Abstract
Chronic fibro-proliferative diseases are associated with nearly 45% of all deaths in the developed world. Matrix metalloproteinase (MMP) mediated remodeling of the extracellular matrix (ECM) plays an important role in disease development. Degradation of type I collagen is considered having a major role in this matter. C1M is a biomarker measuring type I collagen degradation fragments in blood. The aim of the current study was to investigate whether MMP mediated type I collagen degradation (C1M) was predictive of mortality in a large prospective cohort of Danish women aged 48–89 (n = 5855).
Subjects with high serum C1M showed significant increased mortality. The adjusted three year HR was 2.02 [95% CI: 1.48–2.76] for all-cause mortality, 2.32 [95% CI: 1.51–3.56] for cancer and 1.77 [95% CI: 0.98–3.17] for cardiovascular diseases. The adjusted nine year HR was 1.50 [95% CI: 1.28–1.75] for all-cause mortality, 1.49 [95% CI: 1.16–1.90] for cancer and 1.69 [95% CI: 1.27–2.24] for cardiovascular diseases.
High MMP-mediated type I collagen degradation was associated with increased mortality. Subjects with high C1M had a 2-fold increase in mortality compared to subjects with low levels of this collagen degradation product.
Abbreviations: C1M, MMP-mediated type I collagen degradation; CTX-I, Cathepsin K degraded products of C-terminal telopeptides of type I collagen; ECM, Extracellular matrix; MMP, Matrix metalloproteinase; PERF I, Prospective Epidemiological Risk Factor study; PTM, Post-translational modification
Keywords: Extracellular matrix remodeling, Clinical, Type I collagen, Mortality, MMP, Protease activity
Highlights
-
•
High MMP-mediated type I collagen degradation is an independent risk factor associated with a 2-fold increase in mortality.
-
•
A 2.3-fold increase in cancer mortality was found for subjects with high MMP-mediated type I collagen degradation.
-
•
Specific enzymatic processing of type I collagen is essential since only C1M and not CTX-I was associated with mortality.
1. Introduction
It is estimated that women in the European (EU15) countries are expected to live approximately 80% of their lives in good health — resulting in a healthy life expectancy up to 20% shorter than their total life expectancy (Health and Consumer Protection Directorate-General, 2006). A major contributor to the decrease in healthy life expectancy is chronic fibroproliferative diseases such as fibrosis and cancer, and nearly 45% of all deaths in the developed world are associated with some form of tissue remodeling disease (Wynn, 2007, Pinzani, 2008). Tissue remodeling in relation to diagnosis and prognosis is therefore a hot topic, consequent to the prevalence of diseases associated with this remodeling, following decreased healthy life expectancy and premature death.
The common denominator of fibroproliferative diseases is dysregulated tissue remodeling causing an accumulation of extracellular matrix (ECM) components in tissues of different organs (Wynn, 2007, Wynn, 2008, Wynn and Barron, 2010, Schuppan et al., 2001). The ECM consists mainly of collagens, proteoglycans and glycoproteins. Collagens constitute approximately 30% of all proteins in the body, with type I collagen as the most ubiquitous collagen (Muiznieks and Keeley, 2013). Under pathological conditions the normal remodeling balance is disturbed replacing original proteins of the ECM with different matrix components, in turn leading to an altered composition and quality of the matrix (Karsdal et al., 2013a). Emerging evidence suggests that altered components and post-translational modifications (PTMs) of proteins in the ECM may both initiate and drive disease progression (Leeming et al., 2011a).
Matrix metalloproteinases (MMPs) constitute a principal family of enzymes involved in degradation of ECM proteins. The pathological over-expression of MMPs results in small protein fragments holding PTMs, which are released into the blood. These PTM fragments can be referred to as neoepitopes or so-called ‘protein fingerprints’. A neoepitope is a protease-generated PTM, which has potential as a biochemical marker of ECM remodeling (Karsdal et al., 2013b). Despite the notion that MMP-mediated ECM remodeling is a central event in initiation and progression of connective tissue diseases (Wynn, 2007, Pinzani, 2008), technologies for measurement are limited in the diagnostic armamentarium.
Type I collagen may be measured by 4 different epitopes (CTX-I, C1M, ICTP and PINP). Measurement of the pro-peptide of type I collagen (PINP) is a standard marker for bone formation, while a cathepsin K degraded product (CTX-I) is the standard measure of bone resorption (Rosenquist et al., 1998). Bone formation and bone resorption are completely different and opposite directed processes, emphasizing the need in clinical chemistry to measure the right protein in the right way. The marker of the cross-linked carboxyterminal telopeptide of type I collagen (ICTP), is an MMP derived intermediate conformational epitope which has been evaluated for ECM-related diseases (Elomaa et al., 1992). Recently we developed an ELISA assay detecting MMP-mediated type I collagen degradation fragments in serum (termed C1M). The competitive ELISA measures the end product of tissue degradation, i.e. a pool of peptides/proteins all having this specific MMP-mediated binding site as the denominator. The monoclonal antibody recognizes a 6 amino acid sequence at position 764 in the C-terminus of type 1 collagen (Leeming et al., 2011b). The collagen degradation fragment is generated by MMPs 2, 9 and 13 and is destroyed by cathepsin K, making this a soft tissue specific marker not originating from bone turnover.
In the period from 1999–2001 a total of 5855 Danish postmenopausal women participated in the large Prospective Epidemiological Risk Factor (PERF I) study aimed at identifying risk factors associated with age-related diseases. Serum samples originally collected in the PERF I cohort were in the current study analyzed in relation to levels of C1M and combined with register data from Danish national registries describing cause and time of death of the deceased part of the cohort.
We hypothesized that MMP-mediated tissue degradation of type I collagen was predictive for mortality.
2. Methods
2.1. Study Design
The Prospective Epidemiologic Risk Factor (PERF I) study was an observational, prospective follow-up study of Danish postmenopausal women who had previously either participated in clinical randomized placebo-controlled studies or were screened without being randomized for previous studies at the Center for Clinical and Basic Research (CCBR) in either Copenhagen or Aalborg. Invitations for participation were done by including all subjects in the CCBR subject database regardless of their previous medical history, ensuring no overrepresentation of subjects with history of specific diseases. A total of 5855 Danish postmenopausal Caucasian women aged 48-89 were enrolled in the PERF I Study from 1999–2001. The study was carried out in accordance with ICH-GCP with study protocol approval from the local ethics committee.
2.2. Baseline Examinations
Vital signs and fasting serum samples were collected at time of enrollment and serum samples were stored at − 80 °C for later use. Subjects reported on demographic characteristics; smoking status, alcohol consumption, physical activity and level of education as well as hypertension, hyperlipidemia, cancer history and diabetes in a self-reported questionnaire.
2.3. Outcome Variables
The primary end-points were all-cause mortality and cause specific mortality. Date of death of the deceased sub-group of the PERF I cohort (n = 1505) was obtained from the Danish Civil Registration System and cause of death was obtained from the National Danish Causes of Death Registry. Registry data was obtained up to 31st December 2012 leading to an average follow-up period of 12.1 years (11.4–13.1) for censored subjects. Causes of death were classified according to the International Classification of Diseases, tenth revision (ICD10). The primary cause of death was used for further evaluation of serum C1M levels in specific disease groups; cardiovascular diseases (ICD10 codes I00–I99), cancer (C00–C97), lung diseases (J00–J99), and other deaths (remaining ICD10 codes). Subjects who were dead due to external causes (ICD10 codes V01–X59) were excluded from survival analysis (n = 39). The time of survival was defined as the time from date of enrollment to date of death or to 31st December 2012.
2.4. Type I Collagen Degradation
MMP-degraded type I collagen was measured in serum by enzyme-linked immunosorbent assay (ELISA) as described by Leeming et al. (2011b)(n = 5629). The analyte was tested for stability and was considered to be stable after 12 years of storage (− 80 °C). In order to confirm analyte stability, 10 consecutive freeze-thaw cycles were done with no significant change in C1M level. Three year stability studies were performed to validate detection of analyte (C1M), by measuring the same sample in one year intervals. Moreover, the mean level of C1M found in the present study was compared to mean levels of C1M in studies with similar study population conducted at later time points with sample storage of shorter duration.
The cohort was divided into quartiles based on serum C1M level. Q1 (n = 1411): 26.2 ng/mL [21.2–31.3 ng/mL], Q2 (n = 1400): 35.2 ng/mL [31.4–39.5 ng/mL], Q3 (n = 1391): 46.2 ng/mL [39.6–56.0 ng/mL], Q4 (n = 1400): 87.1 ng/mL [56.1–458.8 ng/mL].
Serum CTX-I (n = 5611) was measured by Serum CrossLaps one step ELISA as described by Rosenquist et al. (1998).
2.5. Statistical Analysis
Statistical analysis was conducted using Medcalc® (v 12.3.0) and SAS® (v 9.4). Data are shown as mean ± standard error mean (SEM) if not otherwise indicated. Baseline characteristics of survivors and dead were compared using one-way analysis of variance (ANOVA) for numerical variables while a Chi-square test was used to compare categorical variables (Table 1).
Table 1.
PERF I cohort characteristics. Actual numbers are shown next to percentages.
| Variable | Total |
Alive |
Dead |
P-value |
|---|---|---|---|---|
| (n = 5855) | (n = 4350) | (n = 1505) | Alive vs. Dead | |
| Age (years) | 70.8 ± 0.1 | 69.4 ± 0.1 | 74.9 ± 0.2 | < 0.001 |
| BMI (kg/m2) | 26.2 ± 0.1 | 26.3 ± 0.1 | 25.7 ± 0.1 | < 0.001 |
| Underweight (< 18.5) (%) | 1.6 (90/5637) | 1.1 (46/4226) | 3.1 (44/1411) | |
| Normal (≥ 18.5–25.0) (%) | 41.6 (2343/5637) | 40.5 (1713/4226) | 44.6 (630/1411) | |
| Overweight (> 25.0–30.0) (%) | 39.9 (2248/5637) | 40.9 (1729/4226) | 36.7 (518/1411) | |
| Obese (> 30.0) (%) | 17.0 (956/5637) | 17.5 (738/4226) | 15.5 (219/1411) | |
| Current smoking (%) | 22.5 (1315/5844) | 19.8 (861/4342) | 30.2 (454/1502) | < 0.0001 |
| Exercise (≥ 1 time/week, %) | 68.5 (4003/5843) | 72.9 (3165/4340) | 55.8 (838/1503) | < 0.0001 |
| Alcohol (≥ 7 drinks/week, %) | 32.6 (1896/5812) | 32.7 (1411/4317) | 32.4 (485/4795) | 0.9 |
| Education (%) | 0.03 | |||
| Primary school | 71.5 (4178/5841) | 70.6 (3064/4339) | 74.2 (1114/1502) | |
| High school | 21.4 (1250/5841) | 22.2 (963/4339) | 19.1 (287/1502) | |
| University | 7.1 (413/5841) | 7.2 (312/4339) | 6.7 (101/1502) | |
| Hypertension (%) | 31.0 (1807/5838) | 28.9 (1252/4337) | 37.0 (555/1501) | < 0.0001 |
| Hyperlipidemia (%) | 9.1 (530/5845) | 9.4 (407/4342) | 8.2 (123/1503) | 0.2 |
| Cancer history (%) | 5.2 (301/5808) | 4.1 (178/4313) | 8.2 (123/1495) | < 0.0001 |
| Diabetes (%) | < 0.0001 | |||
| Type 1 | 0.7 (39/5845) | 0.6 (26/4342) | 0.9 (13/1503) | |
| Type 2 | 2.4 (144/5845) | 1.9 (83/4342) | 3.9 (59/1503) | |
| Serum C1M (ng/mL) | 50.7 ± 0.5 | 49.8 ± 0.6 | 53.4 ± 1.0 | 0.001 |
| Serum CTX-I (ng/mL) | 0.44 ± 0.003 | 0.44 ± 0.004 | 0.44 ± 0.007 | 0.7 |
Multivariate Cox proportional-hazard analysis was used to determine proportional hazard ratios for selected risk factors (age, BMI, smoking, exercise, alcohol consumption, education level, hypertension, hyperlipidemia, cancer history and diabetes) (Table 2).
Table 2.
Hazard ratios for risk factors associated with mortality.
All hazard ratios are mutually adjusted.
| Variable | Multivariate analysis |
||
|---|---|---|---|
| Hazard ratio | 95% confidence interval | P-value | |
| Age (years) | 1.13 | 1.12 to 1.14 | < 0.0001 |
| BMI (kg/m2) | |||
| Underweight (< 18.5) | 1.59 | 1.16 to 2.18 | 0.004 |
| Normal (≥ 18.5–25.0) | Reference | ||
| Overweight (> 25.0–30.0) | 0.90 | 0.80 to 1.02 | 0.1 |
| Obese (> 30.0) | 0.86 | 0.73 to 1.01 | 0.08 |
| Current smoking (yes/no) | 1.90 | 1.69 to 2.14 | < 0.0001 |
| Physical inactivity (vs. ≥ 1 time/week) | 1.52 | 1.36 to 1.70 | < 0.0001 |
| Alcohol (≥ 7 drinks/week) | 1.07 | 0.95 to 1.20 | 0.3 |
| Education | |||
| Primary school | Reference | ||
| High school | 0.91 | 0.79 to 1.04 | 0.2 |
| University | 0.90 | 0.73 to 1.11 | 0.3 |
| Hypertension (yes/no) | 1.18 | 1.05 to 1.32 | 0.004 |
| Hyperlipedemia (yes/no) | 1.07 | 0.88 to 1.30 | 0.5 |
| Cancer history (yes/no) | 1.86 | 1.53 to 2.26 | < 0.0001 |
| Diabetes (no/Type 1/Type 2) | |||
| Type 1 | 1.52 | 0.88 to 2.62 | 0.1 |
| Type 2 | 1.88 | 1.41 to 2.51 | < 0.0001 |
Serum C1M values were normalized using log-transformation. Univariate and multivariate Cox proportional-hazard analysis was used to assess the relation between mortality and serum levels of C1M (log-transformed) in the full follow up period. The adequacy of the Cox proportional-hazard analysis was tested by checking the functional form and the assumption of proportional hazards as described by Lin, Wei, and Ying (Lin et al., 1993). The Kolmogorov-type supremum test revealed no misspecification of the functional forms for the continuous covariates. The proportional hazard assumption was violated with log-transformed C1M in the 12 year follow-up period. Therefore, the multivariate Cox proportional-hazard analysis was split in three year intervals (0–3 years, 3–6 years, 6–9 years, 9–12 years) where the relation between serum levels of C1M (log-transformed) and all-cause mortality was assessed assuming conformity with the proportional hazard assumption in each three year time interval (Fig. 1). Risk factors from Table 2 were included in the multivariate analysis. Likewise the relation between serum levels of CTX-I (log-transformed) and all-cause mortality was assessed in each time interval (data not shown).
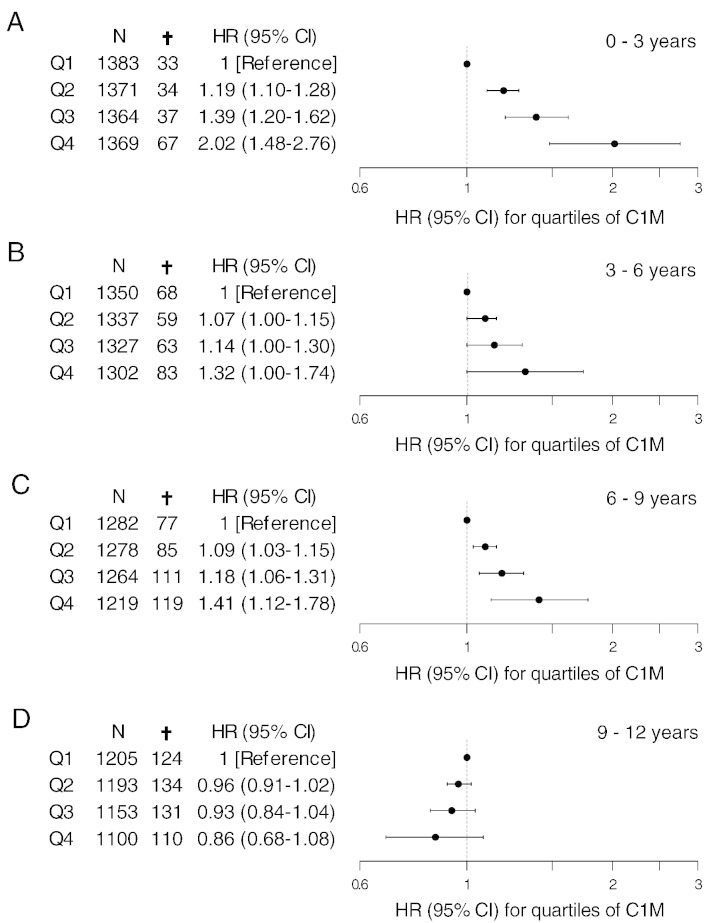
Fig. 1.
Hazard ratios with 95% CI for all-cause mortality in quartiles (Q1–Q4) of C1M. A: 0–3, B: 3–6, C: 6–9 and D: 9–12 years. Values are adjusted for age, BMI, smoking, alcohol consumption, physical inactivity, education level, hypertension, hyperlipidemia, cancer history and diabetes.
Hazard ratios for each quartile of serum C1M was determined by multiplying the parameter estimate of the log-transformed C1M value derived from the multivariate Cox proportional-hazard analysis, with the range between the log-transformed means of serum C1M levels in each quartile, followed by a back-transformation to the original scale using the exponential function. The lower quartile (Q1) was used as reference.
A Kaplan–Meier survival curve was applied to illustrate mortality over time in the four quartiles in the full follow-up period (Fig. 2A) and in three year time intervals (Fig. 2B–E). A log-rank test was used to determine differences between the survival curves.
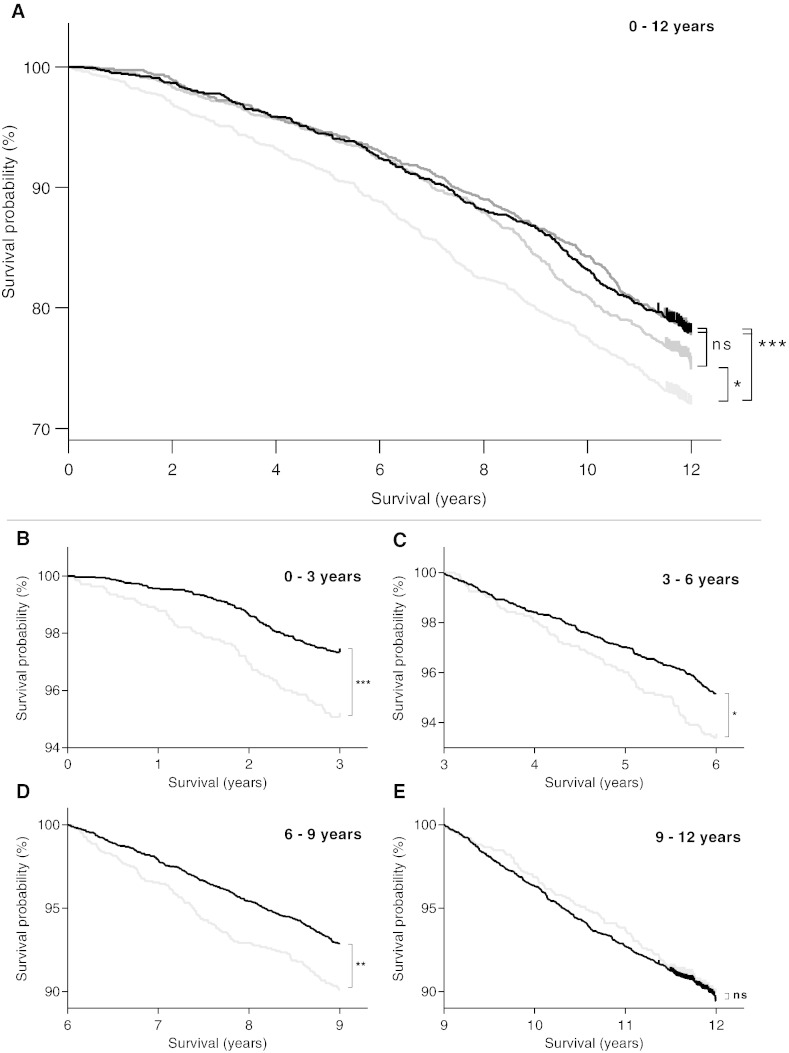
Fig. 2.
Kaplan–Meier survival curves for A: 0–12 years with C1M levels divided into quartiles (Q1 (lowest), Q2, Q3 and Q4). Black: Q1, dark gray: Q2, gray: Q3, light gray: Q4; B: 0–3 years, C: 3–6 years, D: 6–9 years and E: 9–12 years with C1M levels divided into Q1–Q3 (pooled) and Q4 (upper quartile). Black: Q1–Q3, light gray: Q4. *p < 0.05, **p < 0.01, ***p < 0.001, ns = not significant.
Multivariate Cox proportional-hazard analysis was further used to assess levels of serum C1M (log-transformed) in the time interval from 0–9 years (Fig. 3). Risk factors from Table 2 were included in the multivariate analysis.
Fig. 3.
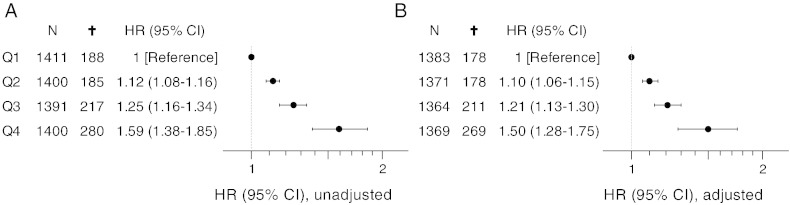
Hazard ratios with 95% CI for all-cause mortality in quartiles (Q1–Q4) of C1M with nine year follow-up. A: unadjusted, B: adjusted. Adjusted values are corrected for age, BMI, smoking, alcohol consumption, physical inactivity, education level, hypertension, hyperlipidemia, cancer history and diabetes.
Hazard ratios were determined for deaths caused by cancer, cardiovascular diseases, lung diseases and other types of death for subjects with serum C1M level in the upper quartile (Q4) versus subjects in the lower quartile (Q1) in time intervals 0–3 years and 0–9 years (Fig. 4). Hazard ratios for cause-specific diseases were calculated solely on the contribution of deaths with the specific diagnose. The remaining part of the deceased population was excluded from the analysis.
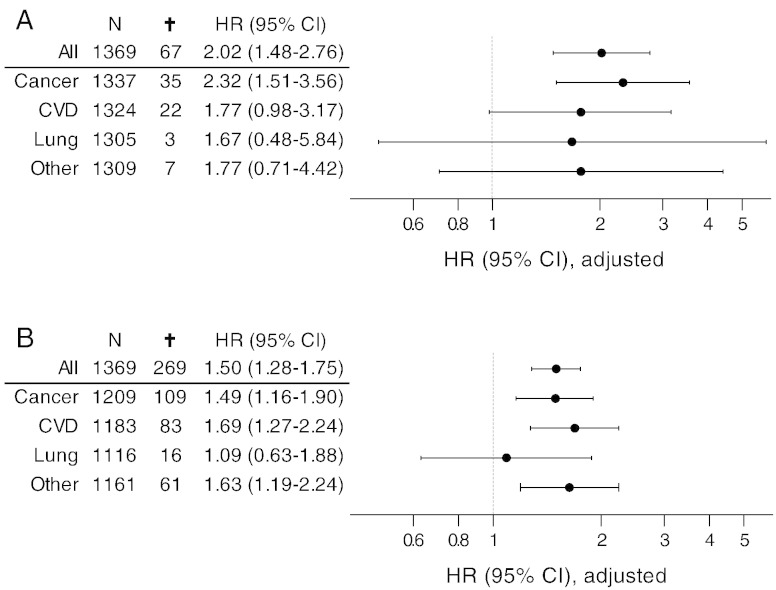
Fig. 4.
Hazard ratios with 95% CI for all-cause mortality and cause specific mortality (cancer, cardiovascular diseases, lung diseases and other diseases) for the upper quartile of the cohort (Q4) in time intervals 0–3 years (A) and 0–9 years (A). Hazard ratios are adjusted for age, BMI, smoking, alcohol consumption, physical inactivity, education level, hypertension, hyperlipidemia, cancer history and diabetes.
3. Results
3.1. PERF I Cohort Characteristics
Table 1 summarizes baseline characteristics of the PERF I cohort stratified in alive and dead subjects, 12 years after initiation of the study. The mean age for the total population was 70.8 years (49.7–88.8). From study entry until 31st December 2012 a total of 1505 subjects died. The age in the deceased subgroup was significantly higher compared to the group of subjects still alive. The entire cohort was characterized by being slightly overweight (BMI 26.2 ± 0.1) with the deceased subgroup having a significantly lower BMI compared to the subjects still alive (25.7 ± 0.1 versus 26.3 ± 0.1) (p < 0.001). The living group was characterized by less smokers (19.8% versus 30.2%), subjects with slightly higher education level (22.2% versus 19.1% high school educated), and a larger proportion of physically active subjects (72.8% versus 55.7%). In the cohort 33% consumed more than 7 drinks/week and the proportion of alcohol-consumers drinking ≥ 7 drinks/week was equal in the living and the deceased group. The deceased group was characterized by having a significantly higher proportion of hypertensive and diabetic subjects, whereas the proportion of subjects with hyperlipidemia did not differ between the two groups. The proportion of subjects with a history of cancer was significantly larger in the deceased part of the cohort (8.2% versus 4.1%).
The serum C1M level was significantly higher (p = 0.001) in the deceased part of the cohort compared to those still alive, while no significant difference was seen in serum levels of CTX-I (p = 0.7).
3.2. Risk Factors for All-cause Mortality
A multivariate Cox proportional-hazard model was used to assess the independent contribution of risk factors (age, smoking, BMI, physical inactivity, alcohol consumption, education level, hypertension, hyperlipidemia, cancer history and diabetes) to mortality in the cohort (Table 2).
All risk factors, except for education level, alcohol consumption (≥ 7 drinks/week), and hyperlipidemia, were associated with mortality.
3.3. Survival
A multivariate Cox proportional-hazard analysis was used to assess hazard ratios for C1M, after adjusting for risk factors listed in Table 2, in different time intervals from blood sampling until time of death. This was done to determine the predictive nature of C1M (Fig. 1).
An increase in mortality with increasing C1M was observed in the 0–3 year interval. This will introduce a “survivor effect” when applying the three year stratification approach in the remaining time intervals, as it will drive the high risk group towards the low risk group over time. Despite confounding from “healthy” survivors in the 3–6, 6–9 and 9–12 year intervals, a trend towards an increase in hazard ratio from the lowest quartile (Q1) to the upper quartile (Q4) was observed in all time intervals from 0–9 years. A 2-fold increase in risk of mortality was observed in the interval from 0–3 years (Fig. 1A, HR 2.02 [95% CI: 1.48–2.76]) for subjects with serum C1M levels in the upper quartile (Q4) compared to the lowest quartile (Q1). The same tendency, however non-significant, was seen in the interval from 3–6 years (Fig. 1B, HR 1.32 [95% CI: 1.00–1.74]) and from 6–9 years (Fig. 1C, HR 1.41 [95% CI: 1.12–1.78]). In the interval from 9–12 years no significant change in hazard ratio could be observed between the four quartiles (Fig. 1D).
Contrary, the other type I collagen degradation product (CTX-I) was not found to be a predictor of all cause mortality in neither of the three year intervals from the multivariate Cox proportional-hazard model (data not shown).
A Kaplan–Meier survival curve was applied to illustrate survival over time (Fig. 2A). Part of the cohort with serum C1M levels in the upper quartile (Q4) had a decreased survival probability compared to the three other quartiles in the entire 12 year follow-up period (p = 0.0001). No significant difference was seen comparing the lowest quartile (Q1) and the two middle quartiles (Q2 and Q3) in the full follow-up period. Pooled data from Q1–Q3 was used for determining the difference in mortality compared to the upper quartile (Q4). A significant difference in mortality was seen in time intervals from 0–9 years; 0–3 years (p = 0.0001), 3–6 years (p = 0.01), and 6–9 years (p = 0.002) (Fig. 2B–D). No difference in mortality was found in the time interval from 9–12 years (p = 0.38, Fig. 2E).
A Cox proportional-hazard analysis was used to assess the mortality risk in part of the follow-up period where an increase in hazard ratio was observed (0–9 year) (Fig. 3). In the univariate Cox proportional-hazard analysis a 59% increased risk of mortality (HR 1.59 [95% CI: 1.38–1.85]) was found, when comparing Q4 to Q1 (Fig. 3A). The increase in mortality risk was 50% (HR 1.50 [95% CI: 1.28–1.75]) within the nine year follow-up period when a multivariate Cox proportional-hazard analysis was applied accounting for risk factors known to impact mortality (Fig. 3B).
3.4. Cause Specific Mortality
Cause specific mortality was assessed in part of the cohort with serum C1M levels in the upper quartile (Q4) versus subjects with serum C1M levels in the lowest quartile (Q1). A multivariate Cox proportional-hazard analysis was used to assess the cause specific mortality risk in the time intervals 0–3 years and 0–9 years (Fig. 4).
In the multivariate Cox regression-analysis, the hazard ratio was 2.32 [95% CI: 1.51–3.56] for cancer, 1.77 [95% CI: 0.98–3.17] for cardiovascular diseases, 1.67 [95% CI: 0.48–5.84] for lung diseases, and 1.77 [95% CI: 0.71–4.42] for other deaths within the 0–3 year interval (Fig. 4A).
In the 0–9 year follow-up interval, the hazard ratio was 1.49 [95% CI: 1.16–1.90] for cancer, 1.69 [95% CI: 1.27–2.24] for cardiovascular diseases, 1.09 [95% CI: 0.63–1.88] for lung diseases, and 1.63 [95% CI: 1.19–2.24] for other deaths (Fig. 4B).
4. Discussion
We have identified MMP-mediated type I collagen degradation (C1M) as an independent risk factor for all-cause mortality. Contrary, we found no association between cathepsin K degraded type I collagen (CTX-I) and all-cause mortality. This suggests that specifically MMP-mediated tissue degradation of type I collagen is associated with mortality.
We found a 2-fold increase in mortality risk in the first three years of follow-up and a 1.5-fold increase was observed with nine year follow-up time in individuals having high MMP-mediated type I collagen degradation compared to individuals with a low serum level of this type I collagen degradation marker.
During pathological remodeling of the ECM excessive levels of tissue- and pathology-specific turnover products are released into the circulation consequently becoming biomarkers. In the present study degradation of type I collagen was measured as a marker for tissue degradation as it is assumed to be a key player in ECM remodeling. Our results emphasize that the enzymatic processing is important since only the MMP-mediated type I collagen degradation was predictive of mortality, not cathepsin K degraded type I collagen. Increased serum C1M levels have previously been shown to be associated with diseases in which chronic inflammation is a key driver, such as ankylosing spondylitis (Bay-Jensen et al., 2012), osteoarthritis (Siebuhr et al., 2014), rheumatoid arthritis (Bay-Jensen et al., 2014), and different types of fibrosis (Leeming et al., 2012, Leeming et al., 2013)- diseases which are all contributing to a decreased healthy life expectancy and ultimately death.
The prognostic nature of C1M was assessed by dividing the follow-up period into three year intervals. A 2-fold increase in risk of mortality was determined within the first three years of the follow up period. The increase in mortality with increasing C1M observed in the 0–3 year interval introduce a “survivor effect” when applying the three year stratification approach. This may explain why the HRs decrease over time, driving the associations towards the null hypothesis in the remaining time intervals (3–12 years). The observed potential association in the intermediate time spans (3–6 and 6–9 years) is therefore very likely, but presumably underestimated since the “survivor effect” will drive the high risk group towards the low risk group over time. Despite this, we believe that C1M may predict an increased risk of mortality up to nine years prior to death for subjects with C1M levels in the upper quartile. A 1.5-fold hazard ratio was determined for the combined 0–9 year interval (Fig. 3). The higher risk in the 0–3 year interval underlines the understanding that an event, in this case death, is easier to predict closer to time of occurrence. Subjects with high MMP-mediated type I collagen degradation may therefore be predisposed to a decreased life expectancy based solely on their degree of type I collagen degradation.
The PERF I cohort comprised slightly overweight elderly women at risk of developing common western-lifestyle diseases such as type II diabetes, hypertension and hyperlipidemia. These lifestyle diseases affect many tissues and organs resulting in chronic low grade inflammation possibly following fibroproliferative changes to the ECM and thereby collagen degradation. The most prevalent primary causes of death in the PERF cohort were cancer and cardiovascular diseases accounting for 34% and 27% of all deaths, respectively. Similarly, the two largest causes of death for women aged 70–74 in the EU, as reported in the European Health Report, are cancer and cardiovascular diseases accounting for 37% and 42% respectively (WHO, 2013). High MMP-mediated type I collagen degradation was associated with both cancer and cardiovascular mortality. At first glance, two markedly different diseases, however with increased tissue turnover being a common denominator of both diseases. The risk of dying from cancer was increased 2.3-fold in the first three years of follow-up and an approximate 1.5-fold increase was observed within the nine year follow-up period in individuals having high MMP-mediated type I collagen degradation. These findings correspond well with the association between ECM remodeling and tumorgenesis (Bonnans et al., 2014, Lu et al., 2012) as ECM remodeling in cancer leads to a dysregulation in tumor growth, inflammation, tissue invasion, and metastasis (Kessenbrock et al., 2010).
In addition, risk of dying from cardiovascular diseases was increased 1.8-fold in the first three years of follow-up and an approximate 1.7-fold increase was observed with nine year follow-up period in individuals having high MMP-mediated type I collagen degradation. Atherosclerosis is a typical hallmark of cardiovascular diseases leading to a disturbance of the ECM homeostasis in the artery wall combined with low-grade inflammation. This results in a disrupted structure of the ECM of the artery wall, ultimately leading to cardiovascular disease and fatal events (Hobeika et al., 2007, Raines, 2000, Galis and Khatri, 2002). Other tissue turnover markers have been associated with mortality; albeit not type I collagen degradation by MMPs. P3NP, a formation marker of type III collagen, was associated with all-cause mortality in the Framingham study (Velagaleti et al., 2010). Endostatin, a degradation fragment of type XVIII collagen, was associated with all-cause, cancer and cardiovascular mortality in two independent cohorts from Sweden (Ärnlöv et al., 2013).
Degradation and formation are interlinked in the tissue turnover balance, making both processes equally important. Determining the better biomarker is therefore not easy. Formation markers, like P3NP, are generated in all tissues comprising type III collagen. However, when measuring a MMP-mediated degradation product, like C1M, it is a prerequisite that the protease is co-expressed in the affected tissue, making this a specific marker for pathologic tissue turnover. When assessing mortality, MMP-mediated type I collagen degradation may possibly either reflect a consequence or a cause of disease leading to mortality (Karsdal et al., 2010). In order to further answer this question, it would be beneficial to have sequential measurements of C1M which could more closely relate diagnosis of disease rather than early prognosis. In the current study it can only be speculated that some individuals may be predisposed for an increased degradation, potentially making them prone to certain diseases and eventually premature death.
Increased serum levels of C1M have shown to be associated with pain and progression of disease in rheumatoid arthritis, and conversely, a decrease by anti-inflammatory modulation (anti-interleukin-6) of more than 35% was associated with protection from disease progression (Siebuhr et al., 2013). This may suggest that attenuation of high remodeling by intervention could be associated with increased life-span. The relation between inflammation and tissue turnover is of particular interest. In autoimmune diseases like rheumatoid arthritis CRP and C1M have been proven to be highly correlated (Siebuhr et al., 2013). In diseases like fibrosis, inflammation may initiate disease, however once present fibrosis can progress without inflammation (Trautwein et al., 2015). The nature and extent of inflammation and ECM remodeling are therefore likely to be very different in different diseases and stages within the same disease. Although this current study identified the prognostic importance of C1M assessment in serum, it remains to be shown whether lowering this marker can result in a reduction of the mortality risk.
Interpreting biochemical markers found in serum is associated with many limitations, as several different tissues at different rates may produce and thus contribute to the total pool of molecular marker. Type I collagen is highly abundant in many tissues throughout the body, and an increase in the serological levels of C1M is a hallmark of several fibroproliferative diseases. Further studies on disease-specific contributions to the total pool of ECM remodeling are therefore needed. Importantly however, measuring increased levels may assist in identifying the sub-groups predisposed for increased ECM remodeling. This could aid in early diagnosis of subjects with high tissue turnover, leading to connective tissue diseases, which may benefit from increased medical attention thereby potentially increasing their lifespan.
This cohort is solely comprised of Danish postmenopausal women and further generalization to other demographics needs to be investigated. However, the risk factors identified in the Cox proportional-hazard analysis (smoking, alcohol consumption, physical inactivity, education level, hypertension, hyperlipidemia and diabetes) had similar associations to risk factors found in the Nurses' Health Study, a cohort of middle-aged women (Baer et al., 2011).
Moreover, as in other epidemiological studies, findings in the present study may be affected by selection bias caused by possible over-representation of relatively healthy subjects in the cohort. One could however argue that this would tend to draw the results in a direction towards the null hypothesis and therefore cannot explain our positive results.
5. Conclusion
We found that increased MMP-mediated tissue degradation, as an independent risk factor, was associated with a 2-fold increase in all-cause mortality within three years of follow-up and a 1.5-fold increase in all-cause mortality up to nine years prior to death.
MMP-mediated tissue degradation may be an important predisposition for cause of disease and subsequent mortality.
Author contributions
Katrine Dragsbæk and Jesper Skov Neergaard: writing, literature search, figures, data and statistical analysis, data interpretation.
Henrik Bo Hansen: data interpretation.
Inger Byrjalsen: statistical analysis, data interpretation.
Stephanie Nina Kehlet: sample analysis, data interpretation.
Anne-Christine Bay-Jensen: writing, sample analysis and stability
Peter Alexandersen and Claus Christiansen: study design, scientific advice.
Morten Karsdal: writing, data interpretation, scientific advice.
Competing interests
Anne-Christine Bay-Jensen, Morten Karsdal and Claus Christiansen are stock owners of Nordic Bioscience.
Acknowledgments
We would like to acknowledge the Danish Research Foundation (Den Danske Forskningsfond) for funding the PERF I study. The foundation had no role in study design, data interpretation or submission of this manuscript. Camilla Sobszyk Christensen is acknowledged for her contribution to the data analysis.
References
- Ärnlöv J., Ruge T., Ingelsson E., Larsson A., Sundstrøm J., Lind L. Serum endostatin and risk of mortality in the elderly: findings from 2 community-based cohorts. Arterioscler. Thromb. Vasc. Biol. 2013;33(11):2689–2695. doi: 10.1161/ATVBAHA.113.301704. [DOI] [PubMed] [Google Scholar]
- Baer H.J., Glynn R.J., Hu F.B. Risk factors for mortality in the nurses' health study: a competing risks analysis. Am. J. Epidemiol. 2011;173(3):319–329. doi: 10.1093/aje/kwq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay-Jensen A., Leeming D., Kleyer A., Veidal S., Schett G., Karsdal M. Ankylosing spondylitis is characterized by an increased turnover of several different metalloproteinase-derived collagen species: a cross-sectional study. Rheumatol. Int. 2012;32(11):3565–3572. doi: 10.1007/s00296-011-2237-8. [DOI] [PubMed] [Google Scholar]
- Bay-Jensen A.C., Byrjalsen I., Siebuhr A.S., Christiansen C., Platt A., Karsdal M.A. Serological biomarkers of joint tissue turnover predict tocilizumab response at baseline. J. Clin. Rheumatol. 2014;20(6):332–335. doi: 10.1097/RHU.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elomaa I., Virkkunen P., Risteli L., Risteli J. Serum concentration of the cross-linked carboxyterminal telopeptide of type I collagen (ICTP) is a useful prognostic indicator in multiple myeloma. Br. J. Cancer. 1992;66(2):337–341. doi: 10.1038/bjc.1992.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Z.S., Khatri J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ. Res. 2002;90(3):251–262. [PubMed] [Google Scholar]
- Health and Consumer Protection Directorate-General . European Commission; 2006. Healthy Aging, A Keystone for a Sustainable Europe — EU Health Policy in the Context of Demographic Change. [Google Scholar]
- Hobeika M.J., Thompson R.W., Muhs B.E., Brooks P.C., Gagne P.J. Matrix metalloproteinases in peripheral vascular disease. J. Vasc. Surg. 2007;45(4):849–857. doi: 10.1016/j.jvs.2006.09.066. [DOI] [PubMed] [Google Scholar]
- Karsdal M.A., Henriksen K., Leeming D.J., Woodworth T., Vassiliadis E., Bay-Jensen A.C. Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers — are they the cause or the consequence of the disease? Clin. Biochem. 2010;43:793–804. doi: 10.1016/j.clinbiochem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Karsdal M.A., Nielsen M.J., Sand J.M. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Assay Drug Dev. Technol. 2013;11(2):70–92. doi: 10.1089/adt.2012.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal M.A., Bay-Jensen A.C., Leeming D.J., Henriksen K., Christiansen C. Quantification of “end products” of tissue destruction in inflammation may reflect convergence of cytokine and signaling pathways — implications for modern clinical chemistry. Biomarkers. 2013;18(5):375–378. doi: 10.3109/1354750X.2013.789084. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Plaks V., Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming D.J., Bay-Jensen A.C., Vassiliadis E., Larsen M.R., Henriksen K., Karsdal M.A. Post-translational modifications of the extracellular matrix are key events in cancer progression: opportunities for biochemical marker development. Biomarkers. 2011;16(3):193–205. doi: 10.3109/1354750X.2011.557440. [DOI] [PubMed] [Google Scholar]
- Leeming D., He Y., Veidal S. A novel marker for assessment of liver matrix remodeling: an enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M) Biomarkers. 2011;16(7):616–628. doi: 10.3109/1354750X.2011.620628. [DOI] [PubMed] [Google Scholar]
- Leeming D.J., Sand J.M., Nielsen M.J. Serological investigation of the collagen degradation profile of patients with chronic obstructive pulmonary disease or idiopathic pulmonary fibrosis. Biomark. Insights. 2012;7:119–126. doi: 10.4137/BMI.S9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming D.J., Byrjalsen I., Jimenez W., Christiansen C., Karsdal M.A. Protein fingerprinting of the extracellular matrix remodelling in a rat model of liver fibrosis-a serological evaluation. Liver Int. 2013;33(3):439–447. doi: 10.1111/liv.12044. [DOI] [PubMed] [Google Scholar]
- Lin D.Y., Wei L.J., Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557–572. [Google Scholar]
- Lu P., Weaver V.M., Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196(4):395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muiznieks L.D., Keeley F.W. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2013;1832(7):866–875. doi: 10.1016/j.bbadis.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Pinzani M. Welcome to fibrogenesis & tissue repair. Fibrogenesis Tissue Repair. 2008;1(1):1. doi: 10.1186/1755-1536-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E.W. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int. J. Exp. Pathol. 2000;81(3):173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist C., Fledelius C., Christgau S., Pedersen B.J., Bonde M., Qvist P. Serum CrossLaps One Step ELISA. First application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopeptides of type I collagen. Clin. Chem. 1998;44(11):2281–2289. [PubMed] [Google Scholar]
- Schuppan D., Ruehl M., Somasundaram R., Hahn E.G. Matrix as a modulator of hepatic fibrogenesis. Semin. Liver Dis. 2001;21(3):351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- Siebuhr A.S., Bay-Jensen A.C., Leeming D.J. Serological identification of fast progressors of structural damage with rheumatoid arthritis. Arthritis Res. Ther. 2013;15(4):R86. doi: 10.1186/ar4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebuhr A.S., Petersen K.K., Rendt-Nielsen L. Identification and characterisation of osteoarthritis patients with inflammation derived tissue turnover. Osteoarthr. Cartil. 2014;22(1):44–50. doi: 10.1016/j.joca.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Trautwein C., Friedman S.L., Schuppan D., Pinzani M. Hepatic fibrosis: concept to treatment. J. Hepatol. 2015;62(1):S15–S24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Velagaleti R.S., Gona P., Sundstrøm J., Larson M.G., Siwik D., Colucci W.S. Relations of biomarkers of extracellular matrix remodeling to incident cardiovascular events and mortality. Arterioscler. Thromb. Vasc. Biol. 2010;30(11):2283–2288. doi: 10.1161/ATVBAHA.110.208462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2013. The European Health Report 2012 — Charting the Way to Well-being. [Google Scholar]
- Wynn T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 2007;117(3):524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A., Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010;30(3):245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]