Abstract
Helminth infections are typically chronic in nature; however, the exact molecular mechanisms by which these parasites promote or thwart host immunity remain unclear. Worm expulsion requires the differentiation of CD4+ T cells into Th2 cells, while regulatory T cells (Tregs) act to dampen the extent of the Th2 response. Priming of T cells requires drainage or capture of antigens within lymphoid tissues, and in the case of intestinal helminths, such sites include the mucosa-associated Peyer's patches (PPs) and the draining mesenteric lymph nodes (MLN). To gain insight into when and where the activation of the adaptive T cell response takes place following intestinal helminth infection, we analyzed Th2 and Treg responses in the PPs and MLN following infection with the murine intestinal helminth Heligmosomoides polygyrus bakeri. Protective Th2 responses were observed to be largely restricted to the MLN, while a greater expansion of Tregs occurred within the PPs. Interestingly, those PPs that formed a contact with the parasite showed the greatest degree of Treg expansion and no evidence of type 2 cytokine production, indicating that the parasite may secrete products that act in a local manner to selectively promote Treg expansion. This view was supported by the finding that H. polygyrus bakeri larvae could promote Treg proliferation in vitro. Taken together, these data indicate that different degrees of Treg expansion and type 2 cytokine production occur within the PPs and MLN following infection with the intestinal helminth H. polygyrus bakeri and indicate that these organs exhibit differential responses following infection with intestinal helminths.
INTRODUCTION
Intestinal helminths infect up to one in four individuals, disproportionately affecting impoverished populations lacking access to adequate water, sanitation, and opportunities for socioeconomic development (1, 2). Following infection, a type 2 immune response is initiated, which involves the rapid activation and engagement of cells belonging to both the innate and the adaptive immune systems (3). The adaptive response is characterized by the induction of CD4+ Th2 cells, which secrete cytokines such as interleukin-4 (IL-4), IL-5, IL-9, and IL-13. Th2 cells in turn promote B cell responses and IgE secretion (4). However, many helminths can additionally drive immunosuppression, allowing the establishment of a chronic infection (5–7). Often, such suppression is not confined solely to the parasite-specific response but also extends to bystander antigens. Indeed, epidemiological and experimental evidence indicates that helminth infection can result in the suppression of immune-mediated disorders, including allergy, autoimmunity, and inflammatory bowel disease (7). One of the mechanisms by which infection by helminths may lead to immunosuppression is their potential to promote regulatory T cell (Treg) expansion (7). However, the molecular mechanisms controlling the expansion and activation of helminth-induced Tregs are only just beginning to be elucidated.
Infection with the natural murine parasite Heligmosomoides polygyrus bakeri is a common experimental model used to study immune responses and chronicity following intestinal helminth infection (8, 9). H. polygyrus bakeri enters the gastrointestinal tract as third-stage infective larvae (L3) and then penetrates the epithelial cell barrier of the small intestine to develop within the submucosa to L4; during this period, the parasite elicits a strong type 2 inflammatory response (10, 11). When it is fully mature, the helminth exits the intestinal mucosa to populate the intestinal lumen, where it establishes a chronic infection as a sexually mature adult (12–14). Subsequent infections of immunocompetent mice result in the rapid trapping of the larvae and abbreviate the infection in a manner dependent on CD4+ T cells, IL-4, macrophages, and the generation of helminth-specific antibodies (14, 15). Although the mechanisms by which H. polygyrus bakeri establishes chronicity following primary infection remain unclear, it is well established that this parasite possesses potent immunomodulatory properties (16). Indeed, it was previously shown that H. polygyrus bakeri can ameliorate various inflammatory diseases, such as allergic asthma (17, 18) and inflammatory bowel disease (18, 19), and promote de novo FoxP3 expression by splenocytes in vitro (20).
The two main inductive sites where immune responses against pathogens dwelling in the upper small intestine can be initiated are the draining mesenteric lymph nodes (MLN) and mucosal Peyer's patches (PPs). PPs are composed of aggregated lymphoid follicles proximal to specialized epithelial cells, M cells, that transport luminal antigens and bacteria to underlying immune cells (21). The MLN lie within the connective tissue of the mesentery and collect antigens from lymphatics draining the entire small intestine and parts of the colon (22). Dendritic cells (DCs) sample enteric antigens in the intestinal lamina propria (LP) and transport them to the MLN, where they are presented to lymphocytes (23). As the life cycle of H. polygyrus bakeri involves stages where the parasite is present in both the small intestinal submucosa and the lumen, we predicted that the immune response was likely to occur in both the MLN and PPs with various kinetics. Surprisingly, however, we noted that effector Th2 cell cytokine production was most prominent in the MLN, while Treg accumulation was greater in PPs. Moreover, we observed that increased Treg expansion and the absence of type 2 cytokine production within PPs were most marked in those patches forming contacts with the invasive larvae. In vitro cocultures revealed the ability of larvae to directly drive the expansion of Tregs. These data indicate that distinct immune responses are initiated in the MLN or PPs depending on the proximity of the organ to invading parasitic larvae.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were approved by the Service de la Consummation et des Affaires Vétérinaires (Epalinges, Canton Vaud, Switzerland) with authorization number 2238. Animal experiments were performed according to institutional guidelines and Swiss federal and cantonal laws on animal protection.
Mice, parasites, and treatments.
C57BL/6 mice were purchased from Charles River and maintained at the École Polytechnique Fédérale de Lausanne (EPFL) animal facility under specific-pathogen-free (SPF) conditions. RAG-1−/− mice (24) were bred and maintained under SPF conditions at the EPFL animal facility. Where indicated, mice were then infected orally with 200 H. polygyrus bakeri L3. For in vivo depletion of CD25+ cells, C57BL/6 mice were injected with 1 mg of anti-CD25 antibody (clone PC61; BioXcell, West Lebanon, NH, USA) via the intraperitoneal route on the day of infection. Control mice were injected with a rat IgG1 isotype control (clone HRPN; BioXcell). PPs were determined to be H. polygyrus bakeri positive when the visible area of the lymphoid tissue formed direct contacts with at least one parasitic larva. Patches that did not form physical contacts with a larva were classified as H. polygyrus bakeri negative.
Mononuclear cell isolation from PPs and MLN.
PPs and MLN were excised and incubated with phosphate-buffered saline (PBS) containing EDTA at 37°C for 15 min. Organs were then smashed and filtered through a 40-μm gauze (BD Biosciences, Franklin Lakes, NJ, USA). Single-cell suspensions were then stained for flow cytometry. Where indicated, single-cell suspensions were enriched for CD4+ T cells by magnetic cell sorting using the CD4+ T cell isolation kit (Miltenyi Biotec, Gladbach, Germany) according to the manufacturer's instructions. The purity of CD4+ T cells was regularly ≥85%.
Flow cytometry.
Antibodies and the corresponding clones (in parentheses) used for flow cytometry, including CD4-Pacific blue (GK1.5), CD103-biotin (M290 or 2E7), CD25-phycoerythrin (PE)-Cy7 (PC61), programmed cell death protein 1 (PD1)-PE (RMP1-30), glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR)–fluorescein isothiocyanate (FITC) (DTA-1), and streptavidin-allophycocyanin (APC), were purchased from BD Biosciences or Biolegend (San Diego, CA, USA). FoxP3-Alexa Fluor 700 (FJK-16a; eBioscience, San Diego, CA) and Ki-67 PE (B56; BD Biosciences) were used in combination with a FoxP3 staining kit (eBioscience). Data were acquired on an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star Inc., Ashland, OR, USA).
Real-time quantitative PCR (RT-qPCR).
Total RNA was isolated from all intestinal specimens using Tri reagent (Molecular Research Center Inc., Cincinnati, OH, USA) and Direct-zol kits (Zymo, Irvine, CA, USA) and reverse transcribed by using RevertAid reverse transcriptase (Fermentas, Thermo Fisher Scientific Inc., Wohlen, Switzerland). Transcribed cDNA was used as a template for PCR. The relative gene expression level was calculated by using GenEx software (MultiD Analyses AB, Göteborg, Sweden) and normalized to the expression level of the housekeeping gene. Data were expressed as means ± standard errors of the means (SEM). Where indicated, data were normalized to values for naive controls. Sequences of the primers used were as follows: 5′-CTTTTCACGGTTGGCCTTAG-3′ and 5′-CCCTGAAGTACCCCATTGAAC-3′ for β-actin, 5′-GGGTGTGAACCACGAGAAAT-3′ and 5′-CCTTCCACAATGCCAAAGTT-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-TGTCATCCTGCTCTTCTTTCTC-3′ and 5′-GCACCTTGGAAGCCCTAC-3′ for IL-4, 5′-TGACAAGCAATGAGACGATGA-3′ and 5′-CCCACGGACAGTTTGATTCTTC-3′ for IL-5, and 5′-TCCAGCCTCCCCGATACC-3′ and 5′-AGCAAAGTCTGATGTGAGAAAGG-3′ for IL-13.
Antibiotic treatment.
Where indicated, C57BL/6 mice were treated with 2.5 mg/ml enrofloxacin in drinking water for 2 weeks, followed by 0.8 mg/ml of amoxicillin and 0.114 mg/ml clavulanic acid in drinking water for a minimum of two further weeks prior to infection. Mice were then infected orally with H. polygyrus bakeri L3 previously washed with enrofloxacin (5 mg/ml), amoxicillin (2 mg/ml), and clavulanic acid (0.2 mg/ml) in PBS for a period of 4 to 6 h. Treatment with amoxicillin and clavulanic acid was continued throughout the experiment. For all experiments, intestinal bacteria were either ablated by antibiotic treatment or dramatically reduced (by a factor of 106), as determined by bacterial plating of cecal contents under aerobic and anaerobic conditions and by microscopic examination of cecal smears stained with Gram's stain and Sytox green nucleic acid stain (Life Technologies). The absence of fungi was also confirmed by staining of cecal smears with calcofluor white (Fluka, Sigma-Aldrich).
In vitro culture of CD4+ splenocytes.
CD4+ T cells were isolated from spleens of naive C57BL/6 mice, and H. polygyrus bakeri L4 were isolated from RAG−/− mice at 4 days postinfection (dpi) using a modified Baermann apparatus and washed with RPMI–10% fetal calf serum (FCS) containing 0.2 mg/ml gentamicin, penicillin, and streptomycin. A total of 8 × 105 cells were then plated in a 24-well plate in the presence or absence of approximately 50 L4. Where indicated, the medium was supplemented with soluble anti-CD3 antibody at a final concentration of 1 mg/ml. After 48 h, cells were stained to assess Treg numbers and proliferation.
Statistics.
Statistical analysis was performed using Student's t test or one-way or two-way analysis of variance (ANOVA) with posttest analysis, as appropriate. P values of <0.05 were considered significant. Graph generation and statistical analyses were performed using Prism version 6.0d software (GraphPad, La Jolla, CA).
RESULTS
Th2 cell responses are largely restricted to the mesenteric lymph nodes following intestinal helminth infection.
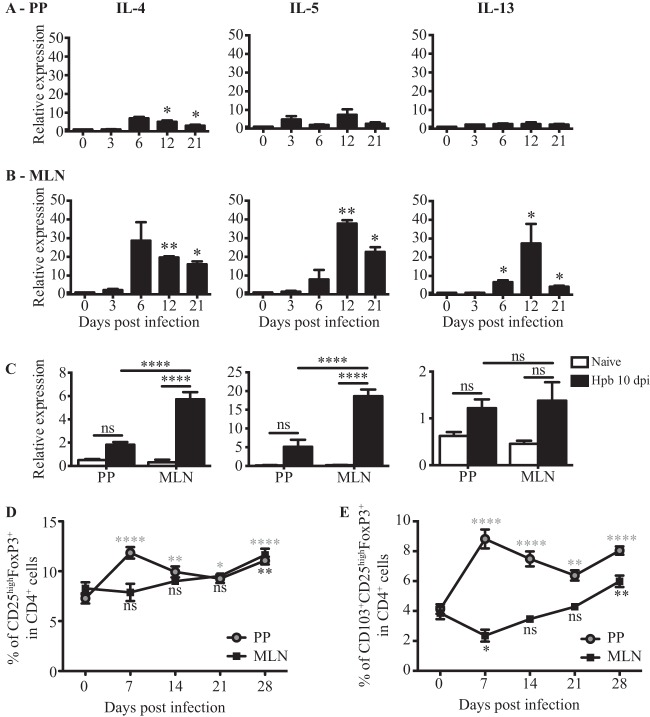
PPs and MLN are secondary lymphoid organs where adaptive immune responses against intestinal antigens are initiated. We therefore analyzed the generation of effector CD4+ T cells in PPs and MLN following H. polygyrus bakeri infection. The immune response to H. polygyrus bakeri is dominated by the production of type 2 cytokines, including IL-4, IL-5, and IL-13 (25). We isolated cells from PPs and MLN at 0, 3, 6, 12, and 21 dpi and measured the expression of IL-4, IL-5, and IL-13 mRNAs by RT-qPCR, since this technique allows us to analyze cytokine expression directly ex vivo. As shown in Fig. 1A, very small, or no, increases in cytokine mRNA expression levels were observed in PPs (P = 0.021 for IL-4, P = 0.1403 for IL-5, and P = 0.2849 for IL-13, as determined by one-way ANOVA), while expression levels of all three cytokines increased significantly in the MLN, peaking at day 12 (P = 0.0276 for IL-4, P = 0.0007 for IL-5, and P = 0.0044 for IL-13, as determined by one-way ANOVA) (Fig. 1B). These data indicate that PPs and MLN differentially support type 2 cytokine production in vivo. We reasoned that one explanation for these data may be the increased proportion of CD4+ T cells present in the MLN compared to that in PPs (20 to 40% of total cells in the MLN versus 10% in PPs [see Fig. S1A in the supplemental material]). We therefore repeated the experiment using purified CD4+ T cells. These data again indicated that type 2 cytokine mRNA expression was barely detectable in PPs of infected mice while being significantly increased in the MLN (P = 0.0008 for IL-4, P = 0.0001 for IL-5, and P < 0.0001 for IL-13, as determined by two-way ANOVA) (Fig. 1C).
FIG 1.
PPs exhibit an early Treg response and a decreased effector T cell response compared to those in MLN following H. polygyrus bakeri infection. (A and B) RT-qPCR analysis of IL-4, IL-5, and IL-13 mRNA expression levels in freshly isolated tissues from PPs (A) or MLN (B) of naive or H. polygyrus bakeri-infected mice at 3, 6, 12, and 21 dpi. Data are normalized to values for β-actin, GAPDH, and naive controls and are from one of two independent experiments (n = 3 per group). Data are expressed as means ± SEM (*, P < 0.05; **, P < 0.01 [as determined by one-way ANOVA with Dunnett's multiple-comparison test]). (C) RT-qPCR analysis of IL-4, IL-5, and IL-13 mRNA expression levels in the CD4+ fraction isolated from PPs or MLN of naive or H. polygyrus bakeri (Hpb)-infected mice (10 dpi). Results are normalized to β-actin and GAPDH values and were pooled from two experiments showing similar responses (cells from 8 to 9 mice per group were pooled for each data point). Data are expressed as means ± SEM (***, P < 0.001; ****, P < 0.0001; ns, not significant [as determined by two-way ANOVA with Tukey correction]). (E and F) Cells were isolated from PPs or MLN of naive or H. polygyrus bakeri-infected mice at 7, 14, 21, and 28 dpi and analyzed by flow cytometry. Data for CD25high FoxP3+ (E) and CD103+ CD25high FoxP3+ (F) cells are shown as a percentage of CD4+ cells. Data from one of two independent experiments are shown (n = 5 per group) and expressed as means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 [as determined by one-way ANOVA with Dunnett's multiple-comparison test]).
Activated CD25high FoxP3+ cells accumulate in PPs early following intestinal helminth infection.
It was previously reported that H. polygyrus bakeri infection promotes the expansion of CD103+ (also known as integrin αE) Tregs in the MLN at late time points following infection (20, 26–28). We therefore additionally investigated the presence of Treg responses in PPs and MLN of H. polygyrus bakeri-infected mice by analyzing the percentages and activation status of FoxP3-positive cells. As shown in Fig. S1B in the supplemental material, the percentage of total FoxP3+ cells increased as early as 7 dpi in PPs (P < 0.0001 by one-way ANOVA), whereas no changes were observed in the MLN (P = 0.0130 by one-way ANOVA). At later time points, the percentages of FoxP3+ cells in the two organs were comparable. Both CD25low (P < 0.0001, as determined by one-way ANOVA) (see Fig. S2A in the supplemental material) and CD25high (P < 0.0001, as determined by one-way ANOVA) (Fig. 1D) FoxP3+ cells were observed to expand in PPs. However, the predominant population of Foxp3+ cells was CD25high.
We next analyzed the expression of CD103, a well-established marker for murine effector/memory-like Tregs which is expressed following their activation in vivo (29). We observed a marked increase in the proportion of CD103+ regulatory T cells within the CD25high FoxP3+, but not the CD25low Foxp3+, subset within PPs (Fig. 1E; see also Fig. S2B in the supplemental material). The increased proportion of CD103+ CD25high FoxP3+ cells in PPs peaked at day 7 following H. polygyrus bakeri infection (P < 0.0001, as determined by one-way ANOVA) and remained until the last time point analyzed, at day 28 (Fig. 1E). In keeping with our above-described data, the percentage of CD103+ CD25high FoxP3+ cells was higher in PPs than in the MLN at all analyzed time points (Fig. 1E), and an increase was observed only for the MLN at 28 dpi. We next analyzed the expression of other activation markers, such as the glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR) and programmed cell death protein 1 (PD1), within the CD25high Foxp3+ population. As shown in Fig. S3A and S3B in the supplemental material, CD25high FoxP3+ cells from PPs of infected mice exhibited increased expression of GITR already at 7 dpi (P < 0.0001, as determined by one-way ANOVA), whereas in the MLN, the expression of GITR was upregulated only at later time points (P < 0.0001, as determined by one-way ANOVA). The expression of PD1 was mostly unchanged in PPs at 7 and 14 dpi (P < 0.0001, as determined by one-way ANOVA) (see Fig. S3C and S3D in the supplemental material) and slightly downregulated in the MLN (P < 0.0001, as determined by one-way ANOVA) (see Fig. S3C and S3D in the supplemental material). We also analyzed the expression of Helios, an Ikaros transcription factor family member, which, although controversial, has been proposed to differentiate thymus-derived from peripherally induced FoxP3+ Tregs in the intestine (30). Of interest, Helios-negative Tregs have been shown to arise in the colon of germfree mice upon colonization (31–33). However, in the context of H. polygyrus bakeri infection, we could not detect any significant difference in the proportions of Helios-negative cells within the CD25high FoxP3+ populations residing in PPs and those residing in the MLN at early time points, whereas later in infection, we observed a decrease in the percentage of Helios-negative cells within the CD25high FoxP3+ populations in both PPs and MLN (P < 0.0001 and P = 0.0001 for PPs and MLN, respectively, as determined by one-way ANOVA) (see Fig. S2E and S2F in the supplemental material).
Taken together, these data indicate that CD25high FoxP3+ cells exhibiting an increased expression level of CD103 accumulate in PPs throughout the course of H. polygyrus bakeri infection, whereas they accumulate in the MLN only at late time points (28 dpi).
Expansion of CD25high FoxP3+ cells contributes to the lower-level Th2 response observed in PPs.
In order to determine the impact of the observed increase in the proportion of CD25high FoxP3+ cells within PPs on the effector Th2 response, we specifically depleted this population using an anti-CD25 monoclonal antibody (PC61). As shown in Fig. S4A and S4B in the supplemental material, PC61 treatment effectively depleted CD25high FoxP3+ Treg cells but had little impact on CD25low FoxP3+ Treg cells in both PPs and MLN. Depletion of CD25high FoxP3+ cells resulted in enhanced production of the type 2 cytokines in PPs at 7 dpi (P = 0.0006 and P = 0.0002 for IL-4 and IL-13, respectively, as determined by one-way ANOVA) (Fig. 2A), with very little difference being observed between the 2 groups by 14 dpi. In the MLN, a trend for increased cytokine production in PC61-treated mice was noted at 7 dpi, and significantly increased IL-5 production was observed at 14 dpi (P < 0.0001, as determined by one-way ANOVA) (Fig. 2B). Despite this trend for increased type 2 cytokine production in both PPs and MLN following PC61 treatment, the level of cytokine mRNA expression in PPs was still lower than that observed for the MLN. Of note, PC61 treatment also led to the depletion of CD25high FoxP3− cells in both the MLN and PPs. We do not know the true identity of these cells, but it is possible that they also contribute to the regulation of type 2 cytokine production.
FIG 2.
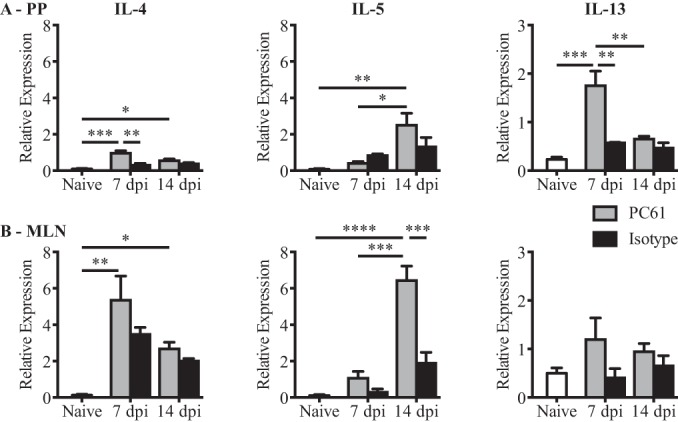
PC61 administration partially restores cytokine production in PPs at 7 dpi. Data from RT-qPCR analysis of IL-4, IL-5, and IL-13 mRNA expression levels in freshly isolated tissues from PPs (A) or MLN (B) of naive or H. polygyrus bakeri-infected mice at 7 and 14 dpi are shown. On the day of infection, mice were treated with PC61 or the isotype control antibody, as indicated. Data were normalized to values for β-actin and are from one of two independent experiments (n = 3 to 4 per group). Data are expressed as means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 [as determined by one-way ANOVA with Dunnett's multiple-comparison test]).
These data indicate that CD25high FoxP3+ cells act to suppress Th2 cytokine production in both PPs and MLN. Moreover, the greater proportion of CD25high FoxP3+ cells in PPs than in the MLN likely contributes to, but does not fully explain, the increased capacity of the MLN to support the development of effector Th2 responses.
Intestinal bacteria do not contribute to the accumulation of regulatory T cells observed in the PPs following helminth infection.
Select bacterial species residing in the intestinal lumen have been described to impact effector and regulatory T cell responses (31, 32, 34–37) and to drive homeostatic proliferation of CD4+ T cells in PPs and MLN (38). Intestinal bacteria are also known to shape the microenvironment of PPs to promote IgA production (39). We therefore sought to investigate whether the presence of intestinal bacteria contributed to the increased accumulation of Tregs within PPs following helminth infection. For this purpose, we treated specific-pathogen-free (SPF) mice with the broad-spectrum antibiotics enrofloxacin, amoxicillin, and clavulanic acid (see Materials and Methods) to reduce the number of intestinal bacteria present. As expected, analysis of control SPF mice at 7 dpi revealed an increased proportion of CD25high FoxP3+ cells in PPs (P = 0.0249, as determined by two-way ANOVA), whereas no increase was observed in the MLN (P = 0.9773, as determined by two-way ANOVA) (Fig. 3A). Antibiotic treatment did not affect the accumulation of CD25high FoxP3+ cells in PPs or the MLN (Fig. 3A); however, a small impact on the proportion of PP cells expressing the activation marker CD103 was noted (P = 0.0232, as determined by two-way ANOVA) (Fig. 3B). These data indicate that intestinal bacteria do not contribute to the increased accumulation of CD25high FoxP3+ cells observed for PPs following H. polygyrus bakeri infection but partially contribute to the activation status of these cells.
FIG 3.
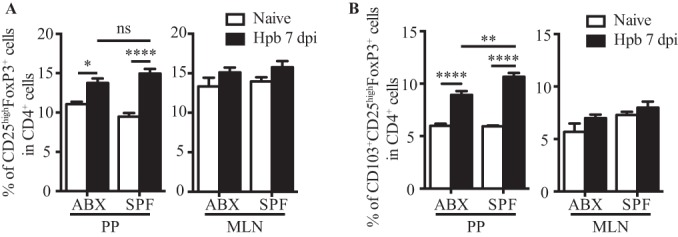
Intestinal bacteria do not contribute to the accumulation of CD25high FoxP3+ cells in PPs of H. polygyrus bakeri-infected mice. Mice belonging to the antibiotic-treated (ABX) group were treated with enrofloxacin and co-amoxicillin for 4 weeks, as described in Materials and Methods. Cells were then isolated from PPs and MLN of naive or H. polygyrus bakeri-infected mice at 7 dpi and analyzed by flow cytometry. Data for CD25high FoxP3+ cells (A) and CD103+ CD25high FoxP3+ cells (B) are shown as a percentage of CD4+ cells. Results are pooled from data from two independent representative experiments of three (n = 4 to 5 per group, per experiment) and expressed as means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 [as determined by one-way ANOVA with Tukey's multiple-comparison test]).
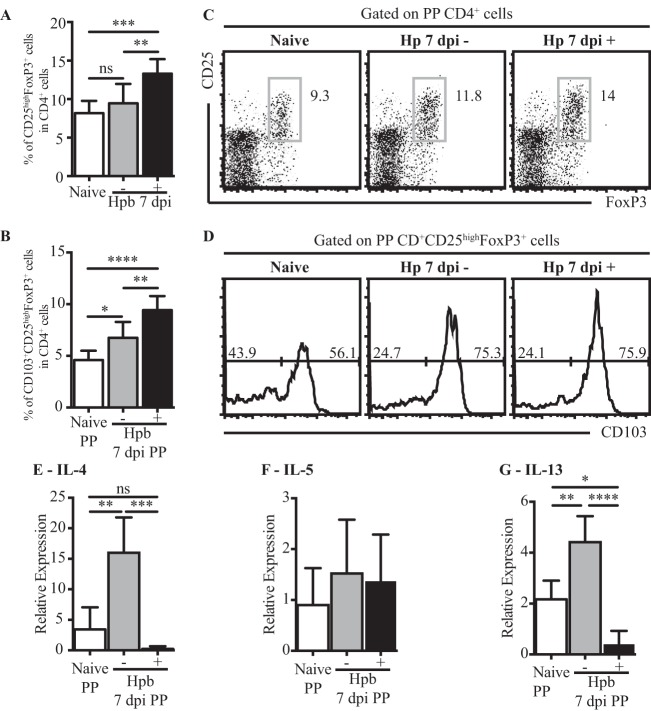
CD25high FoxP3+ cell expansion within Peyer's patches positively correlates with the presence of helminth larvae within, or in contact with, the lymphoid tissue.
It was previously reported that adult H. polygyrus bakeri excretory-secretory (HES) products can induce the conversion of naive T cells into FoxP3-expressing Tregs (20). This observation led us to speculate that the accumulation of Tregs within PPs may result from a close proximity of the lymphoid organ to the parasite and its secreted products. As we had observed that tissue-invasive larvae (L4) were often located in contact with, or even within, PPs, we tested our hypothesis by comparing Treg accumulation in PPs that formed a physical contact with larvae to that in PPs that did not (H. polygyrus bakeri-positive and H. polygyrus bakeri-negative PPs, respectively). As shown in Fig. 4A and B, the percentage of CD25high FoxP3+ cells was strongly increased in H. polygyrus bakeri-positive PPs, whereas no significant changes compared to the percentage in naive mice were noted for H. polygyrus bakeri-negative PPs (P = 0.0001, as determined by one-way ANOVA). We next investigated the changes in the activation status by examining CD103 expression. A strong increase in the proportion of CD103+ CD25high FoxP3+ Tregs was observed for H. polygyrus bakeri-positive PPs (P < 0.0001, as determined by one-way ANOVA), with a smaller increase being observed for H. polygyrus bakeri-negative PPs (Fig. 4C and D). To understand whether the increased proportion of Tregs found in H. polygyrus bakeri-positive PPs correlated with a difference in the Th2 response, we analyzed the expressions of IL-4, IL-5, and IL-13 in H. polygyrus bakeri-positive and H. polygyrus bakeri-negative PPs. Strikingly, we observed that H. polygyrus bakeri infection resulted in increased IL-4 and IL-13 production in H. polygyrus bakeri-negative PPs, while cytokine mRNA levels in H. polygyrus bakeri-positive PPs were decreased even below the levels observed for total PPs from naive mice (P = 0.0003 and P < 0.0001, respectively, as determined by one-way ANOVA) (Fig. 4E and F). The expression of IL-5 was unchanged across all groups (P = 0.55, as determined by one-way ANOVA) (Fig. 4G). These data indicate that contact of PPs with tissue-invasive larvae correlates with an increased accumulation of activated Tregs and decreased type 2 cytokine production. This supports our above-described findings indicating that Tregs can suppress type 2 cytokine production in PPs and indicates that Treg expansion may be driven by direct exposure to parasitic products.
FIG 4.
CD25high FoxP3+ cell accumulation and diminished Th2 responses in PPs correlate with contact with H. polygyrus bakeri larvae. (A to D) Cells were isolated from PPs of naive or H. polygyrus bakeri-infected mice at 7 dpi. In infected mice, PPs were divided according to the presence (+) or absence (−) of the parasitic larvae within the organ. Data from flow cytometric analysis of total CD25high FoxP3+ cells (A) and CD103+ CD25high FoxP3+ cells (C) are shown as a percentage of CD4+ cells. Representative fluorescence-activated cell sorter plots (B) and histograms of CD103 expression (D) are also shown; the same number of events is shown for each histogram. For panels A and C, results are pooled from data from two independent representative experiments of three (n = 4 to 5 per group, per experiment); data are expressed as means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 [as determined by one-way ANOVA with Tukey's multiple-comparison test]). (E to G) Data from RT-qPCR analysis of IL-4 (E), IL-5 (F), and IL-13 (G) mRNA expression levels from one experiment are shown and are representative of data from two independent experiments (n = 4 per experiment for naive mice, and n = 10 per group experiment for H. polygyrus bakeri-infected mice; data for PPs from 2 mice were pooled for each data point); data are expressed as means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 [as determined by one-way ANOVA with Tukey's multiple-comparison test]).
Helminth larvae drive Treg proliferation in vivo and in vitro.
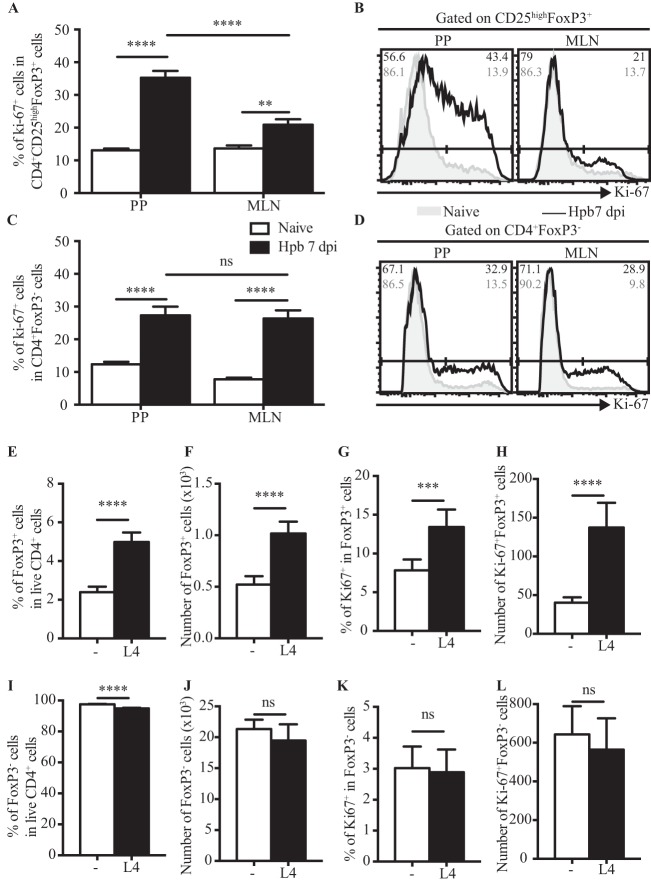
We next determined the impact of helminth larvae on the proliferation of Tregs by assessing the expression of the nuclear protein Ki-67, whose expression is strictly associated with cellular proliferation (40). As shown in Fig. 5A and B, the proportion of Ki-67-positive cells within the CD25high FoxP3+ population was significantly higher in PPs than in MLN (P < 0.0001, as determined by two-way ANOVA). On the contrary, no significant differences were detected in the FoxP3− populations present in PPs or MLN (P = 0.2765, as determined by two-way ANOVA) (Fig. 5C and D). These data indicated that helminth-induced proliferation of CD25high FoxP3+ cells may underlie the increased accumulation of these cells within PPs at early time points (day 7) following infection.
FIG 5.
H. polygyrus bakeri larvae promote the proliferation of CD25high FoxP3+ cells in vitro. (A to D) Ki-67 expression was assessed in cells isolated from PPs and MLN of naive or H. polygyrus bakeri-infected mice at 7 dpi. The percentages of Ki-67+ cells within the CD4+ CD25high FoxP3+ (A) and CD4+ FoxP3− (C) populations as well as representative histograms of Ki-67 expression (B and D) are shown. Results are pooled from data from two independent experiments (n = 3 to 6 per group) and expressed as means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 [as determined by two-way ANOVA with Tukey's multiple-comparison test]). (E to L) CD4+ splenocytes were isolated from naive C57BL/6 mice and incubated for 48 h in presence or absence of H. polygyrus bakeriL4. Percentages (E and I) and total numbers (F and J) of CD25high FoxP3+ (E and F) and FoxP3− (I and J) cells within the CD4+ population are shown. Percentages of Ki-67+ cells within the CD25high FoxP3+ (G) and FoxP3− (K) populations are also shown. Total numbers of Ki-67+ CD25high FoxP3+ (H) and Ki-67+ FoxP3− (L) cells are represented. Results are pooled from data from two independent experiments and expressed as means ± SEM (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 [as determined by Student's t test]). Each condition was analyzed in triplicate.
To test this hypothesis, we cultured naive CD4+ splenocytes in the presence or absence of live L4. Both the proportion (P < 0.0001, as determined by Student's t test) (Fig. 5E) and total number (P < 0.0001, as determined by Student's t test) (Fig. 5F) of CD25high Foxp3+ cells were increased in cultures containing live L4, and this was associated with corresponding increases in the proportion and number of Ki-67+ FoxP3+ cells (P = 0.0004 for percentages and P < 0.0001 for numbers, as determined by Student's t test) (Fig. 5G and H). Of note, a slight decrease in the proportion, but not in the number, of FoxP3− T cells was noted (P < 0.0001 for percentages and P = 0.1693 for numbers, as determined by Student's t test) (Fig. 5I and J). No impact on the proportion or number of cells expressing Ki-67 was noted for FoxP3− T cells (P = 0.7502 for percentages and P = 0.3917 for numbers, as determined by Student's t test) (Fig. 5K and L). These data indicate that H. polygyrus bakeri larvae secrete or shed products that are able to drive the rapid proliferation of CD25high FoxP3+ T cells but not Foxp3− T cells.
DISCUSSION
Tregs are thought to play a dual role during infectious disease, functioning to dampen host immunity (41, 42) and to protect the host against excessive immunopathology (26, 43–45). In the present study, we observed a differential response in PPs and MLN in terms of Treg or effector Th2 immunity following intestinal helminth infection. H. polygyrus bakeri infection resulted in an increased accumulation of regulatory T cells in PPs at both early (days 7 and 14) and late (days 28 and 40) time points following infection, while these cells were observed to accumulate only transiently within the MLN. Tregs from PPs also showed a more activated phenotype, with a greater proportion of CD25high FoxP3+ cells being positive for CD103 and exhibiting a higher surface expression level of GITR. In contrast, PPs exhibited no or little type 2 cytokine production, while the MLN exhibited a strong accumulation of Th2 cells.
The most likely explanation for the dominant regulatory response in PPs is the contact of some of these tissues with invasive larvae, as Treg accumulation was greater in PPs containing larvae than in those located distal to larvae. In support of this conclusion, we observed an ability of H. polygyrus bakeri L4 to promote the proliferation of Tregs in vitro. A previous report demonstrated that adult H. polygyrus bakeri products can promote the conversion of naive T cells into Tregs (20), indicating that H. polygyrus bakeri may expand Tregs through the expansion of thymic Tregs and by eliciting inducible Tregs. That H. polygyrus bakeri can additionally promote the proliferation of thymic Tregs is supported by our observations that helminth-induced accumulation of Tregs within PPs occurred at very early time points postinfection (day 7) and was not associated with a loss of Helios expression. The observed early accumulation of Tregs in PPs also indicates that helminth-induced Treg proliferation may not be antigen specific.
Although we showed that larval products promoted Treg proliferation in vitro, these findings do not rule out the possibility that additional factors contribute to their in vivo expansion following H. polygyrus bakeri infection. One such factor may be tissue damage caused by the invasion of helminth larvae, which could potentially result in the production of soluble factors that instruct Treg expansion. Another possibility is that infection allows concurrent bacterial translocation and that the bacteria contribute to the Treg response. In this regard, our experiments in mice treated with antibiotics indicated that the presence or absence of bacteria did not impact helminth-induced accumulation of Tregs but that bacteria may contribute, at least in part, to the activation status of Tregs within PPs.
Why H. polygyrus bakeri infection should lead to effector Th2 responses in the MLN but not PPs may be partially due to the increased proportion of Tregs within PPs, as increased Treg numbers correlated with an absence of Th2 responses at this site, and depletion of CD25high FoxP3+ cells led to a greater Th2 response. However, Treg depletion did not fully restore type 2 cytokine production in PPs and also impacted Th2 responses in the MLN. As cells can migrate from PPs to the MLN, depletion of regulatory T cells resident in PPs might be expected to impact effector T cell responses in both PPs and MLN. Alternatively, the increased proportion of CD25high FoxP3+ cells in PPs compared to that in the MLN may not be the only factor contributing to different effector responses in these organs. The MLN is known to receive both soluble antigens that are taken up by resident DCs and antigen-loaded DCs that have received stimulatory signals leading to their migration from the intestinal lamina propria (46). In contrast, PPs receive antigens mainly via M cells that are subsequently processed by resident DCs (22). Thus, different DC subsets, and/or activation states, within the MLN versus PPs may contribute to the differential induction of effector Th2 cells in these organs.
As indicated above, helminth infection led to only a small increase in the proportion of Tregs within the MLN, yet Treg depletion augmented Th2 immunity within this organ. This finding indicates that Tregs can actively suppress helminth immunity in the MLN and is in keeping with data from other studies demonstrating increases in the accumulation and suppressor function of MLN Tregs following H. polygyrus bakeri infection (26, 28). The authors of those studies reported an increase in the proportion of MLN Tregs expressing CD103 (26, 28), while we observed only a transient increase in the accumulation of total CD25high FoxP3+ cells and no increased CD103 expression in the MLN. The reasons for this discrepancy are unclear, but they may be related to differences in intestinal bacterial communities across individual breeding colonies, as in the present study, we noted a small impact of intestinal bacteria on CD103 expression by Tregs in PPs. Also, it is not clear whether the functional Tregs present in the MLN arise there or migrate from the intestine or PPs. The migration patterns of Tregs arising in response to intestinal helminth infection would be of interest for future studies.
In summary, our data reveal differential immune responses in PPs and MLN following intestinal helminth infection, with PPs harboring increased numbers of activated Tregs with little or no Th2 responsiveness and the MLN exhibiting a strong Th2 response but limited Treg expansion. The preferential expansion of Tregs and the relative absence of type 2 cytokine production within PPs were closely associated with contact with live parasites. Moreover, parasitic larvae were able to promote Treg proliferation in vitro, indicating that H. polygyrus bakeri may promote local Treg responses in vivo by acting directly on FoxP3+ cells to promote their expansion. Future studies could assess the potential of helminth products to drive intestinal Treg expansion in therapeutic settings, such as in the treatment of food allergies.
Supplementary Material
ACKNOWLEDGMENTS
We thank the École Polytechnique Fédérale de Lausanne (EPFL) animal facility, in particular Gisèle Ferrand, for the advice on antibiotic treatment; Miguel Garcia and the EPFL flow cytometry core facility; and Josephine Uldry for the suggestions on qPCR data analysis.
This work was supported in part by a grant from the Swiss National Science Foundation (310030_133104). L.K.D. is supported by a prize from the Leenaards Foundation, Switzerland. B.V. is supported by funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007-2013 under REA grant agreement no. 316682.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00266-15.
REFERENCES
- 1.Krolewiecki A, Lammie P, Jacobson J, Gabrielli A-F, Levecke B, Socias E, Arias L, Sosa N, Abraham D, Cimino R, Echazú A, Crudo F, Vercruysse J, Albonico M. 2013. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis 7:e2165. doi: 10.1371/journal.pntd.0002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albonico M, Allen H, Chitsulo L, Engels D, Gabrielli A-F, Savioli L. 2008. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl Trop Dis 2:e126. doi: 10.1371/journal.pntd.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez P, Brindley P, Bethony J, King C, Pearce E, Jacobson J. 2008. Helminth infections: the great neglected tropical diseases. J Clin Invest 118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulendran B, Artis D. 2012. New paradigms in type 2 immunity. Science 337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maizels R, Hewitson J, Murray J, Harcus Y, Dayer B, Filbey K, Grainger J, McSorley H, Reynolds L, Smith K. 2012. Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp Parasitol 132:76–89. doi: 10.1016/j.exppara.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds L, Filbey K, Maizels R. 2012. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin Immunopathol 34:829–846. doi: 10.1007/s00281-012-0347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McSorley H, Maizels R. 2012. Helminth infections and host immune regulation. Clin Microbiol Rev 25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camberis M, Le Gros G, Urban J. 2003. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol Chapter 19:Unit 19.12. doi: 10.1002/0471142735.im1912s55. [DOI] [PubMed] [Google Scholar]
- 9.Esser-von Bieren J, Mosconi I, Guiet R, Piersgilli A, Volpe B, Chen F, Gause WC, Seitz A, Verbeek JS, Harris NL. 2013. Antibodies trap tissue migrating helminth larvae and prevent tissue damage by driving IL-4Rα-independent alternative differentiation of macrophages. PLoS Pathog 9:e1003771. doi: 10.1371/journal.ppat.1003771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anthony R, Rutitzky L, Urban J, Stadecker M, Gause W. 2007. Protective immune mechanisms in helminth infection. Nat Rev Immunol 7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris N, Gause W. 2011. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol 32:80–88. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monroy F, Enriquez F. 1992. Heligmosomoides polygyrus: a model for chronic gastrointestinal helminthiasis. Parasitol Today 8:49–54. doi: 10.1016/0169-4758(92)90084-F. [DOI] [PubMed] [Google Scholar]
- 13.Anthony R, Urban J, Alem F, Hamed H, Rozo C, Boucher J-L, Van Rooijen N, Gause W. 2006. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med 12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massacand J, Stettler R, Meier R, Humphreys N, Grencis R, Marsland B, Harris N. 2009. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S A 106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCoy K, Stoel M, Stettler R, Merky P, Fink K, Senn B, Schaer C, Massacand J, Odermatt B, Oettgen H, Zinkernagel R, Bos N, Hengartner H, Macpherson A, Harris N. 2008. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe 4:362–373. doi: 10.1016/j.chom.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Zaiss M, Maslowski K, Mosconi I, Guenat N, Marsland B, Harris N. 2013. IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog 9:e1003531. doi: 10.1371/journal.ppat.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson M, Taylor M, O'Gorman M, Balic A, Barr T, Filbey K, Anderton S, Maizels R. 2010. Helminth-induced CD19+ CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol 40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McSorley H, O'Gorman M, Blair N, Sutherland T, Filbey K, Maizels R. 2012. Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur J Immunol 42:2667–2682. doi: 10.1002/eji.201142161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum A, Hang L, Setiawan T, Urban J, Stoyanoff K, Leung J, Weinstock J. 2012. Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses. J Immunol 189:2512–2520. doi: 10.4049/jimmunol.1102892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grainger J, Smith K, Hewitson J, McSorley H, Harcus Y, Filbey K, Finney C, Greenwood E, Knox D, Wilson M, Belkaid Y, Rudensky A, Maizels R. 2010. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med 207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung C, Hugot J-P, Barreau F. 2010. Peyer's patches: the immune sensors of the intestine. Int J Inflam 2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor R, Williams I. 2005. Lymphoid organogenesis in the intestine. Immunol Res 33:167–181. doi: 10.1385/IR:33:2:167. [DOI] [PubMed] [Google Scholar]
- 23.MacPherson G, Milling S, Yrlid U, Cousins L, Turnbull E, Huang F-P. 2004. Uptake of antigens from the intestine by dendritic cells. Ann N Y Acad Sci 1029:75–82. doi: 10.1196/annals.1309.010. [DOI] [PubMed] [Google Scholar]
- 24.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877. doi: 10.1016/0092-8674(92)90030-G. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J, Min B, Hu-Li J, Watson C, Grinberg A, Wang Q, Killeen N, Urban J, Guo L, Paul W. 2004. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol 5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 26.Finney C, Taylor M, Wilson M, Maizels R. 2007. Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection. Eur J Immunol 37:1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson M, Taylor M, Balic A, Finney C, Lamb J, Maizels R. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med 202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rausch S, Huehn J, Kirchhoff D, Rzepecka J, Schnoeller C, Pillai S, Loddenkemper C, Scheffold A, Hamann A, Lucius R, Hartmann S. 2008. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect Immun 76:1908–1919. doi: 10.1128/IAI.01233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rötzschke O, Borsellino G, Battistini L, Falk K, Kleinewietfeld M. 2009. In vivo-activated CD103+ Foxp3+ Tregs: of men and mice. Blood 113:2119–2120. doi: 10.1182/blood-2008-11-188847. [DOI] [PubMed] [Google Scholar]
- 30.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. 2010. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. 2011. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lathrop S, Bloom S, Rao S, Nutsch K, Lio C-W, Santacruz N, Peterson D, Stappenbeck T, Hsieh CS. 2011. Peripheral education of the immune system by colonic commensal microbiota. Nature 478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer F, Bäckhed F. 2013. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 35.Round J, Lee S, Li J, Tran G, Jabri B, Chatila T, Mazmanian S. 2011. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. 2009. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Ivanov I, Atarashi K, Manel N, Brodie E, Shima T, Karaoz U, Wei D, Goldfarb K, Santee C, Lynch S, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman D. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cording S, Fleissner D, Heimesaat M, Bereswill S, Loddenkemper C, Uematsu S, Akira S, Hamann A, Huehn J. 2013. Commensal microbiota drive proliferation of conventional and Foxp3(+) regulatory CD4(+) T cells in mesenteric lymph nodes and Peyer's patches. Eur J Microbiol Immunol 3:1–10. doi: 10.3923/mj.2013.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massacand JC, Kaiser P, Ernst B, Tardivel A, Burki K, Schneider P, Harris NL. 2008. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS One 3:e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlüter C, Duchrow M, Wohlenberg C, Becker M, Key G, Flad H, Gerdes J. 1993. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol 123:513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S, Yasutomo K. 2004. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med 10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 42.Taylor M, LeGoff L, Harris A, Malone E, Allen J, Maizels R. 2005. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol 174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 43.Kullberg M, Jankovic D, Gorelick P, Caspar P, Letterio J, Cheever A, Sher A. 2002. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med 196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hesse M, Piccirillo C, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever A, Shevach E, Wynn T. 2004. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol 172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 45.Rausch S, Huehn J, Loddenkemper C, Hepworth M, Klotz C, Sparwasser T, Hamann A, Lucius R, Hartmann S. 2009. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur J Immunol 39:3066–3077. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- 46.Jan MH, Sougawa N, Tanaka T, Jirata T, Hiroi T, Tohya K, Guo Z, Umemoto E, Ebisuno Y, Yang BG, Seoh JY, Lipp M, Kiqono H, Miyasaka M. 2006. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol 176:803–810. http://www.jimmunol.org/content/176/2/803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.