Abstract
Airway colonization by the mold Aspergillus fumigatus is common in patients with underlying lung disease and is associated with chronic airway inflammation. Studies probing the inflammatory response to colonization with A. fumigatus hyphae have been hampered by the lack of a model of chronic colonization in immunocompetent mice. By infecting mice intratracheally with conidia embedded in agar beads (Af beads), we have established an in vivo model to study the natural history of airway colonization with live A. fumigatus hyphae. Histopathological examination and galactomannan assay of lung homogenates demonstrated that hyphae exited beads and persisted in the lungs of mice up to 28 days postinfection without invasive disease. Fungal lesions within the airways were surrounded by a robust neutrophilic inflammatory reaction and peribronchial infiltration of lymphocytes. Whole-lung cytokine analysis from Af bead-infected mice revealed an increase in proinflammatory cytokines and chemokines early in infection. Evidence of a Th2 type response was observed only early in the course of colonization, including increased levels of interleukin-4 (IL-4), elevated IgE levels in serum, and a mild increase in airway responsiveness. Pulmonary T cell subset analysis during infection mirrored these results with an initial transient increase in IL-4-producing CD4+ T cells, followed by a rise in IL-17 and Foxp3+ cells by day 14. These results provide the first report of the evolution of the immune response to A. fumigatus hyphal colonization.
INTRODUCTION
The average person inhales hundreds of conidia (spores) of the ubiquitous mold Aspergillus fumigatus each day (1, 2). In the healthy host, conidia are easily cleared by the innate pulmonary defenses such as the mucociliary elevator and pulmonary macrophages (2). However, in patients with chronic pulmonary disease, elimination of inhaled conidia is impaired and germination of conidia occurs, leading to airway colonization with filamentous hyphae. Fungal colonization is associated with a progressive decline in pulmonary function in many patients (3). Aspergillus airway colonization is particularly common in patients with cystic fibrosis (CF), where up to 80% of patients have A. fumigatus isolated from their sputum (4). A minority of these colonized patients (<10%) develop allergic bronchopulmonary aspergillosis (ABPA), characterized by an exuberant T helper 2 (Th2) response with elevated total and A. fumigatus-specific IgE levels, eosinophilia, and progressive reactive airway disease leading to bronchiectasis (5–8). However, the majority of patients colonized with Aspergillus do not develop such a severe hypersensitivity reaction but still manifest a decline in lung function (3). The treatment of these patients with antifungal drugs has been reported to result in improvement of pulmonary function (9), suggesting a role for A. fumigatus in the progression of chronic pulmonary disease.
The natural history of the immune response to A. fumigatus colonization in the absence of ABPA is poorly understood. Repeated challenge with conidia or the administration of Aspergillus antigens in previously sensitized mice produces a condition that resembles ABPA with the production of the Th2 cytokines, such as interleukin-4 (IL-4) IL-5, IL-6, IL-10, and IL-13, accompanied by increased IgE levels, eosinophilic infiltration into the airways, and airway hyperresponsiveness (10–14). However, prolonged hyphal colonization is absent in these animal models and little is known about the immune response and natural history of airway colonization with live A. fumigatus hyphae. Therefore, to better understand the immune response to chronic airway colonization with A. fumigatus hyphae, we have established a chronic colonization model in which A. fumigatus conidia were embedded within agar beads and introduced intratracheally into immunocompetent mice (15, 16). This approach resulted in prolonged airway colonization with live A. fumigatus for up to 28 days. Conidia within the beads developed into hyphae that exited the beads to trigger a robust inflammatory response in the airways, characterized by predominately neutrophilic airway infiltration and the production of chemokines and proinflammatory cytokines. There was an early Th2-type response with an induction of IL-4 and IL-4-secreting CD4+ cells, a modest increase in total IgE levels in serum, and an increase in airway responsiveness following 14 days of colonization. Interestingly, after 14 days of infection, there was a shift from a Th2 to a Th17 and T-regulatory response, which was associated with decreased fungal burden. These data provide the first description of the host response to chronic colonization with A. fumigatus hyphae.
MATERIALS AND METHODS
Mice.
Female C57BL/6J mice, 8 to 12 weeks old, were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Animals were treated in accordance to the Canadian Council for Animal Care, and all experimental procedures were approved by the Animal Care Committees of the McGill University Health Center and McGill University.
Strain and growth conditions.
A. fumigatus strain Af293 was grown on yeast extract-peptone-dextrose (YPD; Difco) agar plates at 37°C, cultured freshly from −80°C stocks before experiments. Conidia were harvested after 6 days of growth with phosphate-buffered saline (PBS) containing 0.1% Tween 80 (vol/vol) (Fisher Scientific), washed, and resuspended at a concentration of 1 × 109 conidia/ml in PBS with 0.1% Tween 80.
Inoculum preparation.
Agar beads (Af beads) were freshly prepared under sterile conditions 36 to 48 h before experiments and kept at 4°C using a modification of the method described for Pseudomonas aeruginosa (15). Five milliliters of 10% YPD–3% agar kept at 52°C was mixed with 5 ml of concentrated conidial suspension (1 × 109 conidia/ml). This mixture was quickly added to prewarmed (52°C) heavy mineral oil (Fisher Scientific) kept in a 150-ml flask and subjected to rapid stirring with a magnetic stirring bar at room temperature for 6 min, followed by 10 min of stirring with cooling of the flask with crushed ice. The oil-agar mixture was centrifuged in Oakridge tubes at 9,000 × g for 20 min at 4°C to pellet the beads. After removal of the oil, beads were washed four times with sterile PBS (HyClone) in polystyrene centrifuge tubes via centrifugation at 400 × g for 10 min at room temperature. The beads were passed through a metal mesh, selecting for beads less than 280 μm in diameter. The number of conidia within the agar beads was determined by homogenizing beads to liberate the conidia, followed by serial dilution onto YPD plates and counting of CFU. The final inoculum was prepared by diluting the Af bead suspension to 5 × 107 conidia/ml. Sterile agar beads without conidia were prepared with YPD agar and PBS with 0.1% Tween 80 as described above. Isolated conidia for infection were prepared by resuspending conidia at a concentration of 5 × 107 conidia/ml in PBS with 0.1% Tween 80.
Scanning electron microscopy of Af beads.
Agar beads containing conidia were incubated for 12 h in phenol red-free RPMI 1640 medium at 37°C and 5% CO2 on glass coverslips. Samples were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at 4°C overnight, sequentially dehydrated in ethanol, and critical-point dried. Samples were then sputter coated with Au-Pd and imaged with a field emission scanning electron microscope (S-4700 FE-SEM; Hitachi).
Intratracheal infection model.
Mice were anesthetized with a mixture of 100 mg/kg of body weight of ketamine (Bionice, Belleville, ON) and 10 mg/kg of body weight of xylazine (Bayer Inc., Toronto, ON) by intraperitoneal injection. Once anesthetized, mice were put in a vertical position under a surgical microscope (Leica). An intratracheal injection of 50 μl of bead suspension containing 2.5 × 106 conidia, sterile beads alone, or conidia alone was performed using a curved 26-G gavage needle (Cadence Science, Staunton, VA).
BAL.
Lungs were lavaged three times with 500 μl of cold sterile PBS (HyClone). Bronchoalveolar lavage (BAL) fluid was spun at 200 × g for 10 min, and the supernatant was removed. The pellet was resuspended with ACK buffer (Gibco) to lyse erythrocytes. The remaining cells were resuspended in 110 μl of sterile PBS (HyClone), counted for total cell counts, and analyzed as detailed below.
Lung homogenates.
Following lavage, extracted lungs were homogenized in 5 ml of sterile PBS (HyClone) and protease inhibitor cocktail (Roche) with a Polytron homogenizer at 26,000 rpm for 45 s. Homogenates were aliquoted and stored at −80°C until being used for cytokine measurements. One milliliter of homogenate was concentrated 10-fold using an Amicon Ultra 0.5 centrifugal filter with a 3,000 nominal molecular weight limit (Millipore, Bedford, MA) immediately before running the Luminex assay.
Lung digest.
Harvested lungs were washed with PBS, minced, and enzymatically digested in 10 ml of digestion buffer (RPMI supplemented with 5% fetal bovine serum [FBS] and 150 U/ml of collagenase [Sigma]) for 60 min at 37°C. The suspension was then passed through a 70-μm cell strainer to produce a single-cell suspension, and an aliquot was removed for fungal burden measurement. After erythrocyte lysis using ACK buffer, cells were resuspended in PBS and counted using a hemocytometer.
Fungal burden and IgE measurements.
The fungal burden was determined by measuring the pulmonary galactomannan content. Three hundred microliters of lung digest was assayed using the Platelia Aspergillus enzyme immunoassay kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. The levels of IgE from serum were measured by an OptEIA enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences, San Diego, CA) according to the manufacturer's instructions.
Cytokine analysis.
The simultaneous measurement of 20 cytokines/chemokines, including IL-1α, -1β, -2, -4, -5, -6, -10, -12, -13, and -17, fibroblast growth factor (FGF) basic, granulocyte-macrophage colony-stimulating factor (GM-CSF), gamma interferon (IFN-γ), IFN-γ-inducible protein 10 (IP-10), cytokine-induced neutrophil chemoattractant (KC), macrophage chemoattractant protein 1 (MCP-1), monokine induced by IFN-γ (MIG), macrophage protein-1 alpha (MIP-1α), tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor (VEGF), from concentrated whole-lung homogenates was performed with the Cytokine 20-Plex Panel kit (Invitrogen) using the Luminex 100 system (Linco Research, Inc.) as per the manufacturer's instructions.
Lung histopathology.
Harvested lungs were inflated with 10% buffered formalin (Fisher Scientific) and fixed overnight, immersed in formalin. Separated halves of the lungs were embedded in paraffin and cut into 5-μm sections collected at different depths of the lung. The sections were then stained with periodic acid-Schiff (PAS) stain and hematoxylin and eosin (H&E).
Pulmonary leukocyte analysis.
Cells from lung digests and BAL fluids were resuspended at a concentration of 106 cells in 1 ml of PBS containing 1 μl of fixable viability dye (eBioscience) and incubated 30 min at 4°C. The cells were washed with staining buffer (PBS + 2% FBS), and Fc receptors were blocked by the addition of unlabeled anti-CD16/32 (FcBlock; BD Pharmingen) for 15 min at 4°C. Cells were stained with antibodies against cell surface components for 30 min at 4°C. The following fluorescently conjugated antibodies purchased at BD were used: CD3-fluorescein isothiocyanate (clone 17A2), CD11c-allophycocyanin (clone HL3), CD11b-phycoerythrin-CF594 (clone M1/70), CD45-allophycocyanin-Cy7 (clone 30-F11), CD19-phycoerythrin-Cy7 (clone 1D3), SiglecF-brilliant violet 421 (clone ESO-2440), and Ly-6G-phycoerythrin (clone 1A8). Cells were washed with staining buffer and fixed with 2% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA) for 30 min on ice. Cells were then washed and resuspended in PBS and kept at 4°C until data acquisition. Data acquisition was performed on an LSR Fortessa (BD) using fluorescence-activated cell sorter (FACS) Diva software (BD). The data were further analyzed using FlowJo software v10.0.7r2 (FlowJo, LLC). Immune cell subsets were defined as follows: neutrophils, CD45+ Ly6G+ CD11c− CD11b+; alveolar macrophages, CD45+ CD11c+ siglecF+ CD11bneg/low; lymphocytes, CD45+ CD11c− Ly6G− CD3+ or CD45+ CD11c− Ly6G− CD19+. Total cells of each population were calculated via the total cell counts of each sample, as determined using a hemocytometer following lung digestion.
Pulmonary T cell subset analysis.
Prior to intracellular staining, cells from lung digest were stimulated in vitro with phorbol myristate acetate (PMA; 50 ng/ml) and ionomycin (1 μg/ml). After 2 h of stimulation, brefeldin A (GolgiPlug; BD) was added for another 3 h to promote the intracellular accumulation of cytokines. The cells were then washed with PBS, resuspended in 1 ml of PBS containing 1 μl of fixable viability dye (eBioscience), and incubated 30 min at 4°C. The cells were then washed and fixed using the BD Cytofix/Cytoperm kit according to the manufacturer's instructions (BD). The following fluorescently conjugated antibodies (purchased from BD) against cell surface components were used: CD4-fluorescein (clone H129.19), CD8-allophycocyanin-Cy7 (clone 53-6.7), and CD45-phycoerythrin-CF594 (clone 30-F11). The following fluorochrome-conjugated antibodies (BD) against cytokines were used: IL-17a-phycoerythrin (clone TC11-18H10), IL-4-allophycocyanin (clone 11B11), and IFN-γ-Alexa Fluor 700 (clone XMG1.2). For measurement of Foxp3-positive T cells, lung digest cells were stained using the Foxp3/transcription factor staining kit (eBioscience) according to the manufacturer's instructions. Lymphocytes were defined as CD45+ CD4+ CD8−, prior to intracellular cytokine analysis.
Measurement of airway responsiveness.
Mice were anesthetized with a mixture of 50 mg/kg of body weight of sodium pentobarbital and 10 mg/kg of body weight of xylazine (Bayer, Inc., Toronto, ON) by intraperitoneal injection. Mice were then tracheotomized using an 18-G cannula and connected to a small animal ventilator (FlexiVent; Scireq, Montréal, Canada). Muscle paralysis was induced with pancuronium bromide (0.2 mg/kg) by intraperitoneal injection. Changes in airway function in response to increasing doses of methacholine (6.25 to 50 mg/ml administered in doubling doses) were recorded. Respiratory system resistance and elastance were determined before challenge and after each dose of methacholine.
Statistical analyses.
One-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison test was used to evaluate differences between groups. Two-way ANOVA followed by Bonferroni posttests was used to evaluate differences between the groups during the time course. P values of <0.05 were considered significant.
RESULTS
A. fumigatus germinates and produces hyphae that emerge from within agar beads.
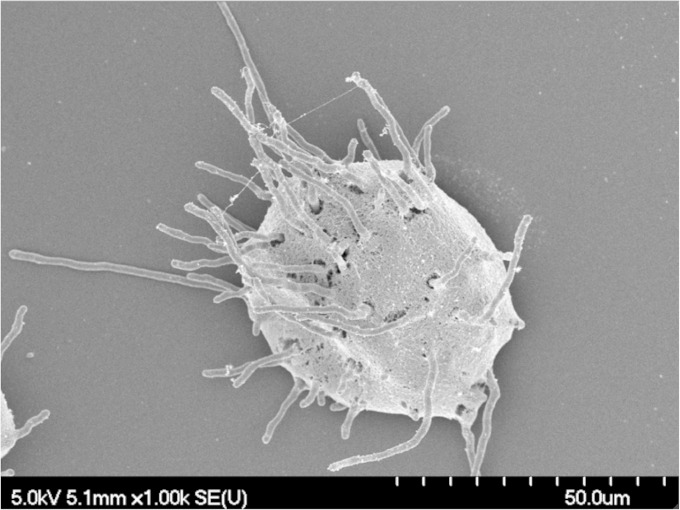
Hyphae are the predominant morphology of A. fumigatus observed within the airways of colonized patients. We therefore investigated whether A. fumigatus conidia embedded within agar beads (Af beads) could germinate and form hyphae when grown in vitro. After 12 h of incubation, numerous hyphae emerged from within agar beads, confirming the ability of A. fumigatus to germinate within, and exit from, agar beads (Fig. 1).
FIG 1.
A. fumigatus conidia germinate and emerge from within agar beads. Beads were grown in RPMI medium for 12 h and then imaged using scanning electron microscopy.
Infection with beads containing A. fumigatus induces colonization with hyphae for up to 28 days.
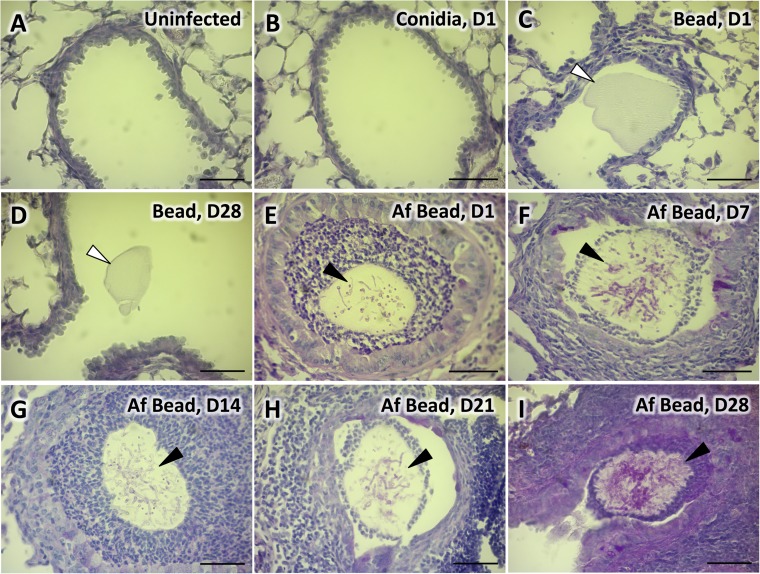
Next, the ability of Af beads to induce colonization of the airways of healthy C57BL/6 mice was determined. Mice were infected with 2.5 × 106 conidia of A. fumigatus in suspension, 2.5 × 106 conidia of A. fumigatus embedded in agar beads, or sterile agar beads by intratracheal injection and sacrificed after 1, 7, 14, 21, and 28 days following infection. Microscopic examination of PAS- and H&E-stained lung tissue was performed at each time point. The histopathologic appearance of lungs from mice infected with A. fumigatus conidia in suspension was indistinguishable from that of uninfected animals at all time points (Fig. 2A and B; see also Fig. S1A to C in the supplemental material). Airways of control mice infected with sterile beads were found to contain empty beads without any evident inflammatory response at all time points (Fig. 2C and D; see also Fig. S1D to F in the supplemental material). In contrast, lungs of mice infected with Af beads revealed the presence of beads within airways containing PAS-positive hyphae at all time points (Fig. 2E to I). In some lesions, hyphae could be visualized exiting the agar beads and interacting with leukocytes within the airways; however, tissue invasion was not seen. Hyphal egress from beads during infection was also confirmed by microscopic analysis of beads retrieved from infected mice using bronchoalveolar lavage (Fig. 3A). Consistent with the findings of noninvasive hyphal growth within the airways, Af bead-infected mice did not manifest signs of invasive disease such as weight loss, ruffling of fur, or decreased survival.
FIG 2.
Colonization of airways with A. fumigatus beads. Histopathology of PAS-stained lung tissue of uninfected C57BL/6 mice (A) or mice infected with conidia in suspension 24 h postinfection (B), sterile beads 24 h postinfection (C), sterile beads 28 days postinfection (D), A. fumigatus beads 24 h postinfection (E), A. fumigatus beads 7 days postinfection (F), A. fumigatus beads 14 days postinfection (G), A. fumigatus beads 21 days postinfection (H), or A. fumigatus beads 28 days postinfection (I); images were taken with a 40× objective lens. White arrows indicate sterile beads. Black arrows indicate PAS-positive hyphae of A. fumigatus within agar beads. Bars, 50 μm. Histopathology of PAS-stained lung tissue of C57BL/6 mice infected with conidia in suspension or sterile beads on days 7, 14, and 21 days postinfection can be found in Fig. S1 in the supplemental material.
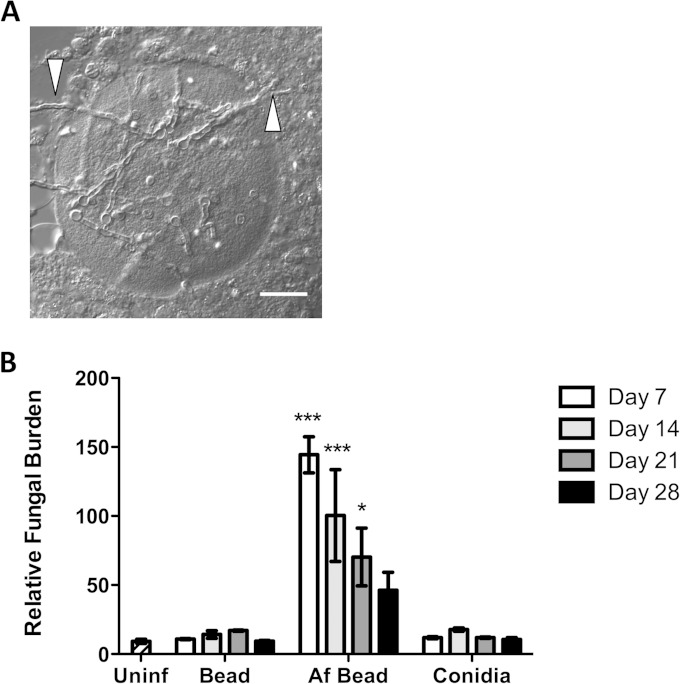
FIG 3.
A. fumigatus conidia within beads develop into hyphae and exit beads in vivo. (A) Differential interference contrast image of AF bead recovered in BAL fluid from a C57BL/6 mouse 3 days postinfection. White arrows indicate hyphae exiting from the agar bead. Scale bar, 20 μm. (B) Time course of pulmonary fungal burden measured from whole-lung digest using the Bio-Rad Platelia GM assay. Bars represent the means ± standard errors of the means from at least two independent experiments (total n = 5 to 8 per group per time point). *, P < 0.05; ***, P < 0.001, compared to uninfected mice.
To confirm these observations and to quantify the kinetics of hyphal growth in vivo, pulmonary fungal burden was measured by determining the concentration of galactomannan (GM) in whole-lung digest. GM is a carbohydrate component of the cell wall produced by hyphae and not conidia and has been used as a surrogate measure of hyphal fungal burden (17–19). Consistent with our microscopic findings of persistence of hyphae within the airways, high levels of GM were detected in pulmonary tissues throughout the 28-day period in mice infected with Af beads but not in those infected with sterile beads (Fig. 3B) or with conidia alone. The level of GM declined over this period, and fungal lesions were less abundant within tissue sections of infected mice at later time points, suggesting that fungi were gradually cleared from the airways.
A. fumigatus embedded in agar beads induces airway inflammation.
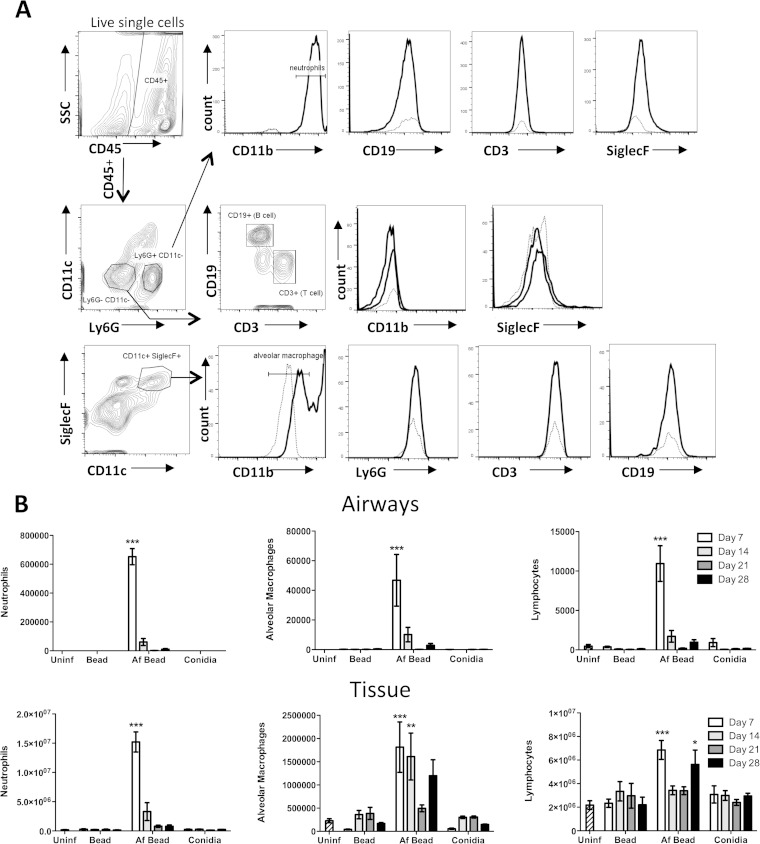
As early as 24 h after infection, microscopic examination of airways from infected mice revealed the presence of inflammation surrounding the A. fumigatus-containing beads within the airways (Fig. 2E). Af beads within airways were surrounded by a ring of leukocytes consisting predominately of neutrophils. By day 7 postinfection, the intraluminal leukocyte infiltration was also accompanied by peribronchial inflammation, composed mostly of mononuclear cells (Fig. 2F). No increase in the number of eosinophils was evident at any time points when H&E-stained sections of the lungs were examined (data not shown). This pattern of inflammation persisted in the lungs of mice up to 28 days postinfection (Fig. 2I), the latest time point studied. To quantify the cellular inflammatory response to Af beads, pulmonary leukocyte infiltration was measured in bronchoalveolar lavage fluid and lung tissue samples using flow cytometry. A significant influx of neutrophils and to a lesser extent macrophages and lymphocytes was observed in both BAL fluid and lungs of mice infected with Af beads (Fig. 4B). This pulmonary leukocyte recruitment was greatest at 7 days following infection and decreased over time. At all time points, higher numbers of neutrophils and macrophages were recovered from BAL fluid and lung tissue from mice infected with Af beads than from the sterile-bead group, the group infected with conidia only, or uninfected controls, although this was statistically significant only at day 7 postinfection (Fig. 4B). Interestingly, two peaks of lymphocyte recruitment were observed in lung digests from mice infected with Af beads, one early peak at 7 days postinfection and a second, later peak at 28 days postinfection (Fig. 4B).
FIG 4.
(A) Gating strategy for leukocyte differential analysis by flow cytometry. Mouse lungs were digested by collagenase, and cells were stained with fluorescently labeled antibodies. Doublet cells and dead cells, determined using a fixable viability dye, were excluded. Histograms show the cell surface staining for a fully stained sample (solid line), as well as the “fluorescence minus one” control (dotted line) for the corresponding channel. (B) A. fumigatus beads induce pulmonary leukocyte recruitment. Total numbers of the indicated leukocyte populations were determined by flow cytometry analysis of BAL fluid (airways) and lung (tissue) samples. Bars represent the means ± standard errors of the means from 4 to 8 mice per group per time point. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared to uninfected mice.
A. fumigatus colonization induces the production of proinflammatory cytokines and chemokines.
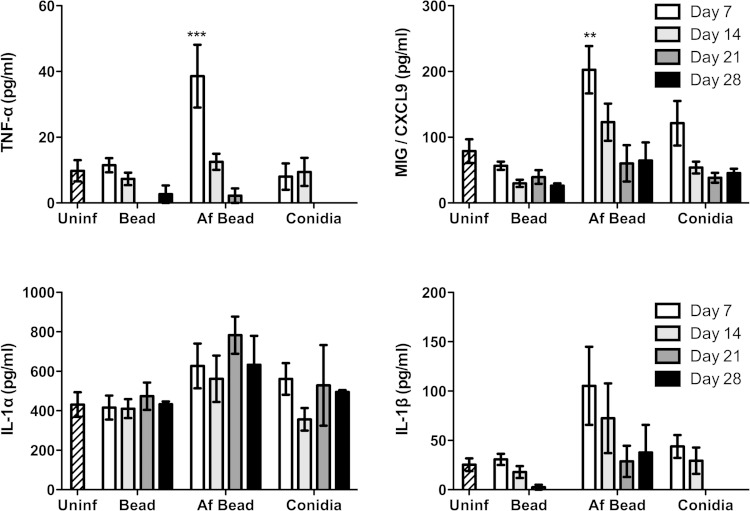
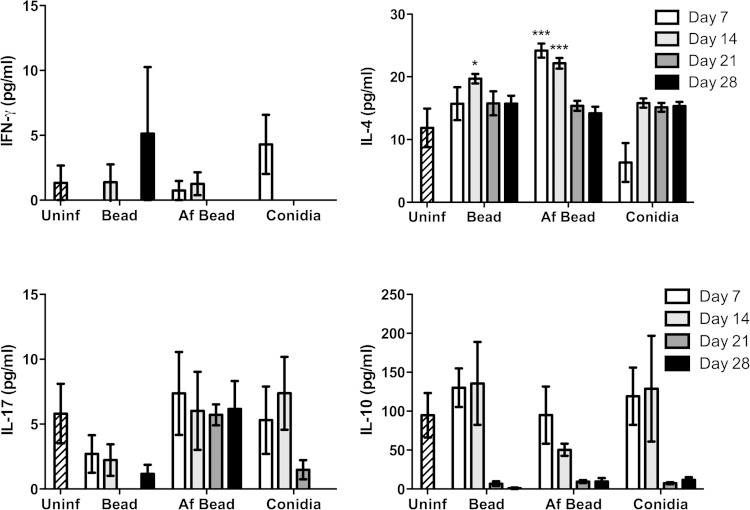
To probe the nature of the inflammatory responses caused by A. fumigatus colonization, the levels of 20 inflammatory cytokines/chemokines were measured from concentrated whole-lung homogenates. After 7 days of colonization, significantly higher levels of the proinflammatory cytokine TNF-α and the epithelium-derived chemokine MIG/CXCL9 were observed in mice infected with Af beads than in uninfected mice, mice infected with sterile beads, or mice infected with conidia alone (Fig. 5). A trend toward increased levels of IL-1β, but not IL-1α, was also observed in Af bead-infected mice, though this was not statistically significant. By day 14 of infection, these significant differences in proinflammatory cytokine expression were less prominent. Low levels of T helper-associated cytokines were also detected at all time points under each of the experimental conditions (Fig. 6). No significant induction of IFN-γ or IL-10 was observed in mice infected with Af beads or conidia alone compared with uninfected mice or mice infected with sterile beads. Mice infected with Af beads developed a significant increase in pulmonary IL-4 levels at 7 and 14 days after infection compared with uninfected mice or mice infected with sterile beads alone. Interestingly, sterile beads induced a lower-level (but significant) increase of IL-4 production at 14 days after infection than uninfected mice, though this was not seen at any other time point. Finally, mice infected with Af beads and conidia alone had higher IL-17 levels than mice infected with sterile beads alone (all time points for Af beads and days 7 and 14 for conidia alone), though these differences were not statistically significant. Collectively, these data suggest that Af beads induce an early Th2 response after infection; however, the low levels of T helper cytokines measured in these samples limit the interpretation of these findings.
FIG 5.
A. fumigatus colonization induces early proinflammatory mediator release. The concentration of the indicated cytokines/chemokines was measured from whole-lung homogenates 7, 14, 21, and 28 days postinfection using the Luminex Multiplex assay. Bars represent the means ± standard errors of the means from at least two independent experiments (total, n = 3 to 20 per group). **, P < 0.01; ***, P < 0.001, compared to uninfected mice. Other cytokines measured are shown in Fig. S2 in the supplemental material.
FIG 6.
Evolution of T-helper-associated cytokines during A. fumigatus colonization. The concentrations of the indicated cytokines/chemokines were measured from whole-lung homogenates at 7, 14, 21, and 28 days postinfection using the Luminex Multiplex assay. Bars represent the means ± standard errors of the means from at least two independent experiments (total, n = 3 to 20 per group). *, P < 0.05; ***, P < 0.001, compared to uninfected mice.
A. fumigatus colonization results in dynamic changes in T cell populations over time.
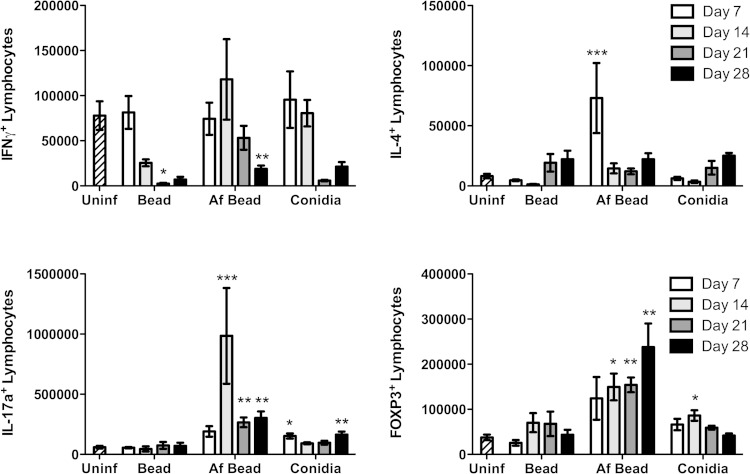
To further characterize adaptive immune responses to A. fumigatus hyphal colonization, the evolution of T cell populations within the lungs of infected mice was studied by intracellular cytokine and marker staining. Mice were infected with conidia, sterile beads, or beads containing A. fumigatus, and their lungs were harvested and digested at 7, 14, 21, and 28 days for characterization of pulmonary lymphocyte populations (Fig. 7). Consistent with our whole-lung cytokine analysis, no significant induction of IFN-γ-producing T cells was detected at any time point after infection, and indeed the numbers of these cells were reduced at later time points in all the mice treated with conidia, sterile beads, and Af beads than in uninfected controls. Mice infected with sterile beads displayed no other significant differences in T cell populations for the duration of the experiment. In mice infected with Af beads, a significant increase in the number of IL-4-producing CD4+ cells was noted at day 7 after infection, which declined by day 14 of infection. At this point, a significant increase in IL-17+ cells was observed. The number of IL-17-producing lymphocytes peaked at day 14 but remained elevated for the duration of the experiment. Foxp3+ T cells were also observed over the course of the experiment and increased over time in an inverse relationship to the decline in fungal burden over time. Mice infected with conidia alone displayed a modest increase in IL-17+ T cell numbers at days 7 and 14, and in Foxp3+ cells at day 14, although this increase was less prominent than in mice infected with Af beads.
FIG 7.
Intracellular lymphocyte staining during A. fumigatus colonization. Absolute number of viable T-cell subsets as determined by flow cytometry analysis of lung tissue digests from mice inoculated as indicated at 7, 14, 21, and 28 days after infection. Bars represent the means ± standard errors of the means from at least two independent experiments combined (total, n = 5 to 15 per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared to uninfected mice.
Mice infected with Af beads have elevated serum IgE.
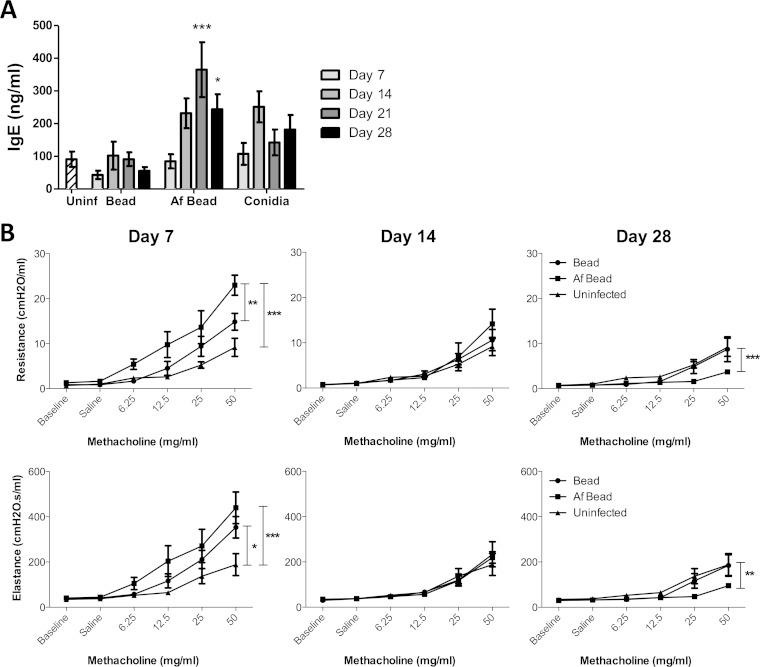
As inhalation of A. fumigatus conidia or extracts have been reported to result in increased total IgE levels in sensitized mice (20, 21), we investigated whether the presence of Af beads triggered similar results. Af bead-infected mice developed higher IgE levels than did sterile-bead-infected, conidium-infected, and uninfected controls; these levels peaked at 21 days postinfection and were significantly different from those of mice infected with sterile beads at 21 and 28 days postinfection (Fig. 8A).
FIG 8.
Mice colonized with A. fumigatus hyphae exhibit signs of an early, limited allergic response. (A) Time course of the levels of IgE from serum measured by ELISA. Bars represent the means ± standard errors of the means from at least three independent experiments (total, n = 5 to 34 per group). (B) Respiratory system resistance and elastance were measured in mice infected with Af beads or sterile beads at 7, 14, and 28 days postinfection using a FlexiVent ventilator. Data points represent the means ± standard errors of the means from 5 to 8 mice per group per time point. *, P < 0.05; **, P < 0.005; ***, P < 0.001, compared to mice infected with sterile beads at the same time point in panel A, or as indicated in panel B.
A. fumigatus colonization causes time-dependent changes in airway responsiveness.
We next assessed whether the increased inflammatory responses to Af beads affect airway responsiveness by challenging mice with methacholine. A significantly higher response to methacholine was observed upon measuring the respiratory resistance and elastance in mice infected with Af beads than in sterile-bead-infected and uninfected controls at day 7 postinfection (Fig. 8B). However, airway hyperreactivity was substantially decreased at day 14 postinfection, suggesting that with decreased fungal burden and subsequent inflammatory responses, mice were able to recover. Interestingly, the airways of mice infected with Af beads were found to be less responsive to methacholine challenge by 28 days of infection than either sterile-bead-infected and uninfected mice, suggesting that the airways of A. fumigatus-colonized mice may become hyporesponsive during recovery from infection.
DISCUSSION
Studies examining the pathogenesis of Aspergillus pulmonary colonization in chronic lung disease have been limited by the lack of an available model in which hyphae grow within the airways of immunocompetent mice. The bead model of A. fumigatus airway colonization described in this paper mimics this type of colonization. Hyphae were observed within the murine airways in the absence of tissue invasion for up to 28 days and elicited a chronic inflammatory response. Further, the growth of hyphae within the beads closely resembled the appearance of hyphae within mucus plugs seen in patients with cystic fibrosis or other chronic lung disease (22). These results are consistent with an earlier report in which invasive aspergillosis was induced through infection of BALB/c mice with A. fumigatus-containing beads for 2 weeks followed by the administration of immunosuppression (16). However, the inflammatory response to colonization was not characterized in any detail in this study.
The development of an animal model in which live hyphae are present within the airway provides the opportunity to study the inflammatory response to chronic airway colonization. While the effects of exposure to live conidia have been previously examined in numerous animal models (23, 24), mature hyphae exhibit important differences in the expression and exposure of pathogen-associated molecular patterns, such as β-glucan and galactosaminogalactan (25, 26, 27), as well as the expression of putative virulence factors such as proteases and the toxins Asp f1 and gliotoxin (28). The effects of these fungal factors on the immune response during airway colonization have remained unstudied, in part due to the lack of an available animal model system that is amenable to the study of live mutant organisms.
The importance of studying infection with live organisms is emphasized by the profile of the inflammatory response seen in mice colonized with Af beads, which differed from those challenged with conidia in suspension and from repeated challenge with conidia or other models that used sensitized mice (23, 29). First, colonization with A. fumigatus hyphae resulted in only a modest Th2-type response early in infection. Low levels of IL-4 and IL-4-producing T cells were observed early after infection, and no significant IL-5 production was detected. IgE levels and airway hyperreactivity were modestly increased in colonized mice; however, all of these responses were much lower than have been reported in other models of ABPA and declined after 2 to 3 weeks of colonization, paralleling the decreases in IL-4 and IL-4-producing T cell numbers. Second, despite the detection of neutrophils and proinflammatory cytokines in the lungs of mice infected with Af beads, IFN-γ levels remained undetectable during colonization, and no induction of IFN-γ-producing T cells within the lungs of infected mice was observed, suggesting that the proinflammatory cytokines observed early after infection are a result of Th1-independent innate inflammatory mechanisms. Collectively, these results provide a model in which a mixed proinflammatory and Th2 response to A. fumigatus colonization occurs early in infection, which is replaced by Th17 and T-regulatory responses over time in association with a reduction in fungal burden. This model is supported by similar trends in the induction of IL-4 and IL-17 that were reported in a study examining the effects of repeated exposure to A. fumigatus conidia on C57BL/6 mice (23). In this study, CD4+ cells from these mice were stimulated ex vivo and examined for intracellular cytokines. Importantly, hyphae were not observed in tissue sections at any point in these experiments. As with our whole-lung cytokine findings in mice colonized with hyphae, the investigators observed an initial increase in IL-4-positive cells that declined over time, while numbers of IL-17-positive cells remained increased. However, in contrast to our findings, they also observed a significant number of IFN-γ-positive and IFN-γ- and IL-17-double-positive cells, whereas IFN-γ and IFN-γ-producing T cells were absent from our studies with hyphal colonization, despite the use of a Th1-prone strain of mice. These observations support the hypothesis that hyphae and conidia induce different types of inflammatory response.
In addition to the early Th2 response, elevated numbers of IL-17-positive T cells were seen during infection in our model. Although Th17 responses are classically associated with protective mucosal immunity against pathogens such as Candida albicans (30), the role of the IL-17 and Th17 response in A. fumigatus infection remains a subject of debate. While an initial study reported that neutralization of IL-17 or IL-23 was associated with enhanced resistance of immunocompetent mice to infection with a high dose of A. fumigatus conidia (31), a study from another group has shown that IL-17 neutralization results in a dramatic increase in fungal burden in a similar mouse model (32). Further, studies of Aspergillus keratitis have reported a protective role of IL-17 (33). In our model of chronic colonization, the development of a Th17 response was associated with a decline in the A. fumigatus fungal burden. Although we did not test the role of the Th17 response in mediating protection to infection in our studies, our findings are more consistent with the hypothesis that Th17 responses are associated with protective immunity against Aspergillus during airway colonization.
We observed that infection with Af beads and, to a lesser extent, sterile agar beads was associated with increased airway reactivity at day 7, which was lost by day 14. This finding coincided well with the rise of IL-4-producing T cells at 7 days of infection. Interestingly, mice infected with Af beads exhibited a decrease in airway responsiveness below baseline at day 28 after infection. This observation parallels the sustained rise in Foxp3+ T cells seen at this time point and may indicate a role for T-regulatory responses in the active downregulation of airway inflammation and hyperreactivity during the resolution of colonization. This hypothesis is consistent with other reports suggesting a role for T-regulatory responses in the suppression of asthmatic responses to inhaled allergens (34, 35).
The immune responses observed in this chronic airway colonization model share several similarities with those reported in patients colonized with A. fumigatus without ABPA. As in this mouse model, a higher number of inflammatory cells, especially neutrophils, are recovered from the BAL fluids of these patients than from those without lower airway disease or those infected with P. aeruginosa alone (36). In addition, other hallmark characteristics of ABPA such as IL-5-mediated eosinophil infiltration into the airway lumen were absent (3). While an early increase in IL-4 levels and a slight increase in total IgE were observed, these were transient and less marked later in infection. It has been suggested that total IgE levels are not specific in differentiating ABPA from colonization, as not all ABPA patients have elevated total IgE levels and increased IgE levels can be observed in some cystic fibrosis (CF) patients with A. fumigatus colonization in the absence of ABPA (37–39). The increase in Th17 cells and IL-17 production observed in this model has also been described in CF patients (40, 41); however, the association between IL-17 and A. fumigatus colonization needs to be further investigated, since these studies did not stratify patients based on A. fumigatus colonization status. Finally, the increase in T-regulatory cells observed during late colonization mirrors a report in which higher percentages of CD4+ Foxp3+ cells were recovered from the blood of CF patients with A. fumigatus colonization without ABPA than from those with ABPA (42).
Although the bead model of chronic airway colonization with A. fumigatus can provide insights into the interactions of A. fumigatus hyphae with the host, there are several important differences between human disease and this mouse model. Healthy mice were used in these experiments, whereas airway colonization with A. fumigatus in humans is normally observed in the context of abnormal airways. In addition, encapsulating the hyphae in agar beads limits hyphal interaction with host cells to emerging hyphae and soluble factors only. This factor may have contributed to the relatively low levels of pulmonary cytokines detected by multiplex enzyme immunoassay (EIA) in lung homogenates. Conversely, however, it is possible that fungi encapsulated within beads may interact with airway cells in a fashion similar to that of hyphae contained within mucus plugs that are seen in chronic airway colonization with A. fumigatus (22, 43). Further, while overt inflammation was not detected in response to infection with agar beads alone, a mild increase in airway hyperreactivity early after inoculation with these beads was observed, suggesting that the agar matrix itself can have subtle immunomodulatory effects. Finally, human airway colonization with A. fumigatus can last for months to years. Although fungal colonization was observed in this model for 28 days, the decline of the fungal burden over this time and the evolution of the inflammatory response are more consistent with a slowly resolving infection.
Our experiments provide the first detailed characterization of the natural history of the immune response to colonization with A. fumigatus hyphae in immunocompetent, nonsensitized mice. These experiments demonstrate that colonization with hyphae induces a chronic inflammatory response associated with early proinflammatory and Th2-type inflammation, which is replaced by Th17 and T-regulatory response later during colonization. This model system may provide the opportunity to study the contribution of candidate host and fungal factors in the pathogenesis of airway colonization with A. fumigatus.
Supplementary Material
ACKNOWLEDGMENTS
We thank Camille Stegen and the Flow Cytometry and Cell Sorting Facility for assistance with the flow cytometry experiments; the facility's infrastructure is supported by the Canada Foundation for Innovation (CFI).
D.C.S. was supported by a Chercheur-Boursier award from the Fonds de la Recherche en Santé du Québec. This work was supported by operating funds from the Canadian Cystic Fibrosis Foundation, the Canadian Institutes of Health Research, and federal funds from the National Institute of Allergy and Infectious Diseases, under contract no. N01-AI-30041 1.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00359-15.
REFERENCES
- 1.Hospenthal DR, Kwon-Chung KJ, Bennett JE. 1998. Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med Mycol 36:165–168. [PubMed] [Google Scholar]
- 2.Latge JP. 1999. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12:310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA, Denning DW, Crameri R, Brody AS, Light M, Skov M, Maish W, Mastella G. 2003. Allergic bronchopulmonary aspergillosis in cystic fibrosis—state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 37(Suppl 3):S225–S264. doi: 10.1086/376525. [DOI] [PubMed] [Google Scholar]
- 4.Rapaka RR, Kolls JK. 2009. Pathogenesis of allergic bronchopulmonary aspergillosis in cystic fibrosis: current understanding and future directions. Med Mycol 47(Suppl 1):S331–S337. doi: 10.1080/13693780802266777. [DOI] [PubMed] [Google Scholar]
- 5.Walker C, Bauer W, Braun RK, Menz G, Braun P, Schwarz F, Hansel TT, Villiger B. 1994. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med 150:1038–1048. doi: 10.1164/ajrccm.150.4.7921434. [DOI] [PubMed] [Google Scholar]
- 6.Knutsen AP, Hutcheson PS, Mueller KR, Slavin RG. 1990. Serum immunoglobulins E and G anti-Aspergillus fumigatus antibody in patients with cystic fibrosis who have allergic bronchopulmonary aspergillosis. J Lab Clin Med 116:724–727. [PubMed] [Google Scholar]
- 7.Kurup VP, Banerjee B, Hemmann S, Greenberger PA, Blaser K, Crameri R. 2000. Selected recombinant Aspergillus fumigatus allergens bind specifically to IgE in ABPA. Clin Exp Allergy 30:988–993. doi: 10.1046/j.1365-2222.2000.00837.x. [DOI] [PubMed] [Google Scholar]
- 8.Chapman BJ, Capewell S, Gibson R, Greening AP, Crompton GK. 1989. Pulmonary eosinophilia with and without allergic bronchopulmonary aspergillosis. Thorax 44:919–924. doi: 10.1136/thx.44.11.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoseyov D, Brownlee KG, Conway SP, Kerem E. 2006. Aspergillus bronchitis in cystic fibrosis. Chest 130:222–226. doi: 10.1378/chest.130.1.222. [DOI] [PubMed] [Google Scholar]
- 10.Kurup VP, Seymour BW, Choi H, Coffman RL. 1994. Particulate Aspergillus fumigatus antigens elicit a TH2 response in BALB/c mice. J Allergy Clin Immunol 93:1013–1020. doi: 10.1016/S0091-6749(94)70050-8. [DOI] [PubMed] [Google Scholar]
- 11.Grunig G, Corry DB, Leach MW, Seymour BW, Kurup VP, Rennick DM. 1997. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J Exp Med 185:1089–1099. doi: 10.1084/jem.185.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurup VP, Guo J, Murali PS, Choi H, Fink JN. 1997. Immunopathologic responses to Aspergillus antigen in interleukin-4 knockout mice. J Lab Clin Med 130:567–575. doi: 10.1016/S0022-2143(97)90106-2. [DOI] [PubMed] [Google Scholar]
- 13.Kurup VP, Murali PS, Guo J, Choi H, Banerjee B, Fink JN, Coffman RL. 1997. Anti-interleukin (IL)-4 and -IL-5 antibodies downregulate IgE and eosinophilia in mice exposed to Aspergillus antigens. Allergy 52:1215–1221. doi: 10.1111/j.1398-9995.1997.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 14.Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. 1998. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med 4:344–355. doi: 10.1007/s008940050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilbault C, Martin P, Houle D, Boghdady ML, Guiot MC, Marion D, Radzioch D. 2005. Cystic fibrosis lung disease following infection with Pseudomonas aeruginosa in Cftr knockout mice using novel non-invasive direct pulmonary infection technique. Lab Anim 39:336–352. doi: 10.1258/0023677054306944. [DOI] [PubMed] [Google Scholar]
- 16.Nawada R, Amitani R, Tanaka E, Niimi A, Suzuki K, Murayama T, Kuze F. 1996. Murine model of invasive pulmonary aspergillosis following an earlier stage, noninvasive Aspergillus infection. J Clin Microbiol 34:1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Gravelat FN, Chiang LY, Chen D, Vanier G, Ejzykowicz DE, Ibrahim AS, Nierman WC, Sheppard DC, Filler SG. 2010. Aspergillus fumigatus AcuM regulates both iron acquisition and gluconeogenesis. Mol Microbiol 78:1038–1054. doi: 10.1111/j.1365-2958.2010.07389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheppard DC, Marr KA, Fredricks DN, Chiang LY, Doedt T, Filler SG. 2006. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis. Clin Microbiol Infect 12:376–380. doi: 10.1111/j.1469-0691.2005.01349.x. [DOI] [PubMed] [Google Scholar]
- 19.Al-Bader N, Vanier G, Liu H, Gravelat FN, Urb M, Hoareau CM, Campoli P, Chabot J, Filler SG, Sheppard DC. 2010. Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence. Infect Immun 78:3007–3018. doi: 10.1128/IAI.00813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogaboam CM, Blease K, Mehrad B, Steinhauser ML, Standiford TJ, Kunkel SL, Lukacs NW. 2000. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am J Pathol 156:723–732. doi: 10.1016/S0002-9440(10)64775-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurup VP, Mauze S, Choi H, Seymour BW, Coffman RL. 1992. A murine model of allergic bronchopulmonary aspergillosis with elevated eosinophils and IgE. J Immunol 148:3783–3788. [PubMed] [Google Scholar]
- 22.Bosken CH, Myers JL, Greenberger PA, Katzenstein AL. 1988. Pathologic features of allergic bronchopulmonary aspergillosis. Am J Surg Pathol 12:216–222. doi: 10.1097/00000478-198803000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Murdock BJ, Shreiner AB, McDonald RA, Osterholzer JJ, White ES, Toews GB, Huffnagle GB. 2011. Coevolution of Th1, Th2, and Th17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect Immun 79:125–135. doi: 10.1128/IAI.00508-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoselton SA, Samarasinghe AE, Seydel JM, Schuh JM. 2010. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Med Mycol 48:1056–1065. doi: 10.3109/13693786.2010.485582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. 2005. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog 1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontaine T, Delangle A, Simenel C, Coddeville B, van Vliet SJ, van Kooyk Y, Bozza S, Moretti S, Schwarz F, Trichot C, Aebi M, Delepierre M, Elbim C, Romani L, Latge JP. 2011. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog 7:e1002372. doi: 10.1371/journal.ppat.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, Xu W, Kravtsov I, Hoareau CM, Vanier G, Urb M, Campoli P, Al Abdallah Q, Lehoux M, Chabot JC, Ouimet MC, Baptista SD, Fritz JH, Nierman WC, Latge JP, Mitchell AP, Filler SG, Fontaine T, Sheppard DC. 2013. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal beta-glucan from the immune system. PLoS Pathog 9:e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gravelat FN, Doedt T, Chiang LY, Liu H, Filler SG, Patterson TF, Sheppard DC. 2008. In vivo analysis of Aspergillus fumigatus developmental gene expression determined by real-time reverse transcription-PCR. Infect Immun 76:3632–3639. doi: 10.1128/IAI.01483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shreiner AB, Murdock BJ, Sadighi Akha AA, Falkowski NR, Christensen PJ, White ES, Hogaboam CM, Huffnagle GB. 2012. Repeated exposure to Aspergillus fumigatus conidia results in CD4+ T cell-dependent and -independent pulmonary arterial remodeling in a mixed Th1/Th2/Th17 microenvironment that requires interleukin-4 (IL-4) and IL-10. Infect Immun 80:388–397. doi: 10.1128/IAI.05530-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 32.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. 2009. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor PR, Leal SM Jr, Sun Y, Pearlman E. 2014. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J Immunol 192:3319–3327. doi: 10.4049/jimmunol.1302235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, Wenzel SE, Moore ML, Peebles RS Jr, Ray A, Ray P. 2012. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med 18:1525–1530. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Wu X, Zhu H, Xie Y, Tang S, Jiang Y. 2015. FOXP3(+)Treg/Th17 cell imbalance in lung tissues of mice with asthma. Int J Clin Exp Med 8:4158–4163. [PMC free article] [PubMed] [Google Scholar]
- 36.Regamey N, Tsartsali L, Hilliard TN, Fuchs O, Tan HL, Zhu J, Qiu YS, Alton EW, Jeffery PK, Bush A, Davies JC. 2012. Distinct patterns of inflammation in the airway lumen and bronchial mucosa of children with cystic fibrosis. Thorax 67:164–170. doi: 10.1136/thoraxjnl-2011-200585. [DOI] [PubMed] [Google Scholar]
- 37.Skov M, McKay K, Koch C, Cooper PJ. 2005. Prevalence of allergic bronchopulmonary aspergillosis in cystic fibrosis in an area with a high frequency of atopy. Respir Med 99:887–893. doi: 10.1016/j.rmed.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Becker JW, Burke W, McDonald G, Greenberger PA, Henderson WR, Aitken ML. 1996. Prevalence of allergic bronchopulmonary aspergillosis and atopy in adult patients with cystic fibrosis. Chest 109:1536–1540. doi: 10.1378/chest.109.6.1536. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz RH, Hollick GE. 1981. Allergic bronchopulmonary aspergillosis with low serum immunoglobulin E. J Allergy Clin Immunol 68:290–294. doi: 10.1016/0091-6749(81)90154-8. [DOI] [PubMed] [Google Scholar]
- 40.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. 2011. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med 184:252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, Pirhonen J, Kolls JK. 2005. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, Alcorn JF, Vyas YM, Aujla SJ, Finelli P, Blanchard M, Zeigler SF, Logar A, Hartigan E, Kurs-Lasky M, Rockette H, Ray A, Kolls JK. 2010. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest 120:3242–3254. doi: 10.1172/JCI42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zander DS. 2005. Allergic bronchopulmonary aspergillosis: an overview. Arch Pathol Lab Med 129:924–928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.