Abstract
Background
To compare life span of persons with and without ocular pseudoexfoliation syndrome (PES).
Methods
The study is based on an epidemiological survey conducted in Sør-Trøndelag county, Norway, in 1985–86. All inhabitants over 64 years of age (2109 individuals) were invited. Mortality information was obtained from The Norwegian Institute of Public Health in 2014, by which time 99% of the participants were deceased.
Results
When adjusting for age and gender, life span was not statistically different in persons with and without PES. Following the diagnosis of PES, patients' survival was up to, and beyond, 30 years.
Conclusions
Our observations suggest that, despite all the systemic aberrations reported in persons with ocular PES, none or only marginal functional changes are caused in extraocular organs and tissues. The present study supports the notion that systemic PES is not a life-threatening condition.
Keywords: Cardiovascular disease, Eye, Life span, Mortality, Ophthalmology, Pseudoexfoliation syndrome, Survey
Highlights
-
•
Over the years several attempts have been made to link pseudoexfoliation syndrome to a number of serious systemic diseases.
-
•
The present study shows no difference in life span between persons with and without pseudoexfoliation in our cohort.
1. Introduction
The classical finding of the pseudoexfoliation syndrome (PES) in the eye, as observed by slit-lamp examination, is characterized by a gray, granular material on the anterior lens surface and along the pupillary border. The first description (Lindberg, 1989) was followed by numerous clinical and morphological studies of the various ocular aspects of the condition over the following decades. For almost sixty years, PES was regarded as being a purely intraocular disease with capsular glaucoma as one of the complications in a proportion of patients.
The view of PES being a purely intraocular disease was challenged by the discovery that the intraocular deposits have their counterparts in various extraocular connective tissues (Ringvold, 1973a, Ritch and Schlötzer-Schrehardt, 2001). PES was subsequently perceived as a connective tissue disorder, whereby the PE-subunits consist of a core protein of aberrant elastic microfibrills (Ringvold, 1973b, Streeten et al., 1986, Thorleifsson et al., 2007) surrounded by various glycoconjugates (Davanger, 1978, Huflejt et al., 2014, Konstas et al., 1990). However, it should be mentioned that immunohistochemical analysis has indicated differences in the carbohydrate composition between intra- and extraocular PE deposits (Hietanen et al., 1995).
Clinically, a number of observations indicate that PES may have functional implications beyond the eye (Bettis et al., 2014, Katsi et al., 2013, Tarkkanen et al., 2008), although opinion is divided with regards to its significance (Andrikopoulos et al., 2014, Gonen et al., 2013, Hietanen et al., 2002, Ringvold, 2001, Schumacher et al., 2001, Tarkkanen, 2008). Recently, the association between ocular PES and vascular disease has been evaluated in a meta-analysis based on sixteen epidemiologic studies (Wang et al., 2014). The authors listed a number of limitations in the included papers while concluding that, overall, the current literature suggests that PES is associated with increased risk of vascular disease. Despite this and numerous morphological, biochemical, genetic, and clinical observations, the question of whether or not PES generates systemic disease, has still not been concluded.
A different approach to evaluate the relationship between PES and possible systemic effects is to study mortality. In general, serious systemic disorders are likely to influence life expectancy. Previous studies have not showed any association between PES and overall mortality (Grødum et al., 2004, Ringvold et al., 1997, Ritland et al., 2004, Shrum et al., 2000, Svensson and Ekström, 2014, Tarkkanen and Kivelä, 2014). If PES has no effect on mortality, affected people will, in general, reach or exceed life expectancy. Interestingly, our study from 1997 indicated “persons with ocular PES are older than those without.” This result was obtained 12 years after the initial examination — with 52% of the participants having died. The present study is a follow-up study, comparing the life span of PES-positive and PES-negative persons, 29 years following the initial observation of PES.
2. Material and methods
This study has been based on figures from the epidemiological survey conducted in three municipalities (Hitra, Holtålen, Rennebu) in the county of Sør-Trøndelag, Norway in 1985–86 (Ringvold et al., 1988). All inhabitants above 64 years of age were invited (2109 persons), 1888 (1018 women, 870 men) were examined, while 221 had chosen not to participate. Informed consent was obtained according to the Helsinki declaration. Conventional slit-lamp examination was performed on a dilated pupil to examine for PES. Due to various ocular diseases (corneal opacities, enucleation, etc.) 43 persons had only one eye examined. “PES-positive” indicated PES in at least one eye.
After approval from the Regional Ethical Committee, mortality information was obtained from The Norwegian Institute of Public Health; As at 01.10.14, all of the 2109 individuals were registered as being alive or deceased. Where appropriate, date of death was documented.
3. Statistical analysis
Results on continuous variables are presented as means and standard deviations, or as medians when appropriate. Results on categorical variables are presented as numbers and percentages.
For the comparison of mean age in two groups, an independent samples t-test was employed, while a chi-square test was used to compare percentages.
Cox regression analysis was used for the analysis of the association between PES (yes vs. no) and mortality, with adjustment for gender and age. Results from this analysis are presented as hazard ratios with 95% confidence intervals and p-values.
Cox regression analysis is also used when comparing the mortality among those who participated in the study with those who were not willing to participate.
The proportional hazard assumptions of the Cox regression analysis was checked for both of these models and found to be adequately met. This assumption was checked by visual inspection of the log–log Kaplan–Meier curves for the groups defined by the independent variables in the models. For each variable in each model the distances between the log–log curves were found to be constant over time.
In order to compare the mortality pattern among the participants with PES with the corresponding mortality pattern during the same time interval among the inhabitants in the Sør-Trøndelag county (306197 inhabitants by 01.01.2014), we calculated the standardized mortality ratio for these two populations, with 95% confidence interval.
A significance level of 5% was used. The statistical analysis was performed by using the software program IBM-SPSS version 20.
4. Results
The clinical screening was conducted in 1985–86. Patient life span was assessed as at 01.10.2014. Of the 1888 examined and 221 not-examined individuals, 98.6% and 98.8% respectively, were deceased by this time.
4.1. Concerning the 1888 examined persons
PES was found in 319 people (16.9%). The prevalence was significantly higher in females than males (19.1% vs. 14.4%; Pearson's chi-square test; p = 0.007), and increased markedly with age (Pearson's chi-square test; p < 0.001), from 11% in the lowest age group to 39% in the highest (Table 1). The collective mean age of both PES positive and negative groups at the time of examination was 74.5 years (range 65–98 years), significantly higher among PES-positive group (77.2 vs. 74.0 years; independent samples t-test; p < 0.001). The relative risk of death when comparing participants with and without PES (reference group) was 1.01 (95% CI: 0.89–1.13; Cox regression analysis; p = 0.932), age and gender adjusted.
Table 1.
PES prevalence in 3 different municipalities in Sør-Trøndelag county, Norway. Examination was performed in 1985–86, and 1888 of 2109 persons above 64 years of age were examined.
| Age groups (years) | PES-negative | PES-positive | Total |
|---|---|---|---|
| 65–69 | 447 (89.0%) | 55 (11.0%) | 502 (100%) |
| 70–74 | 482 (87.2%) | 71 (12.8%) | 553 (100%) |
| 75–79 | 337 (81.6%) | 76 (18.4%) | 413 (100%) |
| 80–84 | 193 (76.0%) | 61 (24.0%) | 254 (100%) |
| 85–89 | 81 (68.6%) | 37 (31.4%) | 118 (100%) |
| 90–98 | 29 (60.4%) | 19 (39.6%) | 48 (100%) |
| Total | 1569 (83.1%) | 319 (16.9%) | 1888 (100%) |
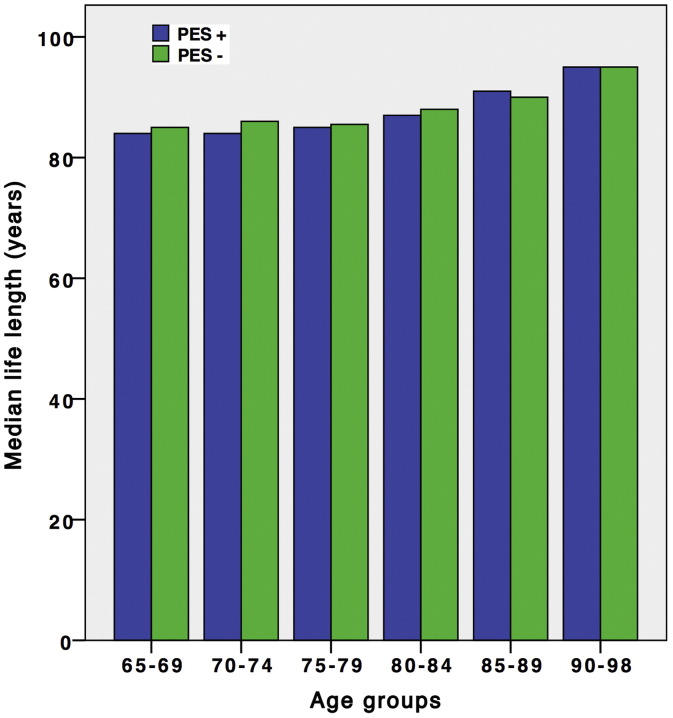
As shown in Fig. 1, median life span as at 01.10.2014 was similar among participants with and without PES, regardless of age at time of observation.
Fig. 1.
Bar chart showing median life length in PES-negative and PES-positive age groups by 01.10.2014, i.e. 29 years after examination. The differences are not statistically significant.
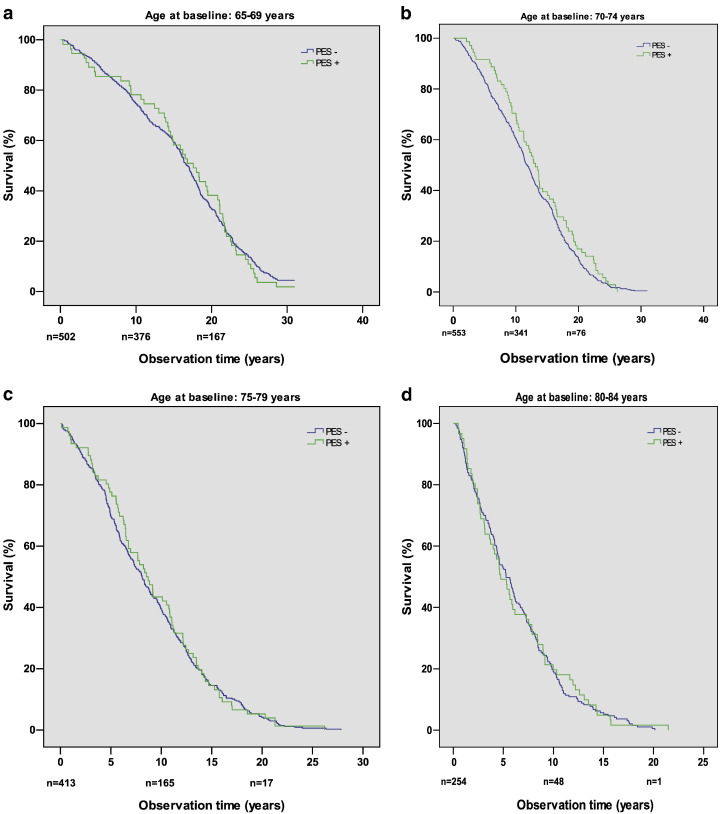
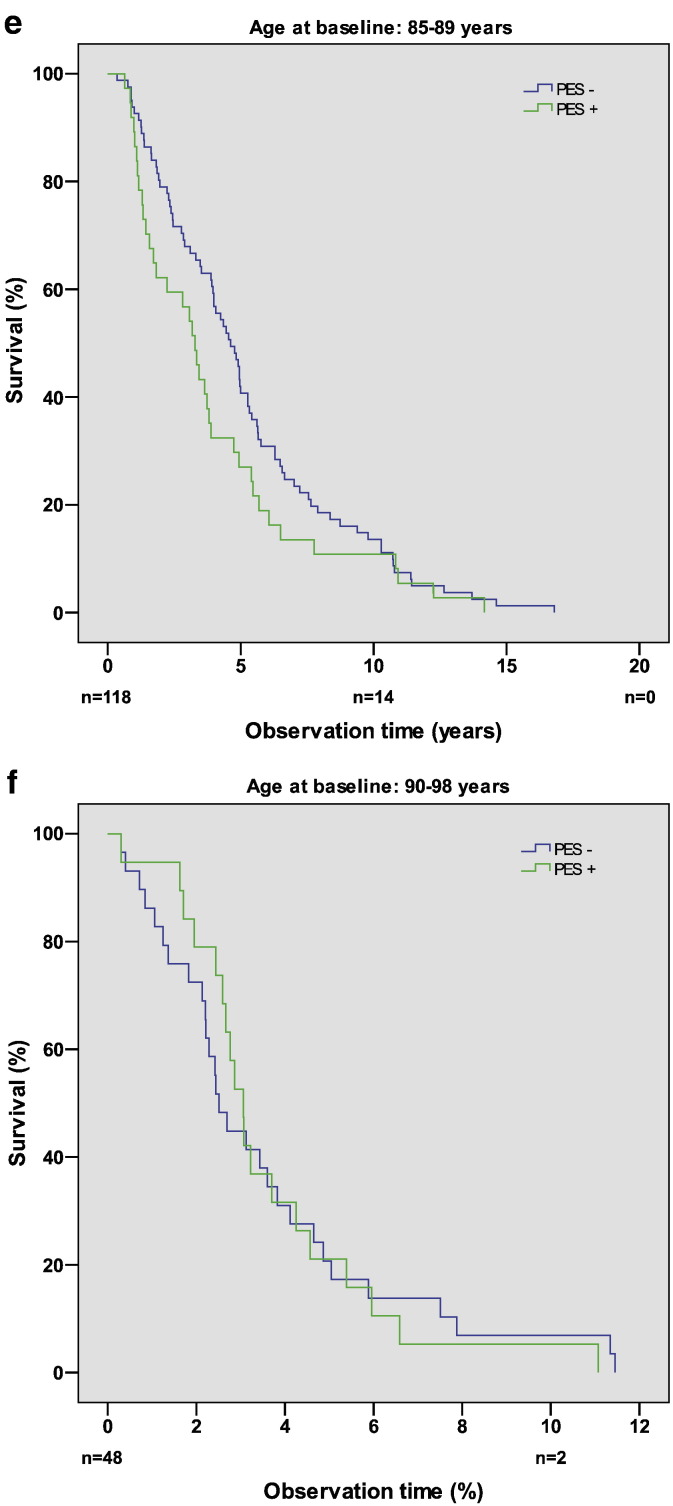
The survival function of PES-positive and PES-negative persons in the age group 65–69 years is shown in Fig. 2a. Similar curves were found in the other age groups, and the differences between the two curves were not statistically significant in any of the age groups (Fig. 2b-f).
Fig. 2.
Kaplan–Meier plots showing survival function of PES-negative and PES-positive persons in the age groups (a) 65–69 years, (b) 70–74 years, (c) 75–79 years, (d) 80–84 years, (e) 85–89 years, and (f) 90–98 years at time of examination (1985–1986). n denotes the number of persons at risk at the time points indicated. The differences between the two curves are not statistically significant.
When comparing the mortality pattern from 1985–86 to 2014 among the 319 PES-positive persons with the corresponding pattern in the population above 64 years of age from the whole county of Sør-Trøndelag, a standardized mortality ratio of 0.98 (95% CI: 0.88–1.09) was found.
In total, 55 PES-positive cases were identified in the 65–69 years old group, of which 21 persons were PES-positive for more than 20 years before their death. Interestingly, one PES-positive person in this group was still alive on 01.03.15 (97 years old), i.e. 30 years after the diagnosis of PES was made.
4.2. Concerning the 221 not-examined persons
Mean age at time of invitation (i.e. 1985–86) was 76.6 (SD 7.1) years in not-examined individuals versus 74.5 (SD 6.5) years in the examined group (1888 people). This difference was statistically significant (independent samples t-test; p < 0.001). Regardless of the presence of PES, the relative risk of death, when comparing inhabitants who participated with those who did not (reference group), was 0.79 (95% CI: 0.69–0.91), after adjustment for age and gender.
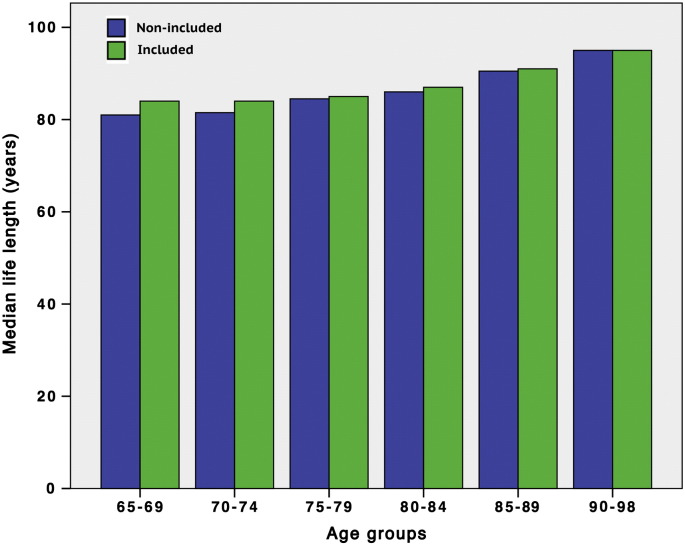
The life span of the 1888 participants versus the 221 non-participants at 01.10.2014 is shown in Fig. 3. Median life span was slightly reduced in the two youngest, non-participating groups (65–69 years; Mann–Whitney test; p = 0.030, and 70–74 years; p = 0.023), whereas the remaining 4 groups showed no difference. The two youngest groups, PES-positive and PES-negative collectively, consisted of 44 and 46 persons, respectively.
Fig. 3.
Bar chart showing median life length in 221 not-examined persons (blue) versus 1888 examined persons (green) by 01.10.2014, i.e. 29 years after the files were collected. The two youngest age groups of the not-examined population (65–69; p < 0.03, and 70–74; p < 0.023) showed slightly reduced life length.
5. Discussion
According to previous studies, mortality is not increased in PES-positive people (Grødum et al., 2004, Ringvold et al., 1997, Ritland et al., 2004, Shrum et al., 2000, Svensson and Ekström, 2014). The aim of the present study was to compare the life span of PES-positive to PES-negative persons. No differences were found between the two groups. This finding was supported by standardized mortality ratio analysis, comparing PES-positive persons with the population of the Norwegian county Sør-Trøndelag.
A different approach to elucidating this issue is to evaluate how long PES-positive persons actually stay alive after debut of the syndrome. Few PES-positive cases were found below 69 years of age in the area surveyed (Table 1). Fig. 1 demonstrates that a number of PES-positive persons in the youngest group (65–69 years) survived at least 20 years after diagnosis. Furthermore, the survival functions in PES-positive and PES-negative persons turned out to be similar (Fig. 2).
PES probably had no impact on life span in the examined population (1888 individuals). With regards to the 221 non-participants (Fig. 3), the slightly reduced life span in the two youngest groups may indicate increased morbidity. Assuming that the PES prevalence pattern is similar among the examined and not-examined persons, i.e. increasing with age, it is noteworthy that reduced life span in the not-examined population is confined to the two youngest age groups with the lowest PES-positive numbers, whereas the remaining four age groups, with significantly higher PES prevalence, showed normal life span. It can be concluded, therefore, that PES is not responsible for a reduced life span.
Taken together, these observations (normal life span along with normal mortality rates in PES-positive people) seem to be inconsistent with most of the recent literature for two reasons:
-
1)
A large number of studies have been presented indicating impaired circulation in various tissues and vital organs in persons with PES (Akarsu and Ünal, 2005, Atalar et al., 2006, Andrikopoulos et al., 2014, Cumurcu et al., 2013, Djordjevic-Jocic et al., 2012, French et al., 2012, Gonen et al., 2013, Katsi et al., 2013, Mitchell et al., 1997, Praveen et al., 2011, Turacli et al., 2007, Visontai et al., 2008). It is unlikely that increased prevalence of serious diseases, like heart attack and stroke, would not influence mortality and life span. Importantly, it should also be added that, although most reports indicate compromised circulation in PES-positive people, some showed no difference (Tarkkanen et al., 2008).
-
2)
Hyperhomocysteinemia in PES-positive persons has been confirmed (Leibovitch et al., 2003, Vessani et al., 2003). In addition, it has been shown that plasma homocysteine levels correlate directly with the prevalence of various arterial occlusive diseases (Boushey et al., 1995, Konecky et al., 1997). Furthermore, there is an association between raised plasma concentration of homocysteine and long-term mortality in patients with coronary artery disease (Martín-Herrero et al., 2007), and also between hyperhomocysteinema and stroke (Hankey and Eikelboom, 2001). Consequently, the reported hyperhomocysteinemia in PES-positive individuals should tend to increase mortality and reduce life span.
The finding that PES had no effect on life span does not necessarily mean that PES has no effect on cause of death. In fact, PES could cause increased vascular-related mortality if it reduced other causes of death. Moreover, our findings only apply to the population studied, and we cannot exclude a different result in populations with different genetic background. Furthermore, these findings only apply to persons that were older than 64 years at enrollment of the study. As the prevalence of pseudoexfoliation increases with age, new cases of pseudoexfoliation may appear in all groups in our material.
The association between PES and a number of systemic diseases listed above is noteworthy. However, despite all the reported pathological aberrations in PES-positive persons, none or only marginal functional changes are induced by PES in extraocular organs and tissues. It is also noteworthy that PES-positive people can live up to and beyond 30 years following their diagnosis. We conclude that systemic PES is not a life threatening condition, as PES-positive persons do not seem to die from, but rather with this disorder.
Author contributions
Jon Klokk Slettedal, M.D., Ph.D., is a senior consultant of ophthalmology and associate professor. He contributed to the design, took part in analysis and interpretation of data, drafting and revising the manuscript critically for intellectual content, approved the final version and has agreed to be accountable for all aspects of the work.
Leiv Sandvik, Ph.D. is an epidemiologist and senior statistician. He contributed to design, analysis and interpretation of data, revising the manuscript critically for intellectual content, approved the final version and has agreed to be accountable for all aspects of the work.
Amund Ringvold, M.D., Ph.D., is a professor of ophthalmology and retired senior consultant. He contributed to the conception and design, did the survey in 1985–86, performed analysis and interpretation of data, drafting and revising the manuscript critically for intellectual content, approved the final version and has agreed to be accountable for all aspects of the work.
Funding
None.
References
- Akarsu C., Ünal B. Cerebral haemodynamics in patients with pseudoexfoliation glaucoma. Eye. 2005;19:1297–1300. doi: 10.1038/sj.eye.6701776. [DOI] [PubMed] [Google Scholar]
- Andrikopoulos G.K., Alexopoulos D.K., Gartaganis S.P. Pseudoexfoliation and cardiovascular diseases. World J. Cardiol. 2014;6(8):847–854. doi: 10.4330/wjc.v6.i8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalar P.T., Atalar E., Kilic H., Abbasoglu Ö.E., Ozer N., Aksöyek S., Övünc K., Ozmen F., Gürsel E. Impaired systemic endothelial function in patients with pseudoexfoliation syndrome. Int. Heart J. 2006;47:77–84. doi: 10.1536/ihj.47.77. [DOI] [PubMed] [Google Scholar]
- Bettis B.I., Allingham R.R., Wirostko B.M. Systemic diseases associated with exfoliation syndrome. Int. Ophthalmol. Clin. 2014;54(4):15–28. doi: 10.1097/IIO.0000000000000044. [DOI] [PubMed] [Google Scholar]
- Boushey C.J., Beresford S.A., Omenn G.S., Motulsky A.G. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- Cumurcu T., Dorak F., Cumurcu B.E., Erbay L.G., Ozsoy E. Is there any relation between pseudoexfoliation syndrome and Alzheimer's type dementia? Semin. Ophthalmol. 2013;28:224–229. doi: 10.3109/08820538.2013.793726. [DOI] [PubMed] [Google Scholar]
- Davanger M. On the interfibrillar matrix of the pseudoexfoliation material. Acta Ophthalmol. (Copenh) 1978;56:233–240. doi: 10.1111/j.1755-3768.1978.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Djordjevic-Jocic J., Jovanovic P., Bozic M., Tasic A., Rancic Z. Prevalence and early detection of abdominal aortic aneurysm in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Curr. Eye Res. 2012;37:617–623. doi: 10.3109/02713683.2012.665120. [DOI] [PubMed] [Google Scholar]
- French D.D., Margo C.E., Harman L.E. Ocular pseudoexfoliation and cardiovascular disease: a national cross-section comparison study. N. Am. J. Med. Sci. 2012;4:468–473. doi: 10.4103/1947-2714.101987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen K.A., Gonen T., Gumus B. Retinal artery stenosis and abdominal aorta aneurysm in patients with pseudoexfoliation syndrome. Eye. 2013;27:735–741. doi: 10.1038/eye.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grødum K., Heijl A., Bengtsson B. Glaucoma and mortality. Graefes Arch. Clin. Exp. Ophthalmol. 2004;242:397–401. doi: 10.1007/s00417-004-0858-2. [DOI] [PubMed] [Google Scholar]
- Hankey G.J., Eikelboom J.W. Homocysteine and stroke. Curr. Opin. Neurol. 2001;14:95–102. doi: 10.1097/00019052-200102000-00015. [DOI] [PubMed] [Google Scholar]
- Hietanen J., Uusitalo M., Tarkkanen A., Kivelä T. Lectin and immunohistochemical comparison of glycoconjugates in the conjunctiva of patients with and without exfoliation syndrome. Brit. J. Ophthalmol. 1995;79:467–472. doi: 10.1136/bjo.79.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen J., Soisalon-Soininen S., Kivelä T., Tarkkanen A. Evaluation of the clinical association between exfoliation syndrome and abdominal aortic aneurism. Acta Ophthalmol. Scand. 2002;80:617–619. doi: 10.1034/j.1600-0420.2002.800611.x. [DOI] [PubMed] [Google Scholar]
- Huflejt M.E., Preiss J.S., Thomson J.E., Gils I.M., Vuskovic M.I. Glycomics, extracellular matrix, and anti-glycan antibodies in exfoliation syndrome. J. Glaucoma. 2014;23:S24–S29. doi: 10.1097/IJG.0000000000000118. [DOI] [PubMed] [Google Scholar]
- Katsi V., Pavlidis A.N., Kallistratos M.S., Fitsios A., Bratsas A., Tousoulis D., Stefanadis C., Manolis A.J., Kallikazaros I. Cardiovascular repercussions of the pseudoexfoliation syndrome. N. Am. J. Med. Sci. 2013;5:454–459. doi: 10.4103/1947-2714.117294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecky N., Malinow M.R., Tunick P.A., Freedberg R.S., Rosenzweig B.P., Katz E.S., Hess D.L., Upson B., Leung B., Perez J. Correlation between plasma homocysteine and aortic atherosclerosis. Am. Heart J. 1997;133:534–550. doi: 10.1016/s0002-8703(97)70148-0. [DOI] [PubMed] [Google Scholar]
- Konstas A.G., Marshall G.E., Lee W.R. Immunogold localization of laminin in normal and exfoliative iris. Br. J. Ophthalmol. 1990;74:450–457. doi: 10.1136/bjo.74.8.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitch I., Kurtz S., Shemesh G., Goldstein M., Sela B.A., Lazar M., Loewenstein A. Hyperhomocystinemia in pseudoexfoliation glaucoma. J. Glaucoma. 2003;12(1):36–39. doi: 10.1097/00061198-200302000-00007. [DOI] [PubMed] [Google Scholar]
- Lindberg J.G. Clinical investigations on depigmentation of the pupillary border and translucency of the iris (1917) Acta Ophthalmol. 1989;67(Suppl. 190):1–96. [PubMed] [Google Scholar]
- Martín-Herrero F., Martín-Moreiras J., Pabón P., Sánchez P.L., Moríñigo-Muñoz J.L., Jimenez-Candil J., Cruz-González I., Alberca I., González-Porras J.R., Martín-Luengo C. Homocysteine and outcome in young patients with acute coronary syndromes. Int. J. Cardiol. 2007;118:183–188. doi: 10.1016/j.ijcard.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Wang J.J., Smith W. Association of pseudoexfoliation syndrome with increased vascular risk. Am J. Ophthalmol. 1997;124:685–687. doi: 10.1016/s0002-9394(14)70908-0. [DOI] [PubMed] [Google Scholar]
- Praveen M.R., Shah S.K., Vasavada A.R., Diwan R.P., Shah S.M., Zumkhawala B.R., Thomas R. Pseudoexfoliation as a risk factor for peripheral vascular disease: a case-control study. Eye. 2011;25:174–179. doi: 10.1038/eye.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringvold A. On the occurrence of pseudo-exfoliation material in extrabulbar tissue from patients with pseudo-exfoliation syndrome of the eye. Acta Ophthalmol. (Copenh) 1973;51:411–418. doi: 10.1111/j.1755-3768.1973.tb06018.x. [DOI] [PubMed] [Google Scholar]
- Ringvold A. A preliminary report on the amino acid composition of the pseudo-exfoliation material (PE material) Exp. Eye Res. 1973;15:37–42. doi: 10.1016/0014-4835(73)90187-5. [DOI] [PubMed] [Google Scholar]
- Ringvold A. Pseudoexfoliation and aortic aneurysms. Lancet. 2001;357:2139. doi: 10.1016/S0140-6736(00)05213-2. [DOI] [PubMed] [Google Scholar]
- Ringvold A., Blika S., Elsås T., Guldahl J., Brevik T., Hesstvedt P., Johnsen H., Hoff K., Høisen H., Kjørsvik S. The Middle-Norway eye-screening study. I. Epidemiology of the pseudo-exfoliation syndrome. Acta Ophthalmol. 1988;66:652–658. doi: 10.1111/j.1755-3768.1988.tb04056.x. [DOI] [PubMed] [Google Scholar]
- Ringvold A., Blika S., Sandvik L. Pseudoexfoliation and mortality. Acta Ophthalmol. Scand. 1997;75:255–256. doi: 10.1111/j.1600-0420.1997.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Ritch R., Schlötzer-Schrehardt U. Exfoliation syndrome. Surv. Ophthalmol. 2001;45:265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Ritland J.S., Egge K., Lydersen S., Juul R., Semb S.O. Exfoliative glaucoma and primary open-angle glaucoma: associations with death causes and comorbidity. Acta Ophthalmol. Scand. 2004;82:401–404. doi: 10.1111/j.1395-3907.2004.00297.x. [DOI] [PubMed] [Google Scholar]
- Schumacher S., Schlötzer-Schrehardt U., Martus P., Lang W., Naumann G.O.H. Pseudoexfoliation syndrome and aneurisms of the abdominal aorta. Lancet. 2001;357:359–360. doi: 10.1016/s0140-6736(00)03645-x. [DOI] [PubMed] [Google Scholar]
- Shrum K.R., Hattenhauer M.G., Hodge D. Cardiovascular and cerebrovascular mortality associated with ocular pseudoexfoliation. Am J. Ophthalmol. 2000;129:83–86. doi: 10.1016/s0002-9394(99)00255-x. [DOI] [PubMed] [Google Scholar]
- Streeten B.W., Gibson S.A., Dark A.J. Pseudoexfoliation material contains an elastic microfibrillar-associated glycoprotein. Trans. Am. Ophthalmol. Soc. 1986;84:304–320. [PMC free article] [PubMed] [Google Scholar]
- Svensson R., Ekström C. Pseudoexfoliation and mortality: a population-based 30-year follow-up study. Acta Ophthalmol. 2014;92:1–3. doi: 10.1111/aos.12402. [DOI] [PubMed] [Google Scholar]
- Tarkkanen A. Is exfoliation syndrome a sign of systemic vascular disease? Acta Ophthalmol. 2008;86:832–836. doi: 10.1111/j.1755-3768.2008.01464.x. [DOI] [PubMed] [Google Scholar]
- Tarkkanen A., Kivelä T.T. Mortality in primary open-angle glaucoma and exfoliative glaucoma. Eur. J. Ophthalmol. 2014;24(5):718–721. doi: 10.5301/ejo.5000450. [DOI] [PubMed] [Google Scholar]
- Tarkkanen A., Reunanen A., Kivelä T. Frequency of systemic vascular disease in patients with primary open angle glaucoma and exfoliation glaucoma. Acta Ophthalmol. 2008;86:598–602. doi: 10.1111/j.1600-0420.2007.01122.x. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G., Magnusson K.P., Sulem P., Walters G.B., Gudbjartsson D.F., Stefansson H., Jonsson T., Jonasdottir A., Jonasdottir A., Stefansdottir G. Common sequence variants in the LOXL 1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- Turacli M.E., Özdemir F.A., Tekeli O., Gökcan K., Gerceker M., Dürük K. Sensorineural hearing loss in pseudoexfoliation. Can. J. Ophthalmol. 2007;42:56–59. [PubMed] [Google Scholar]
- Vessani R.M., Ritch R., Liebmann J.M., Jofe M. Plasma homocycteine is elevated in patients with exfoliation syndrome. Am J. Ophthalmol. 2003;136:41–46. doi: 10.1016/s0002-9394(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Visontai Z., Horvath T., Kollai M., Hollo G. Decreased cardiovagal regulation in exfoliation syndrome. J. Glaucoma. 2008;17:133–138. doi: 10.1097/IJG.0b013e3181379d67. [DOI] [PubMed] [Google Scholar]
- Wang W., He M., Zhou M., Zhang X. Ocular pseudoexfoliation syndrome and vascular disease: a systematic review and meta-analysis. Plos One. 2014;9:1–7. doi: 10.1371/journal.pone.0092767. [DOI] [PMC free article] [PubMed] [Google Scholar]