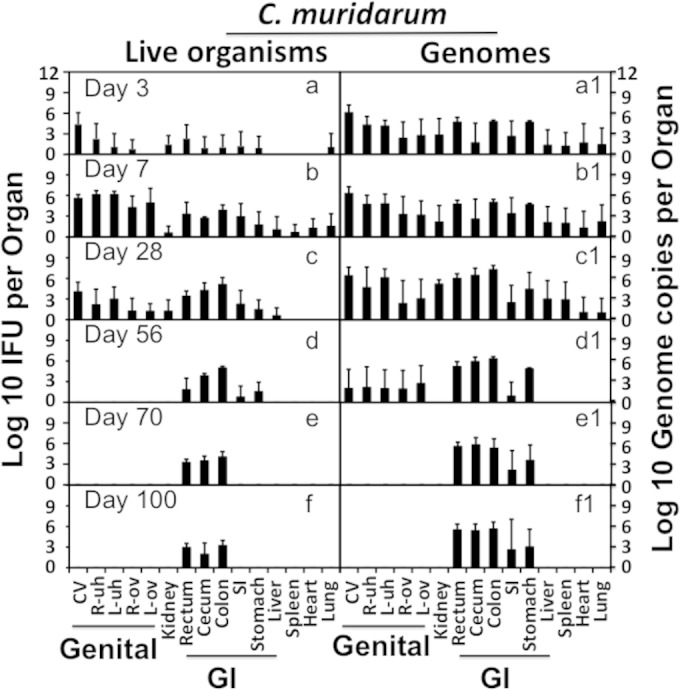
Recovery of live C. muridarum organisms and genomes from organs of mice intravaginally infected with luciferase-expressing C. muridarum. CBA/J mice were intravaginally infected with luciferase-expressing C. muridarum organisms, and on different days after infection as indicated in the corresponding panels, a group of 4 or 5 mice were sacrificed at each time point for harvesting organs as described in the Fig. 2 legend. The same organs after ex vivo imaging (Fig. 2 legend) were subjected to tissue homogenization in 0.5 ml SPG buffer. The homogenates from vagina-cervix (CV), right uterine horn (R-uh), left uterine horn (L-uh), right ovary and oviduct (R-ova), left ovary and oviduct (L-ova), kidney, rectum, cecum, colon, small intestine (SI), stomach, liver, spleen, heart, and lung (listed along the x axis at the bottom of the figure) were titrated for both chlamydial live organisms (a to f) and genome copies (a1 to f1). The titers were expressed as total number per organ/tissue (log10) and are displayed along the corresponding y axis (left for IFUs and right for genome copies). Organs/tissues belonging to the genital and gastrointestinal tracts were marked with “Genital” and “GI,” respectively. Note that both live organisms and genomes were detected in all organs on day 7 after intravaginal infection. By day 28, live organisms were no longer detectable in lung, heart, or spleen. By day 56, live organisms were detected only in the GI tract tissues from stomach to rectum, and by day 70, live organisms were restricted primarily to colon, cecum, and rectum. The detection of chlamydial genomes was more sensitive and remained positive in the entire GI tract tissues by day 100.
The genital C. muridarum spreading to establish a long-lasting infection in the GI tract is independent of mouse strain backgrounds.
Having observed the genital-to-GI tract spreading by C. muridarum in CBA/J mice, we further tested whether the spreading also occurred in other mouse strains. We used the same imaging technology and chlamydial organism detection from the tissue homogenates for monitoring the in vivo distribution of the luciferase-expressing C. muridarum in C57BL/6J mice (Fig. 4). The whole-body in vivo imaging identified the luciferase-generated bioluminescence signal in the abdominal area on days 28, 70, and 100 after intravaginal inoculation. Further ex vivo imaging of the mouse organs revealed the bioluminescence signal in both genital and GI tracts on day 28 but only in the GI tract on days 70 and 100. These observations were validated by titrating both C. muridarum live organisms and genomes from the same organs. The live organisms were detected in both the genital and GI tracts on day 28 but were restricted to the GI tract by days 70 and 100. The C. muridarum genome detection was more sensitive, with a positive detection in all organs on day 28. However, the chlamydial genomes were detectable only in the GI tract by days 70 and 100 after the intravaginal inoculation.
FIG 4.
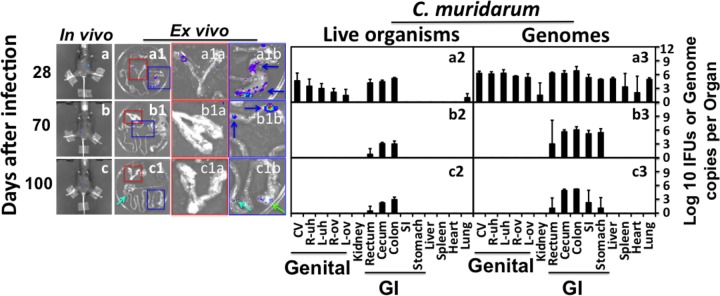
Monitoring the luciferase-expressing C. muridarum infection in C57BL/6J mice. Groups of female C57BL/6J mice were intravaginally infected with luciferase-expressing C. muridarum. On different days after infection as indicated on the left, the whole-body imaging for bioluminescence intensity was taken as described in Fig. 1 legend (a to c; only one representative image is shown). A group of 3 or 4 mice were sacrificed at each time point for harvesting the internal organs for ex vivo imaging as described in Fig. 2 legend (a1 to c1). Areas marked with red or blue squares were enlarged and are presented in panels a1a to c1a (covering the genital tract) or a1b to c1b (GI tract tissues). Blue arrows point to cecum or colon, green arrows to stomach, and greenish blue arrows to small intestines. At each time point, only one representative mouse-derived in vivo whole-body image and one ex vivo imaging of the organs (from the same mouse) are shown. After ex vivo imaging, the same organs were subjected to tissue homogenization as described in Fig. 3 legend. The homogenates from vagina-cervix (CV), right uterine horn (R-uh), left uterine horn (L-uh), right ovary and oviduct (R-ov), left ovary and oviduct (L-ov), kidney, rectum, cecum, colon, small intestine (SI), stomach, liver, spleen, heart, and lung as listed along the x axis at the bottom were titrated for both chlamydial live organisms (a2 to c2) and genome copies (a3 to c3). The results were expressed in total number per organ/tissue (log10) and are displayed along the corresponding y axis. Organs/tissues belonging to the genital and gastrointestinal tracts were marked with “Genital” and “GI,” respectively. Note that by day 28, live organisms were detected mainly in the genital and GI tracts but only in the GI tract by day 56 and thereafter.
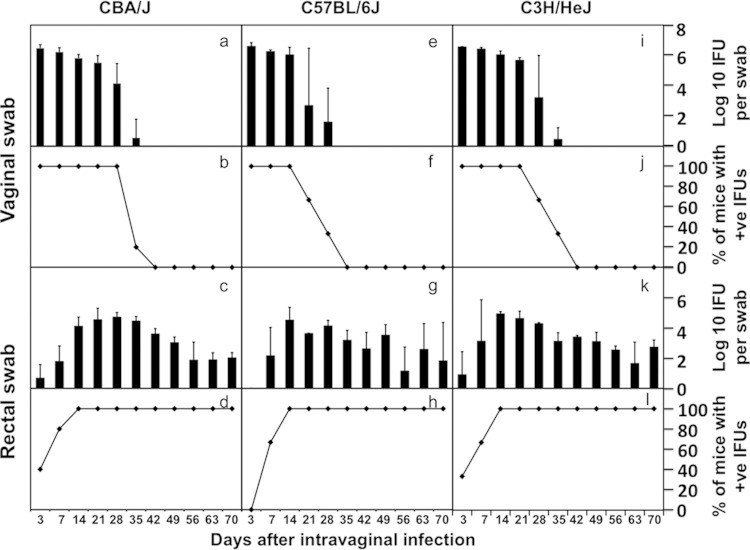
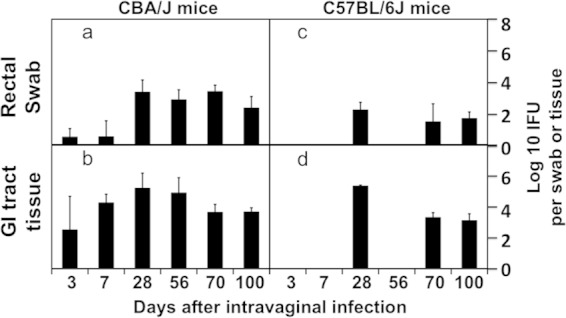
The total number of IFUs recovered from all GI tract segments from a given mouse correlated well with the number of IFUs recovered from the anorectal swab from the same mouse (Fig. 5). This analysis suggests that the GI tract infection with C. muridarum can be monitored by detecting the chlamydial live organisms in the rectal swabs. We then compared the live-organism recoveries from both vaginal and rectal swabs in three mouse strains, including CBA/J, C57BL/6J, and C3H/HeJ (Fig. 6). By day 35 after intravaginal infection, most mice cleared infection in the lower genital tracts regardless of their strain backgrounds. However, significant levels of live organisms were detected in the rectal swabs of all mouse strains throughout the observation period. These findings have demonstrated that the intravaginally inoculated C. muridarum organisms persist in the mouse GI tract for long periods of time regardless of the mouse strain.
FIG 5.
Recovery of live C. muridarum organisms from anorectal swabs for monitoring chlamydial infection in mouse gastrointestinal tract. CBA/J or C57BL/6J mice intravaginally infected with the luciferase-expressing C. muridarum as described in the legends of Fig. 1 and 4 were swabbed from the anorectum on different days after infection as listed along the x axis at the bottom of the figure. The anorectal swabs were assessed for live C. muridarum organisms, expressed as log10 IFUs per swab, shown along the y axis for CBA/1J (a) and C57BL/6J (c) mice. Since groups of mice were sacrificed on different days for making tissue homogenates (see legends of Fig. 3 and 4), the number of mice swabbed from each time point varied from 4 to 31. The number of C. muridarum live organisms recovered from the GI tract tissues harvested at each time was calculated as log10 means and standard deviations and plotted along the same infection course as the swab samples (panels b for CBA/J and d for C57BL/6J). It is clear that the numbers of live organisms detected in the anorectal swabs paralleled with those detected in the GI tract tissue homogenates at the corresponding time points, suggesting that the live-organism recovery from the anorectal swabs can be used for monitoring the GI tract infection.
FIG 6.
Comparison of live C. muridarum organism recoveries from genital versus GI tracts of three different strains of mice intravaginally infected with C. muridarum. CBA/J, C57BL/6J, and C3H/HeJ mice (n = 3 to 5 for each strain) were intravaginally infected with the C. muridarum organisms (clone Nigg3G0.1.1), and both cervicovaginal and anorectal swabs were taken on different days after infection as listed along the x axis at the bottom of the figure. The number of live organisms recovered from the swabs was expressed as log10 IFUs (panels a, e, i, c, g, and k), and the number of mice with positive live-organism shedding (+ve) was expressed as a percentage (b, f, j, d, h, and l) as displayed along the y axis. Note that although live organisms were no longer detectable in the vaginal swabs of most mice 5 weeks after infection, significant levels of live organisms remained in the rectal swabs of most mice for >70 days after infection.
The C. muridarum spreading from the genital to GI tracts is independent of oral or rectal routes.
The C. muridarum genomes were detected in multiple organs during the first 4 weeks (Fig. 3 and 4), and the live infectious organisms were seen in all the organs examined during the first 2 weeks after intravaginal infection (Fig. 3), which is consistent with what was previously reported by Cotter et al. (10) and Perry et al. (11). These observations demonstrated that C. muridarum organisms spread systemically after intravaginal inoculation. However, it remains unknown whether the systemic spreading is responsible for the C. muridarum organism trafficking to the GI tract. This is because following an intravaginal inoculation, live C. muridarum organisms can be shed to the vagina and eventually excreted. The live-organism-containing excretions can be taken into the GI tract by mice either orally or via anorectal contact. To exclude these possibilities, mice wearing Elizabethan collars and singly housed in the netted cage were monitored for genital and GI tract infections following an intravaginal inoculation (Fig. 7). Despite the physical restraining of mice from picking up excretions, all mice still developed long-lasting infection in the GI tract, suggesting that oral uptake is not required for the genital chlamydial organisms to spread to the GI tract. However, the above-described experiment cannot exclude the possibility of cross-contamination between the vaginal and rectal areas, which might also contribute to the observed GI tract spreading. We then further compared the C. muridarum spreading to the GI tract following intravaginal versus intrabursal inoculations. We previously showed that after intrabursal inoculation, there was a delay in live organisms descending to the vagina and being excreted out of the mouse body (8, 38). We expected a significant delay of the spreading of the intrabursally inoculated organisms to the GI tract if oral uptake or anorectal contact of the excreted live organisms was required for the chlamydial spreading. As shown in Fig. 8, the luciferase-generated bioluminescence signal was detected in the vagina/cervix area on day 3 following the intravaginal inoculation. However, the signal from the intrabursally inoculated organisms was restricted to only the left abdominal area, where the initial intrabursal injection was performed, on both day 3 and day 7 after the intrabursal injection, indicating that the intrabursally inoculated organisms failed to reach the lower genital tract area for excretion at these time points. Only when the infections progressed did the signal expand in the abdominal area and to the lower genital tract area. Mice were simultaneously monitored for the live-organism shedding by both vaginal and rectal swabs (Fig. 9). Mice intravaginally infected with C. muridarum developed positive shedding in the vaginal swab on day 3 and in the rectal swab only on day 10 after infection. However, mice intrabursally inoculated with C. muridarum showed no live organism shedding in the vaginal swabs on either day 3 or day 7 after the inoculation, which is consistent with the above-described in vivo imaging result (showing that no bioluminescence signal was detected in the lower genital tract area). Interestingly, the intrabursally inoculated mice showed live organisms in the rectal swabs taken on day 7, suggesting that the intrabursally inoculated C. muridarum organisms successfully spread to the GI tract by day 7, when no live organisms were present in the vagina. These observations together suggest that the intrabursally inoculated organisms spread to the GI tract via a route independent of either oral uptake or anorectal contact. This is because the intrabursally inoculated organisms did not have access to the outside of any mouse housed in the same cage on day 7, which made it impossible for the mice to acquire live organisms into their GI tracts via either oral uptake or anorectal contact. In both groups of mice, the continued presence of live organisms in the rectal swabs long after the organisms were cleared from the vaginal swabs indicates that C. muridarum organisms established long-lasting infections in the GI tracts regardless of how the organisms were delivered to the genital tract.
FIG 7.
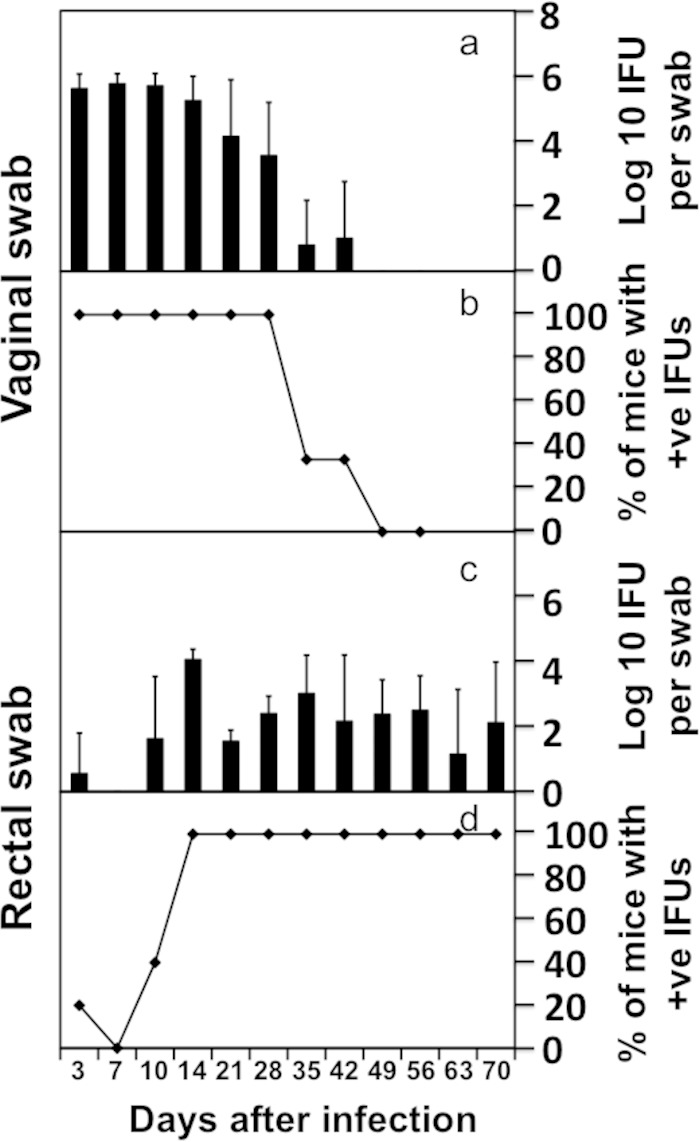
Recovery of live C. muridarum organisms from genital versus GI tracts of C57BL/6J mice wearing an Elizabethan collar. C57BL/6J mice (n = 5, each wearing an Elizabethan collar) were intravaginally infected with luciferase-expressing C. muridarum. Both cervicovaginal and anorectal swabs were taken on different days after infection as indicated along the x axis at the bottom of the figure. The number of live organisms recovered from the swabs was expressed as log10 IFUs (a and c), and the number of mice with positive live-organism shedding (+ve) was expressed as a percentage (b and d) as displayed along the y axis.
FIG 8.
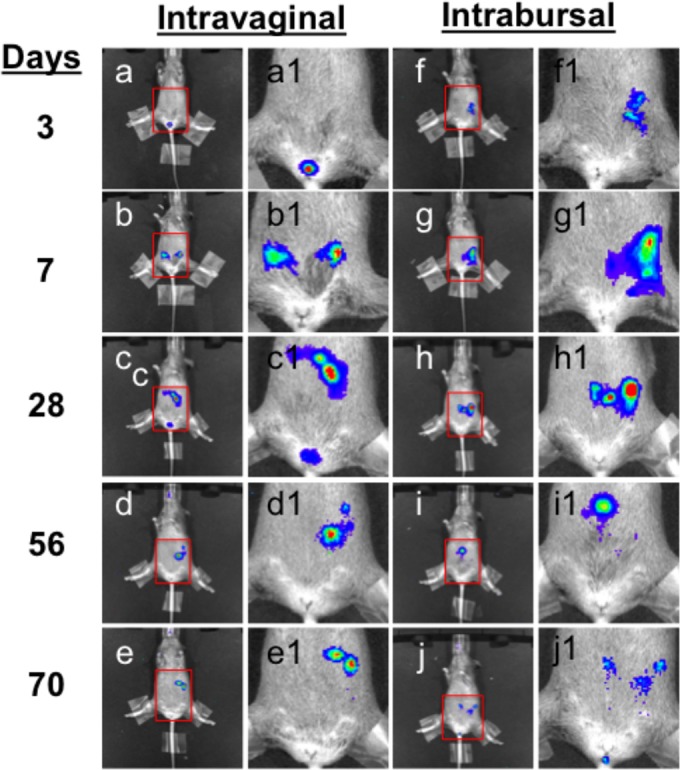
In vivo imaging of C. muridarum traffic in CBA/J mice infected in the lower versus upper genital tracts. CBA/J mice were infected with C. muridarum intravaginally (a to e, n = 5) or intrabursally (f to j, n = 5). On different days after infection as listed on the left, whole-body in vivo imaging was taken on each mouse. Images from one representative mouse are shown. The lower abdominal areas marked with red squares were amplified and are presented as panels a1 to j1. Note that the bioluminescence signal was restricted to the site of injection without descending to the lower genital tract on day 3 or 7 after the intrabursal injection.
FIG 9.
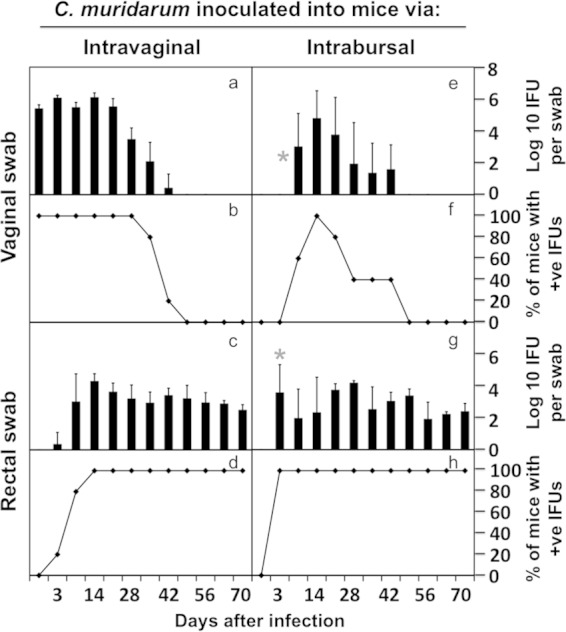
Effect of the upper genital tract inoculation on chlamydial spreading to the GI tract. The same CBA/J mice infected with C. muridarum intravaginally (a to d, n = 5) or intrabursally (e to h, n = 5) as described in the legend of Fig. 6 were swabbed from both cervicovaginal (a, b, e, and f) and anorectal (c, d, g, and h) sites on different days after infection, as indicated along the x axis at the bottom of the figure. The number of live organisms recovered from the swabs was expressed as log10 IFUs (a, e, c, g), and the number of mice with positive live-organism shedding (+ve) was expressed as a percentage (b, f, d, h) as displayed along the y axis. Note that following genital tract inoculation, C. muridarum spread to the GI tract as early as day 7 and persisted in the GI tract regardless of the precise routes of inoculation. However, intrabursal inoculation, although resulting in a delayed vaginal shedding of live organisms (no live organism on day 7, star in panel e), caused a significantly higher level of infection in the GI tract on day 7 (panel g, marked with a star, P < 0.05).
DISCUSSION
In the current study, the in vivo whole-body imaging of the luciferase-expressing C. muridarum infection in mice following a genital tract inoculation has led us to discover that a long-lasting GI tract infection with C. muridarum can be caused by the genital tract-derived chlamydial organisms. This discovery is significant, since it has confirmed the long-observed chlamydial ability to achieve long-term residence in the GI tract and more importantly provided an explanation for the source of the GI tract Chlamydia. Since the mouse chlamydial species C. muridarum can spread from the genital to the GI tracts in female mice, we can now ask whether the human chlamydial species C. trachomatis spreads from the urogenital tract to the GI tract in women. The same logic has been applied for correlating mouse model findings with human chlamydial infection characteristics. For example, the observations that oral or intragastric inoculation caused long-lasting C. muridarum infection in the mouse GI tract (11, 16–18, 22, 32) have been used to support the proposal that women may acquire GI tract C. trachomatis infection via oral intercourse (22). While C. trachomatis organisms can be introduced into the GI tract of a woman via oral intercourse, we should not fix our attention on the sexual behavior pathway only. Women may acquire chlamydial infection in the GI tract via multiple pathways. For example, a positive diagnosis for C. trachomatis in rectal swabs has been reported among diverse woman populations, some of whom may not necessarily practice oral or anal intercourse. A recent cross-sectional study of 604 adult women visiting 25 primary health care facilities in rural South Africa revealed a 7.1% rate of rectal chlamydial infection (21). A separate study reported a >10% prevalence rate for rectal chlamydial infection among 3,055 women who attended 2 Canadian provincial sexually transmitted infection (STI) clinics (27). Sexual behaviors were not considered in either study. It is possible that some of these women may acquire chlamydial infection in the GI tract via sexual behavior-independent pathways. This hypothesis is consistent with a recent report showing that women with urogenital Chlamydia were significantly more likely to have a positive rectal result, and neither anal symptoms nor reported anal sex was associated with a positive rectal Chlamydia test (28). While the contribution of sexual behavior pathways for transmitting C. trachomatis to the GI tract is recognized, the results from our current mouse study have also alerted us to investigate the possibility of the urogenital tract C. trachomatis spreading to the GI tract of women via sexual behavior-independent pathways. A caveat for the above-mentioned conclusion is that even in a population without the habit of practicing oral or anal sex, the chlamydial organisms can also be orally introduced to the GI tract, for example, through oral contamination from secretions on hands or fingers.
How are the C. muridarum organisms spread from the mouse genital tract to GI tract? Since intravaginal inoculation of C. muridarum can result in live-organism shedding from the vagina, mice may pick up the live organisms either orally or via anorectal contact. Elizabethan collars were used to prevent mice from eating their own excretions, which may contain live chlamydial organisms (11). However, mice singly housed wearing the Elizabethan collars still developed robust GI tract infection following intravaginal inoculation. We reproduced this observation in the current study (Fig. 7). These observations suggest that oral uptake is not required for the genital chlamydial organisms to spread to the GI tract. We and others have presented evidence that the intravaginally inoculated C. muridarum can spread to multiple organs, including the GI tract (Fig. 3 and 4) (10, 11). Most importantly, we have demonstrated that the intrabursally inoculated C. muridarum organisms spread to GI tracts in the absence of live-organism shedding (Fig. 8). Thus, we can conclude that C. muridarum can spread from the genital to the GI tracts via a pathway independent of either oral uptake or anorectal contact and that the oral or anorectal contact-independent spreading can lead to long-lasting chlamydial infections in the GI tract. A caveat is that the intrabursal injection may cause spillage of the C. muridarum organisms into the peritoneum, allowing C. muridarum access to the circulation system and GI tract. Thus, more evidence is still required for demonstrating a direct spreading from the genital to the GI tracts. At this moment, let us assume that the genital tract C. muridarum can spread to the GI tract. The next question is what pathways the genital C. muridarum organisms can use for the spreading. We speculate that the progeny EBs produced in the genital tract epithelial cells may enter the circulation or lymphatic systems with or without being taken up by phagocytes or dendritic cells. Since live chlamydial EBs can be recovered from the livers, lungs, or spleens a few weeks after intravenous inoculation (41, 42), direct bacteremia may be a route for the spreading. Given the enriched submucosal blood vessels in the endometrial tissue, it should not be too difficult for the progeny EBs produced in the endometrial epithelial cells to leak into the submucosal area when the infected cells are lysed and further gain access to the bloodstream. Alternatively, the progeny EBs can be taken by or actively infect mucosal phagocytes or dendritic cells and the EB-laden cells may enter the lymphatic system, through which they can access the GI tract. This hypothesis is consistent with the observation that C. muridarum organisms were detected in mesenteric lymph nodes (10). It will be interesting to investigate the precise molecular and cellular mechanisms by which the genital tract C. muridarum organisms initially cross the mucosal barrier.
Regardless of how the chlamydial organisms access the GI tract, the most important question is the biological/medical significance of the long-lasting chlamydial infection in the GI tract. Although genital tract infection with Chlamydia may result in joint pathologies in both humans (43, 44) and mice (45), the association of chlamydial infection with GI tract pathologies is unclear (24–26). In fact, no inflammatory pathology was detected despite the long-lasting presence of the C. muridarum organisms in the GI tract (16). Since chlamydial infection in the GI tract is long lasting (16) and more resistant to antibiotic treatment than infection in the genital tract (17, 22, 46), it has been proposed that the GI tract chlamydial organisms may serve as a reservoir for reinfecting the genital tract and/or causing persistent infection in the genital tract (32, 46), which potentially leads to exacerbation of the upper genital tract pathology. The finding that mucosal infection with C. muridarum can spread to the other mucosae (11) is consistent with this hypothesis. The fact that some women were diagnosed with rectal Chlamydia alone (27) also seems to support this hypothesis. However, we must recognize that this hypothesis has not been directly tested. Questions as to the efficiency of the GI tract chlamydial spread to the genital tract have not been carefully evaluated in either animal model studies or human epidemiological investigations.
We should be cautious in using the findings obtained from the C. muridarum infection in mice to interpret C. trachomatis pathogenicity in humans. Obviously, C. muridarum has been adapted to mice in extra-genital tract organs, including the lung and GI tract. C. muridarum strain Nigg was initially identified as a virus that caused systemic infection in mice (47) and persisted in the respiratory tract of normal mice without causing obvious pathologies (48). Thus, the genital tract might not be the primary target of the C. muridarum organisms (49). However, the C. trachomatis serovars were all isolated from human tissues, including the ocular (50–52) and urogenital tract (53–55) tissues. Thus, the C. trachomatis organisms likely use the ocular or urogenital tract tissues as their primary sites for adapting to the human host. However, the high rates of positive detection of C. trachomatis in the rectal swabs taken from women (21, 27, 28) indicate that C. trachomatis organisms can also infect the GI tract. The widespread GI tract infection in humans has prompted modification of chlamydial treatment guidelines and stimulated the investigation of the transmission pathways and the effects of the chlamydial GI tract infection on chlamydial pathogenesis in the genital tract. It is worth noting that C. trachomatis has long been proposed to persist in infected individuals as a noninfectious form for long periods of time, although there is still lack of direct evidence for supporting the hypothesis. Here, we have found that C. muridarum organisms can persist in the GI tract for long periods of time in the infectious form. The question is whether similar long-lasting active C. trachomatis infection also exists in the human GI tract. As discussed above, the active gut infection may serve as a reservoir for reinfecting the upper genital tracts in women. Testing of these hypotheses should significantly advance our understanding of the pathogenic mechanisms of C. trachomatis infections in humans.
ACKNOWLEDGMENTS
This work was supported in part by grants (to G.Z.) from the U.S. National Institutes of Health. Q.Z. and Y.H. are supported by the Central South University Xiangya Medical Student Exchange Program.
REFERENCES
- 1.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 2.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Maza LM, Peterson EM. 2002. Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs 3:980–986. [PubMed] [Google Scholar]
- 4.Rockey DD, Wang J, Lei L, Zhong G. 2009. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines 8:1365–1377. doi: 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- 5.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Zhou Z, Chen J, Wu G, Yang Z, Zhou Z, Baseman J, Zhang J, Reddick RL, Zhong G. 2014. Lack of long lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum. Infect Immun 82:2688–2696. doi: 10.1128/IAI.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G. 2013. Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One 8:e71649. doi: 10.1371/journal.pone.0071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun 65:2145–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry LL, Hughes S. 1999. Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infect Immun 67:3686–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Lei L, Zhou Z, He J, Xu S, Lu C, Chen J, Yang Z, Wu G, Yeh IT, Zhong G, Wu Y. 2013. Contribution of interleukin-12 p35 (IL-12p35) and IL-12p40 to protective immunity and pathology in mice infected with Chlamydia muridarum. Infect Immun 81:2962–2971. doi: 10.1128/IAI.00161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Fan Y, Brunham RC, Yang X. 1999. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur J Immunol 29:3782–3792. doi:. [DOI] [PubMed] [Google Scholar]
- 14.Perry LL, Feilzer K, Caldwell HD. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol 158:3344–3352. [PubMed] [Google Scholar]
- 15.Li LX, McSorley SJ. 2013. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection. PLoS Pathog 9:e1003707. doi: 10.1371/journal.ppat.1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igietseme JU, Portis JL, Perry LL. 2001. Inflammation and clearance of Chlamydia trachomatis in enteric and nonenteric mucosae. Infect Immun 69:1832–1840. doi: 10.1128/IAI.69.3.1832-1840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeruva L, Melnyk S, Spencer N, Bowlin A, Rank RG. 2013. Differential susceptibilities to azithromycin treatment of chlamydial infection in the gastrointestinal tract and cervix. Antimicrob Agents Chemother 57:6290–6294. doi: 10.1128/AAC.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rank RG, Yeruva L. 2014. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun 82:1362–1371. doi: 10.1128/IAI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kent CK, Chaw JK, Wong W, Liska S, Gibson S, Hubbard G, Klausner JD. 2005. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis 41:67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]
- 20.Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, Read R. 2014. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis 41:589–591. doi: 10.1097/OLQ.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 21.Peters RP, Dubbink JH, van der Eem L, Verweij SP, Bos ML, Ouburg S, Lewis DA, Struthers H, McIntyre JA, Morre SA. 2014. Cross-sectional study of genital, rectal, and pharyngeal Chlamydia and gonorrhea in women in rural South Africa. Sex Transm Dis 41:564–569. doi: 10.1097/OLQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 22.Rank RG, Yeruva L. 2015. An alternative scenario to explain rectal positivity in chlamydia-infected individuals. Clin Infect Dis 60:1585–1586. doi: 10.1093/cid/civ079. [DOI] [PubMed] [Google Scholar]
- 23.Wong HT, Lee KC, Chan DP. 2015. Community-based sexually transmitted infection screening and increased detection of pharyngeal and urogenital Chlamydia trachomatis and Neisseria gonorrhoeae infections in female sex workers in Hong Kong. Sex Transm Dis 42:185–191. doi: 10.1097/OLQ.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dlugosz A, Tornblom H, Mohammadian G, Morgan G, Veress B, Edvinsson B, Sandstrom G, Lindberg G. 2010. Chlamydia trachomatis antigens in enteroendocrine cells and macrophages of the small bowel in patients with severe irritable bowel syndrome. BMC Gastroenterol 10:19. doi: 10.1186/1471-230X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Kruiningen HJ, Stephenson EH, Thayer WR Jr. 1986. A negative search for four infectious agents in inflammatory bowel disease. J Clin Gastroenterol 8:255–257. doi: 10.1097/00004836-198606000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Cartun RW, Van Kruiningen HJ, Pedersen CA, Berman MM. 1993. An immunocytochemical search for infectious agents in Crohn's disease. Mod Pathol 6:212–219. [PubMed] [Google Scholar]
- 27.Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, Bell CA, Drews SJ, Read R. 2015. Evidence for increased Chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 60:398–404. doi: 10.1093/cid/ciu831. [DOI] [PubMed] [Google Scholar]
- 28.Musil K, Currie M, Sherley M, Martin S. 2015. Rectal chlamydia infection in women at high risk of chlamydia attending Canberra Sexual Health Centre. Int J STD AIDS pii:0956462415586317. [DOI] [PubMed] [Google Scholar]
- 29.Trebach JD, Chaulk CP, Page KR, Tuddenham S, Ghanem KG. 2015. Neisseria gonorrhoeae and Chlamydia trachomatis among women reporting extragenital exposures. Sex Transm Dis 42:233–239. doi: 10.1097/OLQ.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazan JA, Carr Reese P, Esber A, Lahey S, Ervin M, Davis JA, Fields K, Turner AN. 2015. High prevalence of rectal gonorrhea and Chlamydia infection in women attending a sexually transmitted disease clinic. J Womens Health (Larchmt) 24:182–189. doi: 10.1089/jwh.2014.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campos-Hernandez E, Vazquez-Chagoyan JC, Salem AZ, Saltijeral-Oaxaca JA, Escalante-Ochoa C, Lopez-Heydeck SM, de Oca-Jimenez RM. 2014. Prevalence and molecular identification of Chlamydia abortus in commercial dairy goat farms in a hot region in Mexico. Trop Animal Health Prod 46:919–924. doi: 10.1007/s11250-014-0585-6. [DOI] [PubMed] [Google Scholar]
- 32.Yeruva L, Spencer N, Bowlin AK, Wang Y, Rank RG. 2013. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis 68:88–95. doi: 10.1111/2049-632X.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell J, Huang Y, Liu Y, Schenken R, Arulanandam B, Zhong G. 2014. Bioluminescence imaging of Chlamydia muridarum ascending infection in mice. PLoS One 9:e101634. doi: 10.1371/journal.pone.0101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto A, Izutsu H, Miyashita N, Ohuchi M. 1998. Plaque formation by and plaque cloning of Chlamydia trachomatis biovar trachoma. J Clin Microbiol 36:3013–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, Wu Y, Zhong G. 2015. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infect Immun 83:1881–1892. doi: 10.1128/IAI.03158-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Chen C, Gong S, Hou S, Qi M, Liu Q, Baseman J, Zhong G. 2014. Transformation of Chlamydia muridarum reveals a role for Pgp5 in suppression of plasmid-dependent gene expression. J Bacteriol 196:989–998. doi: 10.1128/JB.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. doi: 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturdevant GL, Kari L, Gardner DJ, Olivares-Zavaleta N, Randall LB, Whitmire WM, Carlson JH, Goheen MM, Selleck EM, Martens C, Caldwell HD. 2010. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect Immun 78:3660–3668. doi: 10.1128/IAI.00386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, Yang Z, Sun X, Tang L, Ding Y, Xue M, Zhou Z, Baseman J, Zhong G. 2015. Intrauterine infection with plasmid-free Chlamydia muridarum reveals a critical role of the plasmid in chlamydial ascension and establishes a model for evaluating plasmid-independent pathogenicity. Infect Immun 83:2583–2592. doi: 10.1128/IAI.00353-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong GM, Peterson EM, Czarniecki CW, Schreiber RD, de la Maza LM. 1989. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun 57:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampe MF, Wilson CB, Bevan MJ, Starnbach MN. 1998. Gamma interferon production by cytotoxic T lymphocytes is required for resolution of Chlamydia trachomatis infection. Infect Immun 66:5457–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Appel H, Kuon W, Kuhne M, Wu P, Kuhlmann S, Kollnberger S, Thiel A, Bowness P, Sieper J. 2004. Use of HLA-B27 tetramers to identify low-frequency antigen-specific T cells in Chlamydia-triggered reactive arthritis. Arthritis Res Ther 6:R521–R534. doi: 10.1186/ar1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villareal C, Whittum-Hudson JA, Hudson AP. 2002. Persistent Chlamydiae and chronic arthritis. Arthritis Res 4:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baillet AC, Rehaume L, Benham H, O'Meara CP, Armitage CW, Ruscher R, Brizard G, Cg Harvie M, Velasco J, Hansbro P, Forrester JV, Degli-Esposti M, Beagley KW, Thomas R. 2015. High Chlamydia burden promotes tumor necrosis factor-dependent reactive arthritis in SKG mice. Arthritis Rheumatol 67:1535–1547. doi: 10.1002/art.39041. [DOI] [PubMed] [Google Scholar]
- 46.Craig AP, Kong FY, Yeruva L, Hocking JS, Rank RG, Wilson DP, Donovan B. 2015. Is it time to switch to doxycycline from azithromycin for treating genital chlamydial infections in women? Modelling the impact of autoinoculation from the gastrointestinal tract to the genital tract. BMC Infect Dis 15:200. doi: 10.1186/s12879-015-0939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nigg C. 1942. An unidentified virus which produces pneumonia and systemic infection in mice. Science 95:49–50. [DOI] [PubMed] [Google Scholar]
- 48.Nigg C, Eaton MD. 1944. Isolation from normal mice of a pneumotropic virus which forms elementary bodies. J Exp Med 79:497–510. doi: 10.1084/jem.79.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rank RG. 2006. Chlamydial diseases, p 325–348. In Fox GJ, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL (ed), The mouse in biomedical research: diseases, 2nd ed, vol 2 Academic Press, New York, NY. [Google Scholar]
- 50.Tang FF, Chang HL, Huang YT, Wang KC. 1957. Studies on the etiology of trachoma with special reference to isolation of the virus in chick embryo. Chin Med J 75:429–447. [PubMed] [Google Scholar]
- 51.Tang FF, Huang YT, Chang HL, Wong KC. 1957. Isolation of trachoma virus in chick embryo. J Hyg Epidemiol Microbiol Immunol 1:109–120. [PubMed] [Google Scholar]
- 52.Tang FF, Huang YT, Chang HL, Wong KC. 1958. Further studies on the isolation of the trachoma virus. Acta Virol 2:164–170. [PubMed] [Google Scholar]
- 53.Mordhorst CH. 1965. Isolation of the Tric agent from eye and urogenital tract of trachoma patients in Denmark. Acta Pathol Microbiol Scand 63:637–638. [DOI] [PubMed] [Google Scholar]
- 54.Foy HM, Wang SP, Kenny GE, Johnson WL, Grayston JT. 1967. Isolation of TRIC agents and mycoplasma from the cervix of pregnant women. Preliminary results. Am J Ophthalmol 63(Suppl):1053–1056. doi: 10.1016/0002-9394(67)94082-2. [DOI] [PubMed] [Google Scholar]
- 55.Holt S, Pedersen AH, Wang SP, Kenny GE, Foy HM, Grayston JT. 1967. Isolation of TRIC agents and mycoplasma from the genito-urinary tracts of patients of a venereal disease clinic. Am J Ophthalmol 63(Suppl):1057–1064. doi: 10.1016/0002-9394(67)94083-4. [DOI] [PubMed] [Google Scholar]
- 56.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]