Abstract
Streptococcus agalactiae (group B streptococcus [GBS]) colonizes the rectovaginal tract in 20% to 30% of women and during pregnancy can be transmitted to the newborn, causing severe invasive disease. Current routine screening and antibiotic prophylaxis have fallen short of complete prevention of GBS transmission, and GBS remains a leading cause of neonatal infection. We have investigated the ability of Streptococcus salivarius, a predominant member of the native human oral microbiota, to control GBS colonization. Comparison of the antibacterial activities of multiple S. salivarius strains by use of a deferred-antagonism test showed that S. salivarius strain K12 exhibited the broadest spectrum of activity against GBS. K12 effectively inhibited all GBS strains tested, including disease-implicated isolates from newborns and colonizing isolates from the vaginal tract of pregnant women. Inhibition was dependent on the presence of megaplasmid pSsal-K12, which encodes the bacteriocins salivaricin A and salivaricin B; however, in coculture experiments, GBS growth was impeded by K12 independently of the megaplasmid. We also demonstrated that K12 adheres to and invades human vaginal epithelial cells at levels comparable to GBS. Inhibitory activity of K12 was examined in vivo using a mouse model of GBS vaginal colonization. Mice colonized with GBS were treated vaginally with K12. K12 administration significantly reduced GBS vaginal colonization in comparison to nontreated controls, and this effect was partially dependent on the K12 megaplasmid. Our results suggest that K12 may have potential as a preventative therapy to control GBS vaginal colonization and thereby prevent its transmission to the neonate during pregnancy.
INTRODUCTION
Streptococcus agalactiae (group B streptococcus [GBS]) is a Gram-positive organism that is currently the leading infectious agent responsible for neonatal morbidity and mortality in the United States (1). The primary risk factor for newborn disease is maternal GBS colonization within the gastrointestinal or vaginal tract (1). GBS colonization of the vaginal tract during pregnancy may be constant, intermittent, or transient in nature among individual women (2). In lieu of an effective vaccine, the current recommendations from the Centers for Disease Control and Prevention include late-gestational screening and intrapartum antibiotic prophylaxis (IAP) for GBS-positive mothers, which has resulted in reduced early-onset GBS disease and infant colonization (1). However, IAP with ampicillin for GBS-positive mothers has been determined an independent risk factor for ampicillin-resistant Escherichia coli early-onset sepsis (3) and has recently been shown to reduce beneficial Bifidobacterium spp. in week-old newborn intestinal flora (4). There are likely to be other unrecognized negative effects of antibiotic exposure on long-term gut health, which prompts development of alternative prophylactic strategies. Several studies have proposed the use of probiotic strains to control GBS and have observed inhibitory activity in vitro with Lactobacillus (5) and Bifidobacterium (4).
In the present study, we examined the inhibitory capacity of the oral commensal Streptococcus salivarius against GBS. S. salivarius is an early and prolific colonizer of the human oropharyngeal tract, and certain strains have been recently developed for use as oral probiotics due to their production of a diverse assortment of bacteriocins having inhibitory activity that is especially directed against members of other Streptococcus species, including causative agents of strep throat and otitis media (6, 7). Several of these bacteriocins are encoded by megaplasmids which have demonstrated mobility to other S. salivarius strains in vivo (8). S. salivarius strains K12 and M18, developed and marketed by BLIS Technologies Ltd. as probiotic strains BLIS K12 and BLIS M18, contain the fully sequenced megaplasmids pSsal-K12 (6) and pSsal-M18 (9), respectively. These two megaplasmids show greater than 50% alignment, with pSsal-K12 containing loci for salivaricins A2 and B and pSsal-M18 containing loci for salivaricins A2, 9, and MPS (6, 9). Because of their known inhibitory effect on Streptococcus pyogenes and similar Streptococcus species (8), we hypothesize that some of these megaplasmid-encoded bacteriocins will also have activity toward GBS.
In this study, we have utilized a deferred-antagonism test as well as coculture assays to demonstrate that S. salivarius strains possess differing abilities to inhibit the growth of GBS clinical isolates. Furthermore, we have shown that S. salivarius strain K12 is capable of interacting with human vaginal epithelial cells in a manner similar to that of GBS. Our in vitro results are corroborated in a murine model of vaginal colonization in which inoculation with K12 reduces GBS vaginal carriage. Based on our data, we conclude that administration of K12 may have potential as a preemptive treatment to limit GBS vaginal colonization during pregnancy and thereby prevent neonatal transmission.
MATERIALS AND METHODS
Bacterial strains.
Streptococcus agalactiae strains utilized in this study include A909 (ATCC BAA-1138), CJB111 (ATCC BAA-23), COH1 (ATCC BAA-1176), NCTC 10/84 (ATCC 49447), NEM316 (ATCC 12403), 515 (ATCC BAA-1177), 2603 V/R (ATCC BAA-611), and six vaginal isolates obtained from pregnant women (10), which we here denoted DMC-VI-10, DMC-VI-27, DMC-VI-29, DMC-VI-39, DMC-VI-40, and DMC-VI-50. All S. salivarius strains used in this study were from the laboratory collection at BLIS Technologies Ltd. and are given in Table 1. All strains were grown aerobically in Todd-Hewitt broth (THB) (Hardy Diagnostics, Santa Maria, CA) at 37°C with 5% CO2. For coculture assays, all strains were grown in THB supplemented with 1% (wt/vol) glucose and 4 mM calcium chloride. When required, antibiotics were added at the following concentrations: streptomycin (Strep), 1,000 μg/ml; spectinomycin (Spec), 100 μg/ml.
TABLE 1.
Deferred-antagonism test with GBS clinical isolates
| Strain | Description | No. of GBS isolates inhibited | Inhibitionc against indicated GBS isolate |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A909 | CJB111 | COH1 | NCTC 10/84 | NEM316 | 515 | 2603 V/R | DMC-VI-10 | DMC-VI-27 | DMC-VI-29 | DMC-VI-39 | DMC-VI-40 | DMC-VI-50 | |||
| K12 | WT S. salivariusa | 13/13 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| K12ΔpK12 | K12 cured of pSsal-K12 | 0/13 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| M18 | WT S. salivariusa | 2/13 | − | − | − | − | + | − | − | − | − | + | − | − | − |
| M18pK12 | M18 cured of pSsal-M18 expressing pSsal-K12 | 13/13 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Tove R | WT S. salivariusa | 13/13 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| NR | WT S. salivariusa | 5/13 | + | + | − | + | − | − | − | − | − | + | + | − | − |
| 20P3 | WT S. salivariusa | 1/13 | − | − | − | − | − | − | − | − | − | + | − | − | − |
| #5 | WT S. salivariusa | 1/13 | − | − | − | − | − | − | − | − | − | + | − | − | − |
| MPS | WT S. salivariusa | 0/13 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| P | WT S. salivariusa | 0/13 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CCHSS3 | WT S. salivariusb | 0/13 | − | − | − | − | − | − | − | − | − | − | − | − | − |
Human oral isolate.
Human blood isolate.
+, inhibition; −, no inhibition.
Human cell lines.
Immortalized human vaginal epithelial cells (hVEC; VK2/E6E7) were attained from the American Type Culture Collection (ATCC CRL-2616) (11). Cell lines (passages 5 to 25) were cultured in keratinocyte serum-free medium (KSFM) (Life Technologies, Carlsbad, CA) with 0.5 ng/ml human recombinant epidermal growth factor and 0.05 mg/ml bovine pituitary extract at 37°C with 5% CO2.
Deferred-antagonism assays.
Deferred-antagonism assays were carried out as described previously (12). In brief, test strains were inoculated as a 1-cm-wide streak across a Columbia agar base (Difco) supplemented with 5% (vol/vol) human blood and 0.1% (wt/vol) calcium carbonate. After an 18-h incubation, visible growth of the test strain was removed with a glass slide and the agar surface was sterilized with chloroform vapors for 30 min. The plates were then inoculated with liquid indicator strain cultures perpendicular to original test strain growth and incubated for 24 h. Distinct inhibition of indicator strains at the zone of test strain growth was recorded as positive. The indicator strain Micrococcus luteus was used as a positive control. All tests were performed at least in duplicate, and further testing was undertaken if significant discrepancies, such as scattered colonies across the zone of test strain growth, were detected in the inhibition patterns obtained.
Coculture assays.
For coculture experiments, overnight cultures were subcultured to mid-log phase (optical density at 600 nm [OD600] = 0.4). Log-phase cultures (100 μl each) were inoculated into 3 ml of fresh THB supplemented with 1% (wt/vol) glucose and 4 mM calcium chloride either in single culture or in coculture with another strain, as indicated in the figures. At 0 h and 4 h postinoculation, cultures were serially diluted and plated on THB agar plates with added Strep (1000 μg/ml) for GBS selection or Spec (100 μg/ml) for S. salivarius selection.
In vitro cell assays.
Assays of GBS adherence and invasion of hVEC were performed as described previously (13). In short, cells were grown to confluence in 24-well tissue culture plates and washed prior to infection. Bacteria were grown to mid-log phase and added at a multiplicity of infection (MOI) of 1. For adherence assays, after 30 min of infection, monolayers were washed 6 times with phosphate-buffered saline (PBS). Cells were removed from plates with trypsin-EDTA and then lysed with 0.025% Triton X-100. Lysate was serially diluted and plated on THB agar plates to quantify the number of adherent CFU. The total number of adherent CFU was calculated as (total CFU recovered/total CFU of original inoculum) × 100%. To quantify invading bacteria, cells were incubated with bacteria for 2 h, and monolayers were washed 3 times with PBS. Cells were given fresh medium containing antibiotics and incubated for an additional 2 h. Monolayers were washed 3 times with PBS and lysed as described above, and viable intracellular bacteria were determined by serial dilution plating as quantified by adherence assays.
ELISA.
For human enzyme-linked immunosorbent assays (ELISAs), hVEC were infected as described above with several modifications. Bacteria were added at an MOI of 10, and cells were incubated with bacteria for 4 h. Cell supernatants were analyzed for chemokine secretion using a human interleukin 8 (IL-8) ELISA kit (R&D Systems) according to the manufacturer's instructions. For mouse ELISAs, vaginal lavage fluid was collected as described below and analyzed using a mouse KC (CXCL1) ELISA kit (R&D Systems) according to the manufacturer's instructions.
Microscopy.
For light microscopy, hVEC monolayers were propagated on glass coverslips within 24-well plates. After an adherence assay was performed (MOI = 100), coverslips were washed 6 times with PBS, air-dried, heat fixed, and then subjected to a standard Gram stain protocol as described previously (13). All images were taken with a Zeiss upright microscope with an attached Axiocam Icc3 camera at the magnification indicated.
Mouse model of GBS vaginal colonization.
All animal work was approved by the Office of Lab Animal Care at San Diego State University and conducted using accepted veterinary standards. Eight- to 12-week-old female CD1 mice (Charles River Laboratories, Wilmington, MA) were injected intraperitoneally with 0.5 mg 17β-estradiol (Sigma-Aldrich, St. Louis, MO) in 100 μl sesame oil on day −1 (13, 14). For single-challenge experiments, on day 0, mice were vaginally inoculated with 1 × 107 CFU of strain A909 or K12. For probiotic treatment experiments, on day 0, mice were vaginally inoculated with 1 × 107 CFU of A909 or COH1 in 10 μl of PBS (or with PBS as a control for some experiments). On day 1, and all subsequent days, mice were vaginally inoculated with 1 × 108 CFU of K12 or K12ΔpK12 in 10 μl of PBS. Control mice were given 10 μl of PBS. For all mouse experiments, beginning on day 1, prior to inoculation with S. salivarius or PBS, the vaginal lumen was swabbed with a sterile ultrafine swab, and recovered GBS or S. salivarius CFU were enumerated by plating them on THB agar plates with appropriate antibiotics. To collect vaginal lavage fluid for ELISA analysis, on day 2, after swabbing the vaginal lumen but prior to K12 treatment, 20 μl of PBS was pipetted into the vaginal lumen and gently pipetted up and down 4 times. Lavage was conducted twice per mouse, and the resulting 40-μl samples were stored at −20°C.
Statistical analyses.
GraphPad Prism version 5.04 was utilized for statistical analyses. Differences in numbers of recovered bacteria for coculture assays were evaluated using two-way repeated-measures analysis of variance (ANOVA) with Bonferroni's multiple-comparisons posttest. One-way ANOVA with Bonferroni's multiple-comparisons posttest was used for in vitro adherence and invasion assays and for ELISAs. In vivo results for recovered bacteria were analyzed using the Kruskal-Wallis test with Dunn's multiple-comparisons posttest for individual days and two-way repeated-measures ANOVA with Bonferroni's multiple-comparisons posttest for analyses over time. Statistical significance was indicated by a P of <0.05.
RESULTS
Streptococcus salivarius inhibits GBS growth in vitro.
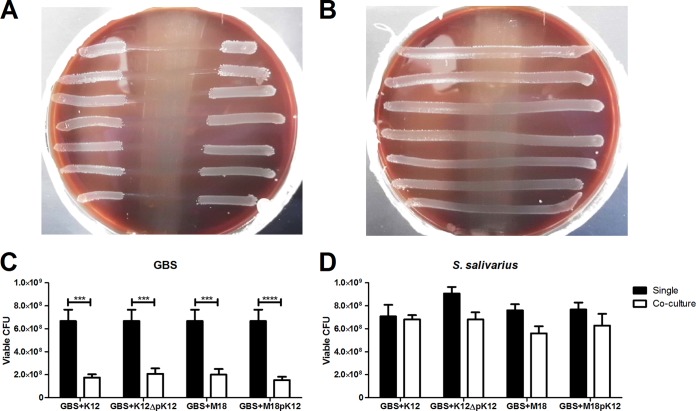
To test the inhibitory capacity of S. salivarius toward GBS, we utilized an established deferred-antagonism test (8). In all, we tested 9 wild-type S. salivarius strains against 13 GBS human clinical isolates (Table 1). We observed a broad range of inhibition, from complete inhibition (all 13 GBS strains inhibited) by strains K12 and Tove R to no inhibition (none of the GBS strains inhibited) by strains MPS, Trev P, and CCHSS3 (Table 1). One GBS vaginal isolate, DMC-VI-29, appeared more susceptible to S. salivarius and was also inhibited by M18, NR, 20P3, and #5 (Table 1). Additional inhibitory activity was observed with strain M18 against GBS NEM316 and with strain NR against GBS A909, CJB111, and NCTC 10/84 (Table 1). Additionally, we observed a dependency of inhibition on the presence of the K12 megaplasmid pSsal-K12, as there was no inhibition of GBS by the K12 megaplasmid-deficient strain K12ΔpK12 (Fig. 1A and B). Furthermore, megaplasmid-dependent inhibitory activity could be transferred to another strain background, with M18pK12 demonstrating complete inhibition (all 13 GBS strains) compared to partial inhibition (2 of 13 GBS strains) observed in the parental M18 strain lacking the pK12 plasmid (Table 1).
FIG 1.
S. salivarius inhibits the growth of GBS in vitro. Images are shown of deferred-antagonism tests with S. salivarius K12 (A) or K12ΔpK12 (B) to examine inhibition of seven GBS invasive clinical isolates, i.e., COH1, A909, NEM316, NCTC 10/84, 515, 2603 V/R, and CJB111 (top to bottom), as described in Materials and Methods. Tests were performed at least twice, and representative results are shown. The numbers of viable GBS A909 CFU (C) or S. salivarius CFU (D) are shown after 4 h postinoculation either in single culture or in coculture. Experiments were carried out at least twice independently with three replicates, and the data for one representative experiment are shown. Data were analyzed using two-way repeated-measures ANOVA (C and D) with Bonferroni's multiple-comparisons posttest. ***, P < 0.001; ****, P < 0.0001.
Because the deferred-antagonism test examines efficacy of secreted bacterial products without the stimulation by the indicator species, we also examined inhibitory activity using live cultures. Briefly, diluted log-phase GBS strain A909 and selected S. salivarius strains were grown in either single culture or coculture for 4 h, and the viable number of CFU was determined with antibiotic selection on THB agar plates. After 4 h of coculture, there was significant reduction in GBS viability when cocultured with either K12, K12ΔpK12, M18, or M18pK12, compared to the viability of single-culture growth of GBS (Fig. 1C). This reduction in viability was not seen in any of the tested S. salivarius strains after 4 h of culture with GBS (Fig. 1D).
Streptococcus salivarius interacts with human vaginal epithelial cells.
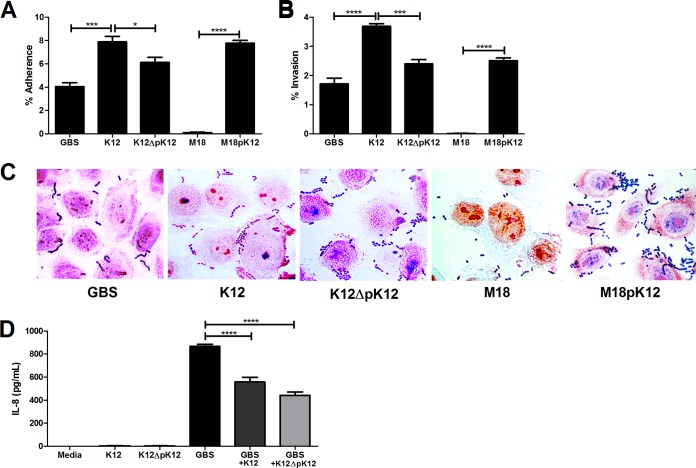
Since S. salivarius is predominantly an oral bacterium (6), we tested the ability of strains K12 and M18 to interact with the human vaginal epithelium in vitro. To assess adherence, bacterial strains were incubated with immortalized human vaginal epithelial cells for 30 min and nonadherent bacteria were removed by washing prior to quantification. We observed that K12 adhered to vaginal cells at significantly higher levels than GBS, whereas M18 demonstrated very little capacity to bind to vaginal cells (Fig. 2A). This phenomenon was not due to an inability of M18 to grow in THB or tissue culture cell assay medium (data not shown). Furthermore, the ability of S. salivarius to adhere to vaginal cells was partially conferred by the pSsal-K12 plasmid, as the K12ΔpK12 strain showed significantly reduced adherence, while M18pK12 achieved adherence levels comparable to those of wild-type K12 (Fig. 2A). We further visualized bacterial adherence to vaginal cells using Gram staining and light microscopy (Fig. 2C). Similarly, we characterized the ability of S. salivarius strains to enter or “invade” vaginal epithelium. As described in Materials and Methods, we recovered and quantified viable intracellular bacteria from vaginal cell lysates after 2 h of infection and a 2-h antibiotic treatment to eliminate extracellular bacteria. The relative invasive capacities of K12 and M18 mimicked their adherence phenotypes, with K12 invading at levels higher than GBS and M18 showing minimal levels of invasion (Fig. 2B). Invasive capacity may also be partially dependent on genes borne on the pSsal-K12 plasmid since its expression in M18pK12 conferred significantly increased invasiveness compared to that of the M18 parental strain (Fig. 2B).
FIG 2.
S. salivarius interacts with human vaginal epithelium in vitro. Adherence (A) and invasion (B) of GBS A909, S. salivarius K12, K12ΔpK12, M18, or M18pK12 to human vaginal epithelial cells (hVEC) were examined at an MOI of 1. (C) Gram stain of hVEC infected for 30 min with GBS A909, S. salivarius K12, K12ΔpK12, M18, or M18pK12 at an MOI of 100. Magnification, ×1,000. (D) Protein expression of IL-8 in hVEC supernatants 4 h postinfection with GBS A909, S. salivarius K12, K12ΔpK12, M18, or M18pK12 at an MOI of 10. Experiments were carried out at least twice independently with three replicates, and data for one representative experiment are shown. Data were analyzed using one-way repeated-measures ANOVA (A, B, and D) with Bonferroni's multiple-comparisons posttest. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
Streptococcus salivarius K12 dampens the host innate immune response in vitro.
Because S. salivarius, particularly K12, exhibited a robust ability to attach to the vaginal epithelium, we next examined whether S. salivarius elicits a host immune response in vitro. To assess immune induction, we quantified the secretion of the neutrophil chemokine IL-8 from human vaginal epithelial cells following incubation with GBS, K12, or both strains together. As seen previously, GBS was a potent inducer of IL-8 secretion from vaginal cells (14), yet K12, either with or without the pSsal-K12 plasmid, did not induce IL-8 production over levels in medium controls (Fig. 2D). Interestingly, when vaginal cells were incubated with both GBS and K12, IL-8 levels were significantly reduced compared to the levels in cells infected with GBS alone, and this reduction was not dependent on expression of pSsal-K12 (Fig. 2D). We also assessed cell viability via trypan blue uptake and did not observe any reduction in viability between infected cells and medium controls (data not shown).
Streptococcus salivarius strain K12 reduces the GBS load and immune response in the vaginal tract.
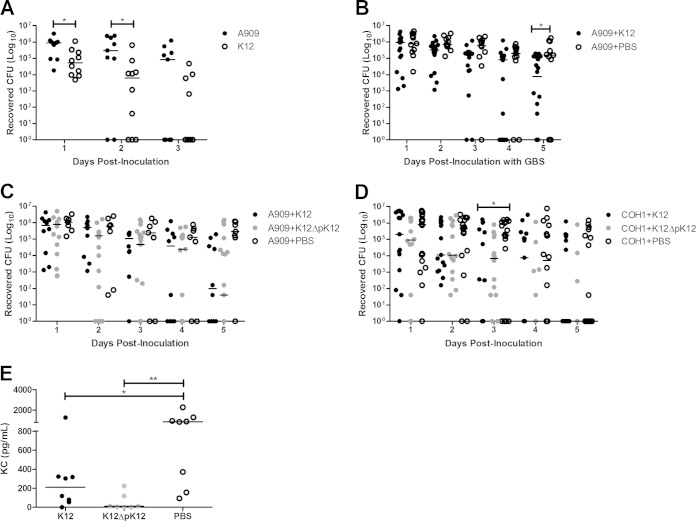
To substantiate our in vitro findings that K12 is capable of interacting with the human vaginal epithelium, we assessed the ability of K12 to colonize the vaginal tract in vivo. Using our previously established model of murine GBS vaginal colonization (13, 14), we monitored the bacterial levels of mice colonized with a single inoculation of either GBS A909 or K12 over time. We detected vaginal colonization with K12 in 100% of mice 1 day postinoculation, and on subsequent days, mice began clearing the bacteria, with approximately half of the animals clearing K12 by 3 days postinoculation (Fig. 3A). In mice colonized with A909, we observed rates of bacterial load and persistence similar to those seen previously (14). Additionally, the amount of K12 recovered from the vaginal vault was 10-fold lower than what was recovered for GBS (Fig. 3A).
FIG 3.
S. salivarius colonizes the vaginal tract and limits GBS colonization in vivo. (A) CD1 mice (n = 9 or 10/group) were colonized with 1 × 107 CFU of GBS A909 or S. salivarius K12 and the vaginal lumen was swabbed daily to determine bacterial loads. (B to D) CD1 mice (n = 7 to 20/group) were colonized with 1 × 107 CFU of GBS and on subsequent days were dosed daily with 1 × 108 CFU S. salivarius K12, K12ΔpK12 (C and D only), or PBS, as described in Materials and Methods. (E) Vaginal lavage fluid samples collected from CD1 mice (n = 7 or 8/group) 2 days postcolonization with 1 × 107 CFU of GBS A909 and 1 day posttreatment with 1 × 108 CFU S. salivarius K12, K12ΔpK12, or PBS were subjected to KC ELISA. Lines represent median values for each group. Data were analyzed using the Mann-Whitney test (A and E) or the Kruskal-Wallis test with Dunn's multiple-comparisons posttest for individual days (B to D). *, P < 0.05; **, P < 0.01.
Based on the inhibitory effect of K12 on GBS growth in vitro, we next examined whether the presence of K12 in the vaginal tract could alter the persistence and/or bacterial levels of GBS. To evaluate this possibility, mice were first inoculated with GBS A909 and allowed 24 h to establish colonization. On subsequent days, mice were first swabbed to assess bacterial loads and then inoculated with K12 or given PBS as a control. By day 5 postinoculation with GBS, mice treated with K12 exhibited significantly lower A909 levels than the control group (P = 0.0146) (Fig. 3B). Since CD1 mice begin clearing A909 about 1 week postinoculation, we did not observe significant differences between K12-treated and control groups at later days (data not shown). Furthermore, we examined the impact of the pSsal-K12 plasmid on reduction of GBS vaginal load and observed that although A909 levels were lower 5 days postinoculation in mice treated with K12ΔpK12, the results of this treatment did not significantly differ from those of the control group (Fig. 3C). Additionally, we examined the effect of K12 treatment on another GBS clinical isolate, COH1 (serotype III), and observed significant reduction of COH1 by day 3 postinoculation with wild-type K12, but not of the K12ΔpK12 strain, compared to the control group (Fig. 3D).
To determine if K12 was able to reduce innate immune responses in vivo as we observed in hVEC in vitro (Fig. 2), we quantified vaginal levels of KC, a mouse IL-8 homologue. We subjected mouse vaginal lavage fluid 2 days postinoculation with A909, corresponding to 1 day posttreatment with K12, to KC ELISA. Mice treated with K12 or K12ΔpK12 had significantly lower levels of KC than the PBS control group (Fig. 3E).
DISCUSSION
GBS prevails as a leading cause of global neonatal disease, yet our ability to control GBS vaginal colonization, the primary route of neonatal transmission, remains limited. The host and mucosal microbiota constituents that govern GBS presence in the vaginal tract are not fully known. Several clinical studies have observed an inverse correlation between levels of Lactobacillus species and GBS in the human vaginal tract (15, 16). Even so, preliminary human studies with Lactobacillus probiotic treatments (16, 17), as well as murine models (5), have failed to show significant reduction of GBS vaginal colonization. While this paper was being reviewed, a recent study was published that showed reduction of GBS vaginal load in mice, but only if Lactobacillus was given prior to GBS colonization (18).
In this current study, we propose a novel use of the probiotic S. salivarius for controlling GBS colonization of the vaginal tract. We observed a range of inhibition of GBS growth by S. salivarius strains, with certain GBS strains appearing more susceptible to inhibition. This phenomenon is likely explained by differences in the repertoires of bacteriocin genes or gene expression across strains, and this is of interest for future studies. In deferred-antagonism tests, GBS growth inhibition by strain K12 was completely dependent on the presence of the pSsal-K12 plasmid and the capability for expression of inhibitory activity could be transferred to another cured plasmid-negative strain, M18, via the plasmid. However, in coculture experiments, we did not observe this same dependency, as K12 and M18 strains equally inhibited GBS growth with or without pSsal-K12. Additionally, the K12ΔpK12 strain was also capable of reducing GBS bacterial levels within the murine vaginal tract, although not to the same degree as K12. Together, these data imply that there are several S. salivarius chromosomal and plasmid-based factors which can affect GBS growth, depending on interactions with secreted products or direct contact between live bacteria. Furthermore, we observed reduced IL-8 secretion in vaginal cells exposed to both GBS and K12. Possible explanations include downregulation of the host immune response by K12 via the NF-κB pathway, as seen previously (19), or a reduction in the total number of GBS CFU over the incubation period through inhibition of growth by K12. Moreover, we also observed reduced KC production in GBS-colonized mice after only one treatment with K12. The exact mechanisms of GBS growth inhibition or host immune modulation by S. salivarius are unknown, and these dynamics are the focus of future work.
Although primarily considered a member of the human oral microbiota, S. salivarius has been isolated from the vaginal tract in approximately 2% of pregnant women (20). Our in vitro data suggest that the ability of S. salivarius to adhere to the vaginal epithelium varies greatly among different strains and that adherence may be conferred by genes borne on megaplasmids such as pSsal-K12. Correspondingly, we were able to demonstrate persistence of K12 within the murine vagina for several days with one inoculation (Fig. 3); however, repeated subsequent doses improved colonization rates (data not shown). Previous work has also demonstrated that S. salivarius is capable of surviving in combination with Lactobacillus at pH values comparable to those in the vagina (21). Importantly, S. salivarius K12 has also been found safe and well tolerated as an oral probiotic in a human, randomized, placebo-controlled clinical trial (7). Taken together, these data support that S. salivarius K12 is capable of persisting within the human vaginal tract and may serve as a beneficial microbe in this environment.
In summary, we have demonstrated that the probiotic S. salivarius K12 can effectively inhibit the growth of GBS in vitro, as well as significantly reduce vaginal GBS levels in a murine colonization model. Because the majority of CD1 mice clear the GBS strains A909 and COH1 in a matter of 1 to 2 weeks (22), we were able to make conclusions on the effectiveness of K12 treatment only in the first few days postinoculation. Further optimization of this murine model that would prolong GBS colonization in control mice would allow for observations of extended K12 treatment, and this is the goal of future studies. Although K12 treatment did not completely remove GBS from the vaginal tract in mice, we did observe a 10-fold to 100-fold reduction after 2 to 4 days of treatment (Fig. 3). Since high vaginal GBS load has been associated with increased risk for early-onset GBS disease (1) and neonatal colonization (23), we suggest that K12 has therapeutic potential to limit GBS vaginal colonization and neonatal transmission when combined with additional preventative measures such as IAP or a maternal GBS vaccine. Future work must examine the efficacy of K12 treatment on GBS levels within the human vaginal tract to help establish the potential of this strategy for eradicating GBS as a pervasive element of neonatal pathogenesis.
ACKNOWLEDGMENTS
We thank the vivarium manager and staff at San Diego State University for support with animal husbandry, Melody Neely, Wayne State University, for vaginal clinical isolate strains, and Laura Cook and Michael Federle, University of Illinois at Chicago, for providing the GBS A909 wild-type streptomycin-resistant strain.
J.R.T., J.D.H., and P.A.W. are employees of, or have financial interest in, BLIS Technologies Ltd., which markets S. salivarius K12 as a probiotic for oral health. The other authors declare no conflict of interest.
K.A.P. is supported by an ARCS scholarship and a fellowship from the Inamori Foundation, and K.S.D. is supported by an R01 grant, NS051247, from the National Institutes of Health.
REFERENCES
- 1.Verani JR, McGee L, Schrag SJ. 2010. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recommend Rep 59(RR-10):1–36. [PubMed] [Google Scholar]
- 2.Brzychczy-Wloch M, Pabian W, Majewska E, Zuk MG, Kielbik J, Gosiewski T, Bulanda MG. 2014. Dynamics of colonization with group B streptococci in relation to normal flora in women during subsequent trimesters of pregnancy. New Microbiol 37:307–319. [PubMed] [Google Scholar]
- 3.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. 2008. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics 121:689–696. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 4.Aloisio I, Mazzola G, Corvaglia LT, Tonti G, Faldella G, Biavati B, Di Gioia D. 2014. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol 98:6051–6060. [DOI] [PubMed] [Google Scholar]
- 5.De Gregorio PR, Juarez Tomas MS, Leccese Terraf MC, Nader-Macias ME. 2014. In vitro and in vivo effects of beneficial vaginal lactobacilli on pathogens responsible for urogenital tract infections. J Med Microbiol 63:685–696. doi: 10.1099/jmm.0.069401-0. [DOI] [PubMed] [Google Scholar]
- 6.Barretto C, Alvarez-Martin P, Foata F, Renault P, Berger B. 2012. Genome sequence of the lantibiotic bacteriocin producer Streptococcus salivarius strain K12. J Bacteriol 194:5959–5960. doi: 10.1128/JB.01268-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton JP, Cowley S, Simon RR, McKinney J, Wescombe PA, Tagg JR. 2011. Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: a randomized, placebo-controlled, double-blind study. Food Chem Toxicol 49:2356–2364. doi: 10.1016/j.fct.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Wescombe PA, Burton JP, Cadieux PA, Klesse NA, Hyink O, Heng NC, Chilcott CN, Reid G, Tagg JR. 2006. Megaplasmids encode differing combinations of lantibiotics in Streptococcus salivarius. Antonie Van Leeuwenhoek 90:269–280. doi: 10.1007/s10482-006-9081-y. [DOI] [PubMed] [Google Scholar]
- 9.Heng NC, Haji-Ishak NS, Kalyan A, Wong AY, Lovric M, Bridson JM, Artamonova J, Stanton JA, Wescombe PA, Burton JP, Cullinan MP, Tagg JR. 2011. Genome sequence of the bacteriocin-producing oral probiotic Streptococcus salivarius strain M18. J Bacteriol 193:6402–6403. doi: 10.1128/JB.06001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang NY, Patras KA, Seo HS, Cavaco CK, Rosler B, Neely MN, Sullam PM, Doran KS. 2014. Group B streptococcal serine-rich repeat proteins promote interaction with fibrinogen and vaginal colonization. J Infect Dis 210:982–991. doi: 10.1093/infdis/jiu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fichorova RN, Rheinwald JG, Anderson DJ. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod 57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 12.Hyink O, Wescombe PA, Upton M, Ragland N, Burton JP, Tagg JR. 2007. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol 73:1107–1113. doi: 10.1128/AEM.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheen TR, Jimenez A, Wang NY, Banerjee A, van Sorge NM, Doran KS. 2011. Serine-rich repeat proteins and pili promote Streptococcus agalactiae colonization of the vaginal tract. J Bacteriol 193:6834–6842. doi: 10.1128/JB.00094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patras KA, Wang NY, Fletcher EM, Cavaco CK, Jimenez A, Garg M, Fierer J, Sheen TR, Rajagopal L, Doran KS. 2013. Group B streptococcus CovR regulation modulates host immune signalling pathways to promote vaginal colonization. Cell Microbiol 15:1154–1167. doi: 10.1111/cmi.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota T, Nojima M, Itoh S. 2002. Vaginal bacterial flora of pregnant women colonized with group B streptococcus. J Infect Chemother 8:326–330. doi: 10.1007/s10156-002-0190-X. [DOI] [PubMed] [Google Scholar]
- 16.Ronnqvist PD, Forsgren-Brusk UB, Grahn-Hakansson EE. 2006. Lactobacilli in the female genital tract in relation to other genital microbes and vaginal pH. Acta Obstet Gynecol Scand 85:726–735. doi: 10.1080/00016340600578357. [DOI] [PubMed] [Google Scholar]
- 17.Hanson L, Vandevusse L, Duster M, Warrack S, Safdar N. 2014. Feasibility of oral prenatal probiotics against maternal group B Streptococcus vaginal and rectal colonization. J Obstet Gynecol Neonatal Nurs 43:294–304. doi: 10.1111/1552-6909.12308. [DOI] [PubMed] [Google Scholar]
- 18.De Gregorio PR, Juarez Tomas MS, Leccese Terraf MC, Nader-Macias ME. 2015. Preventive effect of Lactobacillus reuteri CRL1324 on group B Streptococcus vaginal colonization in an experimental mouse model. J Appl Microbiol 118:1034–1047. doi: 10.1111/jam.12739. [DOI] [PubMed] [Google Scholar]
- 19.Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, Yu LL, Pistolic J, Falsafi R, Tagg J, Hancock RE. 2008. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun 76:4163–4175. doi: 10.1128/IAI.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabe LK, Winterscheid KK, Hillier SL. 1988. Association of viridans group streptococci from pregnant women with bacterial vaginosis and upper genital tract infection. J Clin Microbiol 26:1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall VM, Cole WM, Phillips BA. 1985. Fermentation of milk by Streptococcus salivarius subspecies salivarius and Streptococcus salivarius subspecies thermophilus and their use to the yoghurt manufacturer. J Appl Microbiol 59:147–151. [Google Scholar]
- 22.Patras KA, Rosler B, Thoman ML, Doran KS. 8 April 2015. Characterization of host immunity during persistent vaginal colonization by group B Streptococcus. Mucosal Immunol doi: 10.1038/mi.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berardi A, Rossi C, Guidotti I, Vellani G, Lugli L, Bacchi Reggiani ML, Ferrari F, Facchinetti F, Ferrari F. 2014. Factors associated with intrapartum transmission of group B Streptococcus. Pediatr Infect Dis J 33:1211–1215. doi: 10.1097/INF.0000000000000439. [DOI] [PubMed] [Google Scholar]