Abstract
Antigen-presenting cells (APCs) are key players in the induction and regulation of immune responses. In Plasmodium falciparum malaria, determination of which cells and pathways are activated in the network of APCs remains elusive. We therefore investigated the effects of a controlled human malaria infection in healthy, malaria-naive volunteers on the subset composition and activation status of dendritic cells (DCs) and monocytes. While subsets of monocytes increased in frequency during blood-stage infection, DC frequencies remained largely stable. Activation markers classically associated with peptide presentation to and priming of αβT cells, HLA-DR and CD86, were upregulated in monocytes and inflammatory CD16 myeloid DCs (mDCs) but not in the classical CD1c, BDCA2, or BDCA3 DC subsets. In addition, these activated APC subsets showed increased expression of CD1c, which is involved in glycolipid antigen presentation, and of the immune complex binding Fcγ receptor III (CD16). Our data show that P. falciparum asexual parasites do not activate classical DC subsets but instead activate mainly monocytes and inflammatory CD16 mDCs and appear to prime alternative activation pathways via induction of CD16 and/or CD1c. Changes in expression of these surface molecules might increase antigen capture and enhance glycolipid antigen presentation in addition to the classical major histocompatibility complex class II (MHC-II) peptide presentation and thereby contribute to the initiation of T-cell responses in malaria. (This study has been registered at Clinicaltrials.gov under registration no. NCT01086917.)
INTRODUCTION
Infection with the malaria parasite Plasmodium falciparum causes severe morbidity and mortality worldwide, especially in sub-Saharan Africa (1). Dendritic cells (DCs) are dedicated antigen-presenting cells (APCs) that orchestrate the immune system and are among the first immune cells to encounter the parasite after its inoculation into the skin by infected mosquitoes. Different DC subsets have been described, some being derived from or related to monocytes. Myeloid DCs (mDCs) are defined by the expression of CD1c (BDCA-1), CD141 (BDCA-3), or CD16 (Fcγ receptor III), while the marker for plasmacytoid DCs (pDCs) is CD303 (BDCA-2) (2–4).
Previous studies investigating the effect of P. falciparum exposure on DCs have shown somewhat contradictory results. In general, DC function is considered to be impaired during acute malaria, leading to immune tolerance (5, 6), based on a variety of definitions of impairment related to DC frequency (7), antigen uptake (8), viability (8), major histocompatibility complex class II (MHC-II) and costimulatory molecule expression (7, 9–11), and cytokine production (10, 11). In murine models, cross-presentation (12) and the ability to form stable interactions with T cells (13) were shown to be impaired in DCs that were exposed to P. berghei or P. chabaudi. In in vitro experiments, the effects of P. falciparum blood-stage parasites on DCs are dependent on the parasite dose used (11, 14), while differential results in studies of natural infections may be explained by ethnicity (15) and age and/or previous exposure (7).
Of note, the vast majority of studies have focused on classical DC subsets and classical markers of DC activation, while inflammatory mDCs expressing Fcγ receptor III (CD16) (3) during malaria infection have to our knowledge been included in the analysis of DC subsets in only two studies (15, 16). Moreover, we previously established that during malaria, monocytes and DC subsets, in particular CD16+ CD14− inflammatory DCs, increase surface B-cell activating factor (BAFF) expression and thus might potentially facilitate B-cell responses (17). Although BAFF production alone cannot be used as a marker for normal APC function or activation, this adds to the hypothesis that APCs are not impaired by a malaria infection per se but might merely gain alternative functions. In addition to HLA-peptide recognition, protection against malaria is associated with γδT cells expressing invariant T-cell receptors, which can be activated by lipid-presenting molecules of the CD1 family (18, 19). Antigen uptake for presentation is further mediated not only by endocytosis, as examined previously (8, 16), but also, for instance, by phagocytosis of immune complex-associated antigens, which can be facilitated by the FcγIII receptor CD16 (20).
Here, we took advantage of the controlled human malaria infection (CHMI) model, which provides an ideal condition to study human immune cells after a first in vivo exposure to P. falciparum at defined time points postinfection, to investigate parasite-mediated classical activation as well as potential changes in CD1c and CD16 expression across DC and monocyte subsets in human volunteers.
MATERIALS AND METHODS
Study subjects and controlled human malaria infection.
Eighteen adult malaria-naive Dutch volunteers were exposed to a CHMI at the Radboud University Medical Center (21). This phase I clinical trial was approved by the Central Committee for Research Involving Human Subjects of The Netherlands (CCMO NL31858.091.10) and has been registered at Clinicaltrials.gov (NCT01086917). All volunteers provided written informed consent. Infection was initiated by intradermal injection of 2,500, 10,000, or 25,000 (n = 6 per group) cryopreserved P. falciparum NF54 sporozoites (PfSPZ Challenge) (21). Volunteers were monitored daily for symptoms and signs of infection and hematological and biochemical parameters during outpatient clinical visits beginning 5 days after inoculation of PfSPZ Challenge. As soon as parasites were detected by microscopic examination of thick blood smears (TS), volunteers were treated with atovaquone and proguanil (1,000 and 400 mg per day, respectively) for 3 days. Cure was confirmed by results showing two consecutive parasite-negative blood slides. Volunteers who did not develop parasitemia by day 21 after challenge were presumptively treated with the same regimen. Fifteen volunteers developed TS-detectable parasitemia. Neither the prepatent period results determined by quantitative PCR nor the TS or peak parasite densities differed between the three groups. Therefore, all TS-positive (TS+) volunteers were analyzed as one group. Three volunteers remaining TS− after CHMI were analyzed in parallel. Those volunteers displayed no changes in APC subset frequency or activation marker expression at any of the time points analyzed (see Fig. S1 in the supplemental material).
PBMC isolation, cryopreservation, and staining.
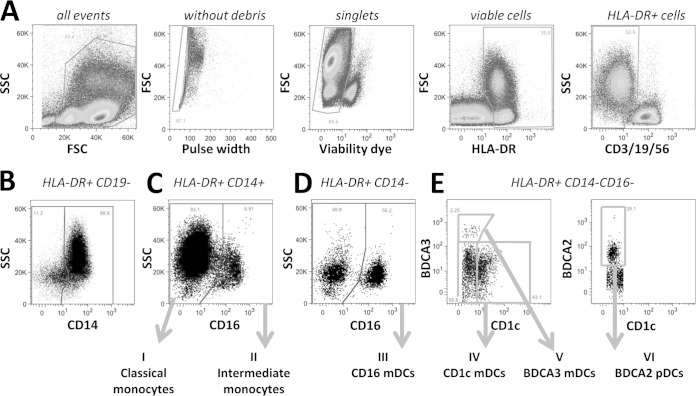
Peripheral blood mononuclear cells (PBMCs) were collected the day before challenge infection (C −1), during liver-stage infection (C +5) and early blood-stage infection (C +7 and C +9), at the time of thick-smear positivity just prior to drug treatment (day of treatment [DT]), 3 days after the day of treatment (DT +3), and after convalescence (C +35 and C +140). PBMCs were isolated and cryopreserved as described previously (17). Phenotypic analyses of the sequential PBMC samples were conducted simultaneously for each individual donor to avoid day-to-day interexperimental variation. Following thawing and washing in phosphate-buffered saline (PBS), 1,000,000 cells/well were incubated in 96-well v-bottom plates with LIVE/DEAD-fixable dead-cell Aqua stain (Invitrogen)–PBS for 30 min on ice. Cells were washed twice with staining buffer (PBS containing 0.5% bovine serum albumin [Sigma]) and stained for 30 min on ice with BDCA-1 (CD1c) fluorescein isothiocyanate (FITC) (AD5 to 8E7), BDCA-2 biotin (AC144), and BDCA-3 allophycocyanin (AD5 to 14H12) (all Miltenyi) and with CD16 phycoerythrin (PE) (3G8), CD3 peridinin chlorophyll protein (PerCP)-Cy5.5 (UCHT1), CD19 PerCP-Cy5.5 (HIB19), CD56 PerCP-Cy5.5 (HCD56), HLA-DR APC-Cy7 (L243), CD14 PeCy7 (HCD14), and CD86 PacBlue (IT2.2) (all BioLegend). Secondary-surface staining was performed with ECD (Beckman Coulter). Cells were kept in PBS–1% paraformaldehyde on ice until analyzed. Acquisition of 50,000 to 200,000 events per sample was performed using an ADP 9-color flow cytometer (Dako/Beckman Coulter). Data were analyzed using FlowJo v9.6 software. HLA-DR+ and CD3 CD19 CD56− APCs were subdivided into classical monocytes (CD14+ CD16−) and intermediate monocytes (CD14+ CD16+) and four CD14− DC subsets based on expression of CD16 (CD16 mDCs), CD1c (type 1 mDCs or BDCA-1 DCs), BDCA-3 (type 2 mDCs), and BDCA-2 (pDCs) (Fig. 1).
FIG 1.
Antigen-presenting-cell (APC) gating strategy. (A) Single, viable PBMCs positive for HLA-DR and negative for the lineage markers CD3, CD19, and CD56. FSC, forward scatter; SSC, side scatter. (B) HLA-DR+ CD3− CD19− PBMCs were distinguished into CD14+ monocytes and CD14− APCs. (C) Monocytes were subdivided based on CD16 expression into CD14+ CD16− classical monocytes (I) and CD14+ CD16+ intermediate monocytes (II). (D) CD14− APCs were gated based on CD16 expression to distinguish CD14 CD16+ myeoloid dendritic cells (DCs) (III) (CD16 mDCs). (E) CD14− CD16− APCs were further subdivided into CD14− CD16− CD1c+ (IV) (type 1 myeloid DC [CD1c mDCs], also known as BDCA1 mDCs), CD14− CD16− BDCA3+ (V) (type 2 mDCs or BDCA3 mDCs), and CD14− CD16− BDCA2+ (VI) (plasmacytoid DCs [pDCs]).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism v5. Cell counts and frequencies and surface marker expression (geometric mean fluorescence intensity [MFI]) were analyzed by repeated measures of one-way analysis of variance (ANOVA) with Dunnett's post hoc test to compare data from all time points to the C −1 baseline data. The majority of data were normally distributed as determined by the D'Agostino-Pearson omnibus normality test. The correlation between peak parasitemia and immunological outcome was tested using Pearson's correlation and log-transferred data for parasitemia. Statistical tests were performed for volunteers (n = 15) at all time points except for the DT because values were missing for three volunteers at that time point. Changes on the DT were therefore calculated for only 12 volunteers, but all other time points were taken into account for the multiple-comparison corrections.
RESULTS AND DISCUSSION
Upregulation of classical activation markers during CHMI is restricted to monocytes and CD16 mDCs.
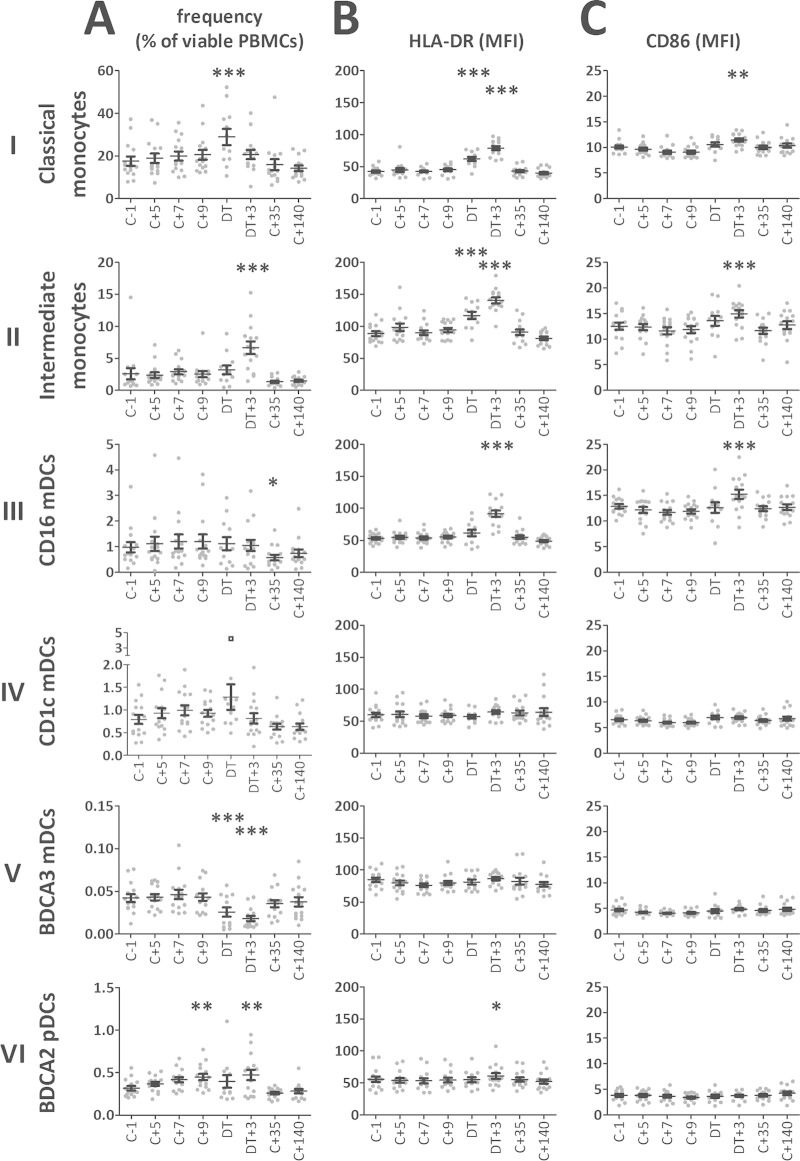
During blood-stage parasitemia, absolute leukocyte and lymphocyte counts (17), as well as combined monocyte and lymphocyte counts (see Fig. S2A in the supplemental material), decreased to their nadirs 3 days after treatment (DT +3) (P < 0.001). Assessments of APC subset proportions within the PBMC compartment for the 15 volunteers who were infected after intradermal injection of P. falciparum sporozoites (21) showed that no changes in DC and monocyte frequencies were evident during liver-stage infection and early blood-stage infection (C +5, C +7, and C +9) (Fig. 2A), except for a slight increase in the pDC frequency at C +9 (P < 0.01). Upon reaching detectable blood-stage parasitemia and after treatment, monocytes increased in frequency: classical monocytes peaked on the DT (P < 0.001) and intermediate monocytes on DT +3 (P < 0.001). Such transient increases in monocyte frequencies during acute infection that resolve after treatment have also been observed in P. vivax-infected patients (22). While pDC levels remained slightly increased on DT +3 (P < 0.01), BDCA-3 mDCs showed a significant decrease in frequency on the DT (P < 0.001) and at DT +3 (P < 0.01). At C +35, monocyte and DC frequencies had recovered to baseline values. The frequency of CD16 mDCs did not change during infection but showed a slight decrease on C +35 compared to baseline (P < 0.01) which was recovered on C +140. Absolute numbers of classical monocytes as well as of CD1c and BDCA3 mDCs showed a significant drop 3 days after treatment, while the numbers of intermediate monocytes increased and the numbers of CD16 mDCs remained stable (see Fig. S2B in the supplemental material).
FIG 2.
Kinetics of APC subset frequencies and expression of activation markers during and after CHMI. (A) Kinetics of APC subset frequency (percentages of viable PBMCs). (B) Kinetics of HLA-DR expression (geometric mean fluorescence intensity [MFI]) of the six APC subsets. (C) Kinetics of CD86 expression (MFI) of the six APC subsets. Data are presented for each individual donor (gray dots) and as means (n = 15) with standard errors of the means (SEM) (black error bars). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (one-way ANOVA with Dunnett's post hoc test compared to baseline [C −1]). Inclusion of the outlier (black square) in the CD1c mDC frequency in the statistical analysis renders the increase on the DT significant. C, challenge; DT, day of treatment.
We next assessed the activation status of APCs (Fig. 2B and C). In both classical and intermediate monocytes, expression of the peptide antigen-presenting molecule HLA-DR significantly increased during blood-stage infection (P < 0.001 at the DT and DT +3) and the costimulatory molecule CD86 was significantly upregulated at DT +3 (P < 0.001). Activation appeared slightly stronger in the CD14+ CD16+ intermediate monocyte subset than in the classical CD14+ CD16− monocytes, which is in line with a previous study on monocytes in P. vivax infection (22). Among the DCs, the CD1c, BDCA-2, and BDCA-3 DCs did not upregulate expression of HLA-DR or CD86 during parasitemia. In contrast, CD16 mDCs showed a significant increase of HLA-DR and CD86 expression at DT +3 (P < 0.01). Of note, we previously found that, together with classical and intermediate monocytes, CD16+ CD14− inflammatory DCs were also the main BAFF-producing APC subset during CHMI, showing much stronger induction of surface BAFF than the only other BAFF-producing DC subset, namely, classical CD1c mDCs (17). These data are suggestive of a functional distinction between CD16 mDCs and the other DC subsets.
Data on inflammatory CD16 mDCs in naturally exposed individuals are sparse and controversial. Similarly to our findings, a study in Mali that examined only the proportions of CD16 mDC found no difference between infected and uninfected individuals (15). In contrast, a field study in Papuan adults showed a reduction in absolute numbers of circulating CD16 mDCs in acute P. falciparum malaria (16). Finally, levels of CD16hi CD14lo monocytes were found to be elevated in malaria-infected compared to uninfected individuals in a Malawian cohort (23), but it is unclear how far this subset overlaps the CD14+ CD16+ intermediate/inflammatory monocytes or the CD14− CD16 mDCs examined here. Whether malaria infection was associated with increased or decreased phenotypic activation in the different DC subsets depended very much on ethnicity in the Malian study. In both ethnic cohorts, however, CD16 mDCs showed reduced HLA-DR expression. In line with this, HLA-DR expression on CD16+ CD14lo monocytes increased upon curative treatment of P. vivax infection (22). On the other hand, CD16+ CD14lo monocytes in these P. vivax-infected patients showed the highest levels of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) transcription, indicating an activated status of these cells (22). Further studies are needed to resolve these discrepancies, which might also relate to the degree of parasitemia. Moreover, future studies should investigate whether these CD16 mDCs might be constituted of so-called 6-sulfo LacNAc+ dendritic cells (slanDCs), an inflammatory DC type with unique capacities in immune complex-associated antigen capture, T-cell priming, and antibody-dependent cellular cytotoxicity (20, 24, 25). These cells are specifically recruited to sites of inflammation (25), which might explain field findings of reduced circulating CD16 mDC frequencies during more-prolonged infection in areas of endemicity (16).
Expression of the glycolipid-presenting molecule CD1c on APC subsets is increased during CHMI.
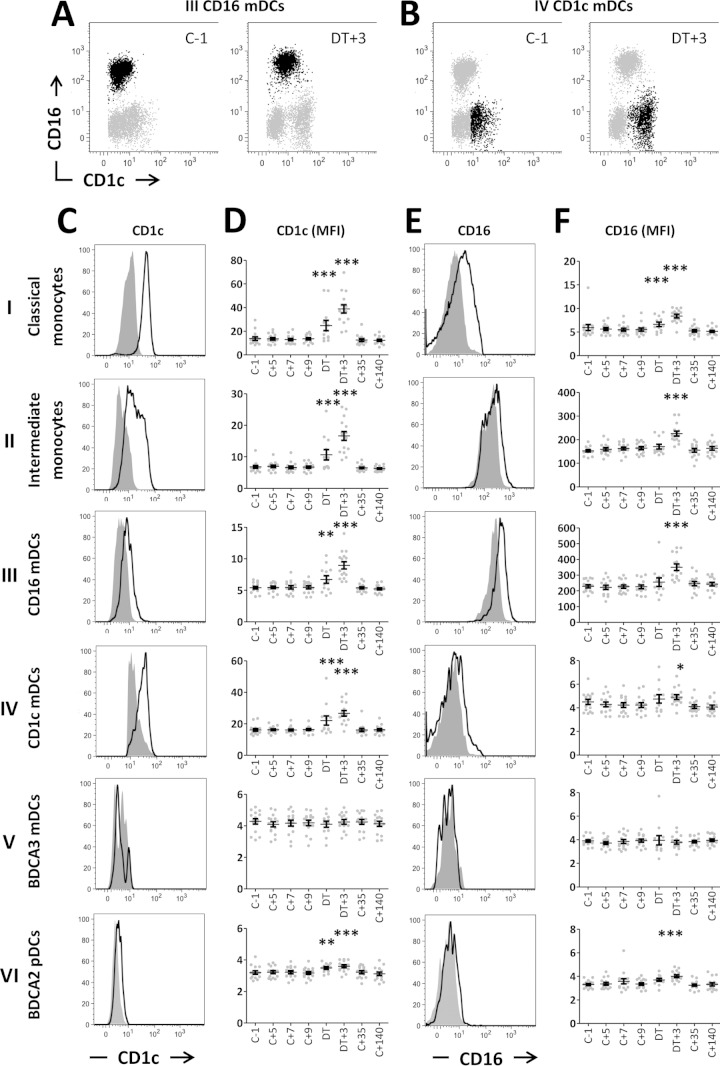
While all four DC subsets were mutually distinct at baseline (Fig. 3A and B and data not shown), CD1c expression during blood-stage parasitemia was upregulated not only in monocytes and CD1c mDCs but also in CD16 mDC and pDC subsets (Fig. 3A, C, and D) (on the DT, classical monocytes, P < 0.001; intermediate monocytes, P < 0.001; CD16 mDCs, P < 0.01; CD1c mDCs, P < 0.001; and pDCs, P < 0.01; at DT +3; all five subsets, P < 0.001). Of note, CD1c expression in the pDC subset was almost absent at baseline and the increase during infection was only marginal. BDCA-3 mDCs showed no change in CD1c expression. CD1c expression returned to baseline values at C +35 for all cell types. Although we found significant correlations between peak parasitemia and CD1c expression on several APC subsets on the DT and/or DT +3, the R2 was always <0.5 (data not shown), indicative of only weak correlations between parasitemia and CD1c expression. Nevertheless, induction of CD1c expression was clearly linked to exposure to blood-stage parasites, since no such upregulation was seen in volunteers who remained TS and PCR negative after CHMI (see Fig. S3A in the supplemental material).
FIG 3.
CD1c and CD16 expression on APCs during CHMI. (A and B) Representative plots showing CD1c and CD16 expression on CD16 mDCs (black dots) (A) or CD1c mDCs (black dots) (B) at baseline (C −1) and peak activation (DT +3). Gray dots in panels A and B show all DCs. (C) Expression of CD1c on APC subsets. (D) Expression (geometric mean fluorescence intensity [MFI]) of CD1c on APC subsets over time. (E) CD16 expression on APC subsets. (F) Expression (MFI) of CD16 on APC subsets over time. Panels C and E show representative histogram plots from one donor at baseline (gray) and DT +3 (bold line). Data in panels D and F are presented for each individual donor (gray dots) and as means (n = 15) with SEM (black error bars).*, P < 0.05; **, P < 0.01; ***, P < 0.001 (one-way ANOVA with Dunnett's post hoc test compared to baseline [C −1]). C, challenge; DT, day of treatment.
CD1c was originally described as presenting mycobacterial glycophospholipids (26), synergizes with CD1d in α-galactosylceramide presentation to NK-T cells expressing an invariant T-cell receptor (TCR) (27), and is able to present a diverse range of ligands to αβT or γδT cells (28). The latter are activated and expanded during controlled and natural malaria infection (19, 29), and schizont-stimulated pDCs are able to activate γδT cells (10). The degree to which this expansion and activation of γδT cells may be related to altered expression of CD1c on APCs remains to be investigated. It is further unknown which glycophospholipids of the malaria parasite or human erythrocytes are presented by CD1c. However, a subset of γδT cells has been identified that recognizes CD1c independently of foreign lipid or glycolipid antigens (30). Therefore, parasite-induced upregulation of CD1c (presenting self-lipids) might be sufficient to activate γδT cells without the need for malaria antigen processing and presentation, circumventing potential inhibition of this presentation. The early upregulation of CD1c expression might be specifically responsible for initiating the cascade of the early activation and expansion of the γδT-cell compartment and therefore the early production of gamma interferon (IFN-γ) (19), leading to further activation of αβT cells (30) and the induction of memory responses.
We found that the increased expression of CD1c on APCs during blood-stage infection is most pronounced on monocytes, CD16 mDCs, and CD1c mDCs (Fig. 3D and panels I to iv). For monocytes, this was shown previously in thalassemia B patients (31). To our knowledge, however, induction of CD1c expression on CD16 mDCs either during inflammation or in other disease settings has not been described before. Generally, CD16-positive blood DCs are described as phenotypically and functionally distinct and as not overlapping other myeloid blood DCs, based on detailed analysis in noninflammatory circumstances (2–4). One study in healthy individuals, however, described a subset of DC-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-expressing DCs, 50% of which also expressed CD16 as well as low levels of CD1c (32). Other studies exploring DC populations in healthy volunteers or during in vivo infection or inflammation determined only CD1c and CD16 expression in separated gating strategies (15, 33). In bacteriologically confirmed TB patients, no upregulation of CD1c in CD14− CD16+ cells occurred (34). Whether the upregulation of CD1c in CD16 mDCs is malaria specific or rather a general feature of activation of CD16 mDCs in certain infections remains to be investigated.
APC subsets show temporarily increased expression of FcγIII receptor CD16 during CHMI.
Not only CD1c but also the FcγIII receptor CD16 was significantly upregulated on classical and intermediate monocytes as well as CD16 mDCs during peak activation (Fig. 3E and F). Again, no such upregulation was seen in volunteers who remained TS and PCR negative after CHMI (see Fig. S3B in the supplemental material). CD1c mDCs (Fig. 3B) and BDCA-2 pDCs (data not shown), defined as CD16− negative at baseline, also showed upregulation of CD16, albeit only very marginal upregulation, and therefore did not merge into the CD16+ DC subset. Whether this only very slight induction of CD16 expression on CD1c mDCs and pDCs can have any functional relevance remains to be determined. CD16 binds immune complexes, and the binding triggers effector functions such as phagocytosis and proinflammatory cytokine secretion (35). Immune complexes are formed particularly during episodes of severe malaria, a condition associated with higher frequencies of CD16+ monocytes (36). Moreover, following malaria infection, elevated CD16 expression on monocytes is associated with enhanced TNF production, as well as with a reduced hemoglobin level (36). This suggests that CD16 cross-linking by malaria-induced immune complexes can trigger inflammatory responses and erythrophagocytosis. Our findings warrant future studies into the effect of temporarily increased CD16 expression, particularly on monocytes and inflammatory CD16 mDCs, even during uncomplicated, early malaria infection, on downstream immune responses. Increased capture of immune complex-associated antigens might, for instance, enhance antigen presentation (20). The recovery of baseline CD16 expression levels on APCs after convalescence observed after CHMI would be consistent with shedding of CD16 upon maturation, as shown for CD16+ slanDCs (25).
Finally, our findings highlight the importance of careful phenotypic analysis of DC subsets in circumstances of immune activation. CD16 and CD1c define two functionally separate subclasses of DCs that are phenotypically distinct under steady-state conditions (2, 3). When cells are activated, however, both markers can be upregulated on both subsets. Classification of activated DCs as defined under noninflammatory circumstances may therefore lead to misinterpretation of the results of direct comparisons of noninflammatory and inflammatory time points. The strong upregulation of CD1c expression, particularly on CD16 mDCs, the only DC subset showing activation marker expression during CHMI, highlights the need to include CD16 as a marker in assessing different DC subsets during immune activation, to avoid assigning phenotypic changes or functional features to the wrong DC subset.
Concluding remarks.
In conclusion, we show that monocytes and inflammatory CD16 mDCs are activated upon exposure to the blood-stage malaria parasite and that these APCs upregulate expression of CD16 and CD1c upon malaria infection in vivo. These data warrant future studies to examine whether potentially increased antigen capture in monocytes and a specialized DC subset, as well as enhanced glycolipid antigen presentation in addition to the classical MHC-II peptide presentation, might contribute to the initiation of T-cell responses in malaria.
Supplementary Material
ACKNOWLEDGMENTS
We thank the trial volunteers and the staff from the Clinical Research Centre Nijmegen, the Radboud University Medical Center, and the Sanaria Manufacturing Team, all of whom made this study possible. We thank Rob Hermsen for performing the quantitative PCR analysis.
This work was supported by Top Institute Pharma (Grant T4-102) and the FP7-founded European Virtual Institute of Malaria Research (Grant 242095). A.C.T. received a European Vaccine Initiative EMVDA Ph.D. scholarship. A.S. received an EMBO long-term postdoctoral fellowship. Development and manufacturing of PfSPZChallenge was supported by SIBR grants R44AI058375-03, -04, -05, and -05S1 from the NIAID/NIH and grant 07984 (PATH Malaria Vaccine Initiative, with funds from the Bill and Melinda Gates Foundation).
A.C.T. and A.S. conducted experiments; A.C.T. designed experiments and analyzed data; M.R. and E.M.B. performed the clinical study and collected clinical data; S.L.H. contributed vital reagents; A.C.T, R.W.S., and A.S. interpreted the data and wrote the manuscript; and M.R., E.M.B., and S.L.H. critically revised the manuscript.
S.L.H. is Chief Executive and Scientific Officer at Sanaria Inc., which manufactured PfSPZ Challenge, and thus does have a potential conflict of interest. The other authors declare no commercial or financial conflict of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00473-15.
REFERENCES
- 1.WHO. 2013. World malaria report: 2013. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, Bardelli M, Montagna D, Locatelli F, Wack A. 2007. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood 109:5371–5379. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. 2002. Characterization of human blood dendritic cell subsets. Blood 100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 4.Lindstedt M, Lundberg K, Borrebaeck CA. 2005. Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J Immunol 175:4839–4846. doi: 10.4049/jimmunol.175.8.4839. [DOI] [PubMed] [Google Scholar]
- 5.Wykes MN, Good MF. 2008. What really happens to dendritic cells during malaria? Nat Rev Microbiol 6:864–870. [DOI] [PubMed] [Google Scholar]
- 6.Scholzen A, Minigo G, Plebanski M. 2010. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol 26:16–25. doi: 10.1016/j.pt.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Urban BC, Cordery D, Shafi MJ, Bull PC, Newbold CI, Williams TN, Marsh K. 2006. The frequency of BDCA3-positive dendritic cells is increased in the peripheral circulation of Kenyan children with severe malaria. Infect Immun 74:6700–6706. doi: 10.1128/IAI.00861-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodberry T, Minigo G, Piera KA, Amante FH, Pinzon-Charry A, Good MF, Lopez JA, Engwerda CR, McCarthy JS, Anstey NM. 2012. Low-level Plasmodium falciparum blood-stage infection causes dendritic cell apoptosis and dysfunction in healthy volunteers. J Infect Dis 206:333–340. doi: 10.1093/infdis/jis366. [DOI] [PubMed] [Google Scholar]
- 9.Urban BC, Mwangi T, Ross A, Kinyanjui S, Mosobo M, Kai O, Lowe B, Marsh K, Roberts DJ. 2001. Peripheral blood dendritic cells in children with acute Plasmodium falciparum malaria. Blood 98:2859–2861. doi: 10.1182/blood.V98.9.2859. [DOI] [PubMed] [Google Scholar]
- 10.Pichyangkul S, Yongvanitchit K, Kum-arb U, Hemmi H, Akira S, Krieg AM, Heppner DG, Stewart VA, Hasegawa H, Looareesuwan S, Shanks GD, Miller RS. 2004. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J Immunol 172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 11.Elliott SR, Spurck TP, Dodin JM, Maier AG, Voss TS, Yosaatmadja F, Payne PD, McFadden GI, Cowman AF, Rogerson SJ, Schofield L, Brown GV. 2007. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect Immun 75:3621–3632. doi: 10.1128/IAI.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, de Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. 2006. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol 7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 13.Millington OR, Gibson VB, Rush CM, Zinselmeyer BH, Phillips RS, Garside P, Brewer JM. 2007. Malaria impairs T cell clustering and immune priming despite normal signal 1 from dendritic cells. PLoS Pathog 3:1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban BC, Ferguson DJ, Pain A, Willcox N, Plebanski M, Austyn JM, Roberts DJ. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 15.Arama C, Giusti P, Bostrom S, Dara V, Traore B, Dolo A, Doumbo O, Varani S, Troye-Blomberg M. 2011. Interethnic differences in antigen-presenting cell activation and TLR responses in Malian children during Plasmodium falciparum malaria. PLoS One 6:e18319. doi: 10.1371/journal.pone.0018319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinzon-Charry A, Woodberry T, Kienzle V, McPhun V, Minigo G, Lampah DA, Kenangalem E, Engwerda C, Lopez JA, Anstey NM, Good MF. 8 July 2013, posting date Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J Exp Med doi: 10.1084/jem.20121972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholzen A, Teirlinck AC, Bijker EM, Roestenberg M, Hermsen CC, Hoffman SL, Sauerwein RW. 19 March 2014, posting date BAFF and BAFF receptor levels correlate with B cell subset activation and redistribution in controlled human malaria infection. J Immunol doi: 10.4049/jimmunol.1302960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen NR, Garg S, Brenner MB. 2009. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol 102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 19.Inoue SI, Niikura M, Mineo S, Kobayashi F. 2013. Roles of IFN-gamma and gammadelta T cells in protective immunity against blood-stage malaria. Front Immunol 4:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Döbel T, Kunze A, Babatz J, Tränkner K, Ludwig A, Schmitz M, Enk A, Schäkel K. 2013. FcgammaRIII (CD16) equips immature 6-sulfo LacNAc-expressing dendritic cells (slanDCs) with a unique capacity to handle IgG-complexed antigens. Blood 121:3609–3618. doi: 10.1182/blood-2012-08-447045. [DOI] [PubMed] [Google Scholar]
- 21.Roestenberg M, Bijker EM, Sim BK, Billingsley PF, James ER, Bastiaens GJ, Teirlinck AC, Scholzen A, Teelen K, Arens T, van der Ven AJ, Gunasekera A, Chakravarty S, Velmurugan S, Hermsen CC, Sauerwein RW, Hoffman SL. 13 November 2012, posting date Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg doi: 10.4269/ajtmh.2012.12-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonelli LR, Leoratti FM, Costa PA, Rocha BC, Diniz SQ, Tada MS, Pereira DB, Teixeira-Carvalho A, Golenbock DT, Goncalves R, Gazzinelli RT. 2014. The CD14+ CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS Pathog 10:e1004393. doi: 10.1371/journal.ppat.1004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaworowski A, Kamwendo DD, Ellery P, Sonza S, Mwapasa V, Tadesse E, Molyneux ME, Rogerson SJ, Meshnick SR, Crowe SM. 2007. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis 196:38–42. doi: 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz M, Zhao S, Schakel K, Bornhauser M, Ockert D, Rieber EP. 2002. Native human blood dendritic cells as potent effectors in antibody-dependent cellular cytotoxicity. Blood 100:1502–1504. [PubMed] [Google Scholar]
- 25.Schäkel K, Kannagi R, Kniep B, Goto Y, Mitsuoka C, Zwirner J, Soruri A, von Kietzell M, Rieber E. 2002. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity 17:289–301. doi: 10.1016/S1074-7613(02)00393-X. [DOI] [PubMed] [Google Scholar]
- 26.Brigl M, Brenner MB. 2004. CD1: antigen presentation and T cell function. Annu Rev Immunol 22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 27.Fox LM, Miksanek J, May NA, Scharf L, Lockridge JL, Veerapen N, Besra GS, Adams EJ, Hudson AW, Gumperz JE. 2013. Expression of CD1c enhances human invariant NKT cell activation by alpha-GalCer. Cancer Immun 13:9. [PMC free article] [PubMed] [Google Scholar]
- 28.Adams EJ. 2013. Diverse antigen presentation by the group 1 CD1 molecule, CD1c. Mol Immunol 55:182–185. doi: 10.1016/j.molimm.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teirlinck AC, McCall MB, Roestenberg M, Scholzen A, Woestenenk R, de Mast Q, van der Ven AJ, Hermsen CC, Luty AJ, Sauerwein RW. 2011. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog 7:e1002389. doi: 10.1371/journal.ppat.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. 2000. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med 191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloma I, Zilber MT, Charron D, Girot R, Tamouza R, Gelin C. 2004. Upregulation and atypical expression of the CD1 molecules on monocytes in sickle cell disease. Hum Immunol 65:1370–1376. doi: 10.1016/j.humimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Engering A, Van Vliet SJ, Geijtenbeek TB, Van Kooyk Y. 2002. Subset of DC-SIGN(+) dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood 100:1780–1786. doi: 10.1182/blood-2001-12-0179. [DOI] [PubMed] [Google Scholar]
- 33.Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, Harrison LC. 2011. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol 186:6207–6217. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- 34.Castano D, Garcia LF, Rojas M. 2011. Increased frequency and cell death of CD16+ monocytes with Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 91:348–360. doi: 10.1016/j.tube.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Ravetch JV, Bolland S. 2001. IgG Fc receptors. Annu Rev Immunol 19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 36.Ogonda LA, Orago AS, Otieno MF, Adhiambo C, Otieno W, Stoute JA. 2010. The levels of CD16/Fc gamma receptor IIIA on CD14+ CD16+ monocytes are higher in children with severe Plasmodium falciparum anemia than in children with cerebral or uncomplicated malaria. Infect Immun 78:2173–2181. doi: 10.1128/IAI.01078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.