Abstract
Background
Cytomegalovirus (CMV) infection in transplant recipients is reported to replicate with a doubling time of 1.2–2 days, and weekly screening is recommended for early diagnosis. We re-evaluated these features in our cohort of transplant recipients.
Methods
The CMV doubling time of the first CMV infection in the first year post-transplant could be calculated for 193 recipients of haematopoietic stem cell or solid organ transplantation. Factors determining the proportion of recipients with a high diagnostic CMV viral load (≥ 18,200 IU/mL) were explored using mathematical simulation.
Findings
The overall median doubling time was 4.3 days (IQR 2.5–7.8) and was not influenced by prior CMV immunity, or type of transplantation (p > 0.4). Assuming a fixed doubling time of 1.3 days and screening intervals of 7 or 10 days, 11.1% and 33.3% were projected to have a high CMV viral load at diagnosis, compared to 1.4% and 4.3% if the doubling time varies as observed in our cohort. Consistently, 1.9% of recipients screened weekly had a high diagnostic virus load.
Interpretation
Screening intervals can be extended to 10 days in cohorts with comparable CMV doubling time, whereas shorter than 7 days is required in cohorts with shorter doubling times to maintain pre-emptive screening quality.
Keywords: Cytomegalovirus, PCR, Solid organ transplantation, Haematopoietic stem cell transplantation, Pre-emptive treatment, Doubling time
Highlights
-
•
CMV doubling time was longer than previously reported, and not influenced by type of transplantation or prior CMV immunity.
-
•
In cohorts with comparable CMV doubling time, intervals between screening with CMV PCR may be extended from 7 to 10 days.
1. Introduction
Cytomegalovirus (CMV) infection is an important cause of complications in transplant recipients. If untreated, it may progress to CMV disease, a condition associated with increased morbidity and mortality. A pre-emptive strategy, comprising of regular screening with CMV PCR to detect and treat CMV infection before it causes clinical disease, has therefore become generally accepted (Kotton et al., 2013, Razonable and Humar, 2013, Andrews et al., 2011, Tomblyn et al., 2009).
A series of longitudinal studies, using measurements of CMV viral load with PCR in transplant recipients, have described the viral dynamics of CMV in vivo (Bowen et al., 1998, Gor et al., 1998, Cope et al., 1997, Hassan-Walker et al., 1999, Ghisetti et al., 2004). Subsequent studies established CMV as a rapidly replicating virus in the human host, with a doubling time ranging from 1 (Emery et al., 1999, Emery et al., 2000, Emery et al., 2002) to 2.3 days (Mattes et al., 2005, Nebbia et al., 2007, Atabani et al., 2012, Funk et al., 2007, Buyck et al., 2010, Munoz-Cobo et al., 2011). Based on the assumption of a rapid doubling time, current guidelines recommend — based on empiric evidence — weekly screening with CMV PCR when recipients are managed pre-emptively (Kotton et al., 2013, Razonable and Humar, 2013, Andrews et al., 2011).
“The Management of Post-Transplant Infections in Collaborating Hospitals” (MATCH) programme was introduced at Rigshospitalet in Copenhagen, Denmark in 2011, with the aim to reduce the risk of severe viral diseases in transplant recipients (unpublished data; da Cunha-Bang, C. et al.). MATCH constitutes a platform for collaboration between the transplantation units and the Department of Infectious Diseases, and the associated database contains data on a large cohort of consecutive transplant recipients of both solid organ transplantation (SOT) and haematopoietic stem cell transplantation (HSCT). Consistent with the current guidelines, weekly screening intervals for CMV were applied in MATCH. However, when using this screening approach, very few recipients with high viral load at time of diagnosis of the CMV infection were detected. This raised the question whether the previously determined CMV doubling time estimates were valid in our cohort.
In this study, the reproducibility of the published doubling time estimates was investigated. Furthermore, the rationale of a weekly screening interval with CMV PCR measurements in transplant recipients, managed pre-emptively, was evaluated.
2. Recipients and Methods
2.1. Recipients and Definition of CMV Infection
Consecutive recipients in the MATCH database transplanted between January 1 2003 and August 27 2013 (n = 2344), who developed a first CMV infectious episode within the first 12 months following transplantation, were eligible for inclusion (n = 329). All applicable regulatory and ethical approvals related to the project are obtained in accordance with the national legislation.
In order to calculate the CMV doubling time, the episodes needed to be recorded with ≥ 2 quantifiable and increasing CMV PCR measurements taken within 14 days of each other (see section Calculation of CMV Doubling Time and Adjustment for Anti-CMV Treatment) (n = 193). Although the MATCH programme was initiated in 2011, it was possible to reconstruct course of events including relevant laboratory assessments for all recipients since 2003 stored electronically into the MATCH database. A CMV infectious episode was defined as two consecutive quantifiable CMV PCR values ≥ 273 IU/mL (i.e. 300 copies/mL) taken within 2 weeks of each other, or one measurement ≥ 2730 IU/mL (da Cunha-Bang et al., 2011). The first of two subsequent consecutive negative CMV PCRs following an infectious episode defines the end of that episode. Only the first CMV episode was eligible for inclusion, i.e. the number of included recipients equals the CMV infectious episodes.
2.2. CMV IgG Serostatus
The CMV IgG serostatus for donor and recipient was determined pre-transplant, and the eligible combinations for inclusion were D +/R −, D +/R + or D −/R +. The recipients were stratified according to risk of CMV infection depending on donor (D)/recipient (R) CMV IgG serostatus (positive (+)/negative (−)) prior to transplantation, as either high-, intermediary- or low risk. The high risk group constituted of D +/R − for SOT recipients, and D −/R + for HSCT recipients. D +/R + constituted the intermediary risk group for both types of transplantations, and the low risk group D −/R + for SOT and D +/R − for HSCT. Due to the small number of recipients in our cohort with low risk serostatus (n = 13), these recipients were analysed together with the recipients at intermediary risk.
2.3. CMV DNA Surveillance and Anti-CMV Treatment
This study is based on measurements of CMV in plasma by PCR, performed on a semi-regular basis as a part of surveillance of CMV in the MATCH programme. The COBAS Amplicor kit (DiDomenico et al., 1996) was used until 2011, and since 2011 the COBAS AmpliPrep/COBAS TaqMan has been used. The Department of Clinical Microbiology simultaneously tested the two PCR kits, and determined the conversion factor between the COBAS Amplicor kit and the COBAS AmpliPrep/COBAS TaqMan to be a factor 1:1. Thus, to make our results more widely applicable we have converted our virus loads into IU/mL using the conversion factor for the COBAS AmpliPrep/COBAS TaqMan (1 copy/mL corresponding to 0.91 IU/mL).
The SOT recipients received (val)ganciclovir prophylaxis for 3 months following transplantation and were subsequently treated pre-emptively with (val)ganciclovir in case of CMV infection. In general, the SOT recipients with serostatus D +/R −, D +/R + or D −/R + were screened monthly in the prophylaxis phase (month one to three post-transplant), and then weekly in months four to six post-transplant. Hereafter the recipients are tested monthly until month 12 post-transplant.
The HSCT recipients were monitored weekly by CMV PCR from week four to week 17 post-transplant and then at week 19, 26 and 52, except in case of graft-versus-host disease where weekly monitoring was continued.
In case of CMV infection (see definition) treatment with (val)ganciclovir, or in case of resistance, foscovir (Cunha-Bang et al., 2013) was initiated.
2.4. Calculation of CMV Doubling Time and Adjustment for Anti-CMV Treatment
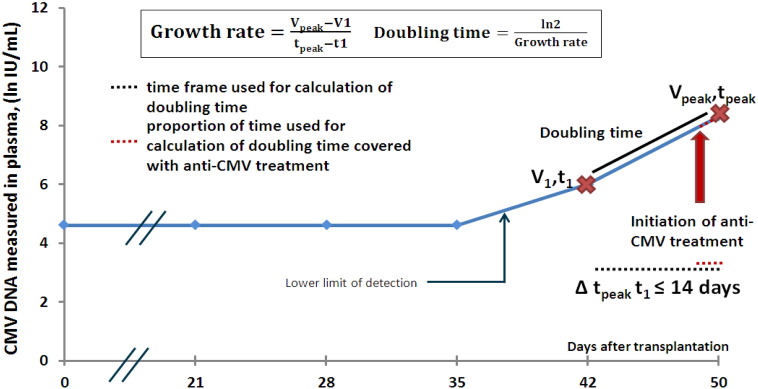
An algorithm was constructed to detect the first positive sample of the infectious episode. This sample is termed V1, and corresponds to the time t1 (Fig. 1). The algorithm was then constructed to find the highest positive sample within 14 days of the V1 sample; this sample is termed Vpeak and the time at which it occurs is termed tpeak. The doubling time is calculated as previously described (Emery et al., 1999, Atabani et al., 2012). First the CMV growth rate is determined from the slope of virus over time:
Fig. 1.
Calculation of doubling time and adjustment of anti-CMV treatment.
An algorithm was constructed to detect the first positive sample of the infectious episode, termed V1 and corresponding to the time t1. The highest sample within 14 days of the V1 sample was then detected; this sample is called Vpeak, and it corresponded to the time tpeak. The doubling time could then be calculated.
The proportion of time used for calculation of doubling time covered with anti-CMV treatment was determined based on data on anti-CMV treatment collected from patient files.
The doubling time can then be calculated using the standard exponential function:
Out of the 329 infection episodes, 193 had ≥ 2 increasing CMV PCR measurements taken within 14 days of each other. Thus, this formula was applied to these episodes and the doubling time was calculated.
When calculating the CMV doubling time, it is necessary to adjust for any administration of anti-CMV treatment. Information on anti-CMV treatment was systematically collected for the included CMV infections from patient files. For each infectious episode, the proportion of time on which the calculation of doubling time was based on and that was covered with anti-CMV treatment, was determined (Fig. 1). Thus this variable can be between 0% (recipients who didn't receive any anti-CMV treatment during the time used for calculation for doubling time) and 100% (recipients who were initiated in anti-CMV treatment the before or the same day as the V1 sample).
2.5. Modelling CMV Screening Intervals
A mathematical simulation model was constructed to determine factors that influence the optimal screening interval for preemptive treatment. A diagnostic viral load ≥ 18,200 IU/mL was defined as undesirably high, based on previous clinical experiences and observations of the prevalence of CMV disease at diagnosis of CMV infection (unpublished data; Lodding, I. et al.). We decided a priori that any monitoring strategy had to result in ≤ 5% of the newly developed CMV infections to be diagnosed at or above this undesirable level. The lower limit of detection for the CMV PCR assay was set at 273 IU/mL, and the results were available 24 h after blood draw. The CMV infection was assumed to emerge randomly within the screening interval, and the doubling time for the infection was either set at 1.3 days (as reported in the literature Funk et al., 2007) or allowed to vary as observed in our cohort (see Resultssection). The observed distribution of doubling time was either fitted as observed or from best Chi Square fit (five degrees of freedom). Based on observations from our cohort, CMV replication was most likely to occur during the first 3 months after transplantation, or in case of successfully administered primary prophylaxis, during the first 3 months after the prophylaxis was discontinued. The model is presented in detail in Appendix A.
The mathematical projection allowed us to explore the impact of different cut-offs for undesirably high virus load, different estimations of doubling time (estimations based of our cohort as well as previously described), and different screening intervals.
2.6. Validation of the Output From the Mathematical Model
In order to illustrate the impact of a weekly screening interval in our own cohort, the number of first-time infections that were preceded by a previous negative CMV PCR exactly 7 days earlier, was identified. Among these, the percentage diagnosed with an undesirably high viral load was calculated.
2.7. Statistical Methods
Differences in doubling time were explored using standard descriptive statistics, including correlation analyses and Mann Whitney U. Double sided p-values were used, and results were considered statistically significant at a level of < 0.05.
3. Results
3.1. Patient Characteristics and Features of CMV Infection
The CMV doubling time could be estimated for 193 of the 329 recorded infectious episodes in MATCH. The excluded infectious episodes had a larger proportion of females (43% vs 33%), and SOT recipients (56% vs 38%) than the included infectious episodes (online Appendix B). Distribution of CMV IgG serostatus and virological profiles were comparable between the groups.
38% of the included 193 infectious episodes represented SOT recipients, and 62% HSCT (Table 1). The median age at transplantation was 46 years (IQR 32–58), with a majority of males (69%). Due to the different strategies for prevention of CMV disease in the SOT and HSCT recipients (SOT recipients receiving primary chemoprophylaxis with (val)ganciclovir for the first 3 months followed by pre-emptive strategy, and HSCT recipients managed solely pre-emptively), the time of emergence of the CMV infections occurred later in SOT recipients compared to the HSCT recipients (109 days vs 50 days, respectively, Table 1). Conversely, the median number of days between the V1 and the Vpeak samples was 7 days and the median viral load of the V1 sample was 1183 IU/mL for all groups. SOT recipients reached a higher median Vpeak compared to the HSCT recipients (p = 0.05).
Table 1.
Characteristics of transplant recipients at the time of transplantation and clinical parameters of subsequent CMV infection, according to type of transplantation.
| All recipients | Type of transplantation |
p-Value | ||
|---|---|---|---|---|
| Solid organ | Bone marrow | |||
| Patient characteristics at the time of transplantation | ||||
| Demographics | ||||
| Number (%) of recipients | 193 (100%) | 74/193 (38%) | 119/193 (62%) | |
| Median age (IQR), years | 46 (32–58) | 51.5 (38–59) | 45 (23–56) | 0.03 |
| Gender (% male) | 133/193 (69%) | 48/74 (65%) | 85/119 (71%) | 0.3 |
| Risk of CMV infection1 | ||||
| Number of recipients at high risk of CMV infection | 96/193 (50%) | 34/74 (46%) | 62/119 (52%) | 0.5 |
| Number of recipients at intermediary/low risk of CMV infection | 97/193 (50%) | 40/74 (54%) | 57/119 (48%) | 0.6 |
| Factors describing CMV infection | ||||
| CMV infection | ||||
| Median time-span from tx to the V1 sample2 (IQR), days | 55 (42–105) | 109 (46–151) | 50 (40–61) | < 0.0001 |
| Median time-span from the V1 sample to Vpeak sample2 (IQR), days | 7 (4–11) | 7 (5–11) | 7 (4–11) | 0.4 |
| Median viral load (IU/mL), V1 sample (IQR) | 1183 (546–3913) | 1183 (455–8099) | 1183 (601–3367) | 0.6 |
| Median viral load (IU/mL) of Vpeak sample (IQR) | 5460 (1820–26,390) | 9965 (1911–40,040) | 4550 (1729–15,470) | 0.05 |
| Anti-CMV treatment | ||||
| Strategy for prevention of CMV disease | 3 months prophylaxis + preemptive treatment | Preemptive treatment | ||
| Number of patients not receiving treatment during the period of infection used for calculation of doubling time3 | 56/193 (29%) | 29/74 (39%) | 27/119 (23%) | 0.01 |
| Number of patients experiencing infectious breakthrough during prophylaxis or treatment | 21/193 (11%) | 15/74 (20%)4A | 6/119 (5%)4B | 0.002 |
| Median proportion of time during CMV infection on treatment (IQR)3 | 50% (0–80%) | 18% (0–85%) | 57% (0–80%) | 0.1 |
| Symptomatic CMV infection5 | ||||
| Number of patients with symptomatic CMV infection | 59/188a (31%) | 33/74 (45%) | 26/114a (23%) | 0.002 |
| Risk factors for development of CMV infection | ||||
| Allograft rejection or graft versus host disease6 | 36/193 (19%) | 8/74 (11%) | 28/119 (24%) | 0.04 |
Abbreviations: CMV; cytomegalovirus, tx; transplantation, IQR; interquartile range.
Risk of CMV infection according to donor (D)/recipient (R) CMV IgG serostatus (+/−) at the time of transplantation. For solid organ transplantation recipients D +/R − is associated with high risk of CMV infection, while D −/R + is associated with low risk. Among bone marrow transplant recipients, D −/R + is associated with a high risk of CMV infection, whereas D +/R − is associated with a low risk. For both types of transplantation, D +/R + is associated with intermediary risk of CMV infection. Due to the low number of low risk patients in our cohort (N = 13), intermediary and low risk patients are grouped together in this table.
V1 is the first positive CMV PCR of the infectious episode, Vpeak is the highest measured CMV PCR sample within 14 days of V1.
When calculating CMV doubling time, the V1 sample and the Vpeak sample, are used. Based on this time span, the proportion of time during CMV infection on treatment has been determined for each patient.
A. Of these, 10 kidney- and two lung recipients had prophylaxis breakthrough; for one lung and two kidney recipients treatment was initiated before confirmation of positive CMV PCR due to strong clinical suspicion of CMV infection. B. The latter was also the case for the six HSCT patients.
Defined as either CMV syndrome (fever, leukopenia or malaise due to CMV infection) or CMV disease (pneumonia, enteritis, hepatitis, encephalitis or retinitis verified to be caused by CMV).
Allograft rejection or graft versus host disease confirmed with biopsy within 30 days prior to the first positive CMV PCR to the date of the highest measured CMV PCR.
For five HSCT recipients, these data were unavailable.
Risk of CMV infection was evenly distributed, overall 50% had high risk and 50% had intermediary or low risk of CMV infection. This distribution was not influenced by type of transplantation.
A total of 56 recipients did not receive any treatment during the follow-up time when the CMV PCR measurements used to determine the doubling time was determined. For an additional 21 patients, treatment was provided throughout this follow-up time. Among the remaining 116 recipients, treatment was provided during some of the follow-up time.
The median proportion of time that was used for calculation of doubling time and that was covered with anti-CMV medication was 50% (IQR 0–80%) (Table 1). For 21 recipients medication was ongoing throughout the entire period used for doubling time estimation either because of breakthrough on primary prophylaxis (ten kidney and two lung transplant recipients) or because of initiation of empiric therapy due to strong clinical suspicion (six HSCT and two SOT). Out of the 12 SOT recipients with breakthrough on prophylaxis, eight progressed to CMV disease, and in one case, sub-optimal dosages of the prophylactic treatment lead to the selection of ganciclovir-related drug mutations. This case was published in Am J of Transplantation in 2013 (Cunha-Bang et al., 2013). Overall, 59 (31%) of the recipients developed CMV disease (Table 1).
3.2. CMV Doubling Time
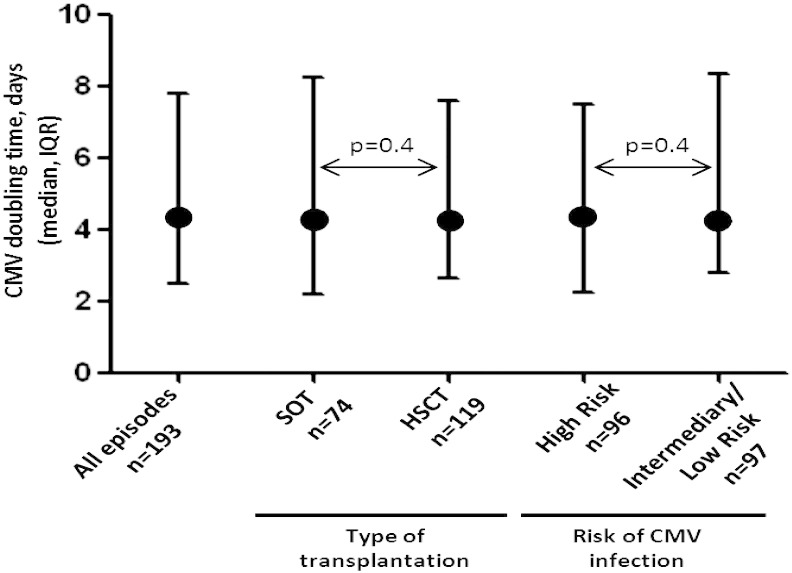
The median doubling time was 4.3 days (IQR 2.5–7.8) (Fig. 2). This estimate persisted after stratifying for risk of CMV infection according to pre-transplant CMV IgG D/R serostatus, and type of transplantation (p > 0.4). The CMV doubling time was similar for different years of transplantation (p > 0.05, data not shown). There was no correlation between the doubling time and the Vpeak (r = − 0.02), and this was also observed after stratifying for type of transplantation as well as risk of CMV infection associated with CMV IgG serostatus (p > 0.2). When calculating the CMV doubling time for the 56 infectious episodes that didn't receive any treatment during the interval used for calculation of doubling time was 4.1 (IQR 2.2–10.7) days. Comparing the doubling time of the 56 patients without treatment with the doubling time of the 137 recipients that did receive treatment at some point during the time used for calculation of doubling time, this was statistically insignificant (p = 0.99).
Fig. 2.
The estimated CMV doubling time for all infectious episodes, stratified for type of transplantation and risk of CMV infection.
Circles indicate the median doubling times and bars show the interquartile ranges.
Abbreviations: SOT; solid organ transplantation, HSCT; heamatopoietic stem cell transplantation.
A total of 59 patients developed CMV disease. The doubling time in those that developed versus those that did not develop CMV disease was 3.96 (1.72–7.39 IQR) days and 4.38 (2.88–7.77) days, respectively (p = 0.1).
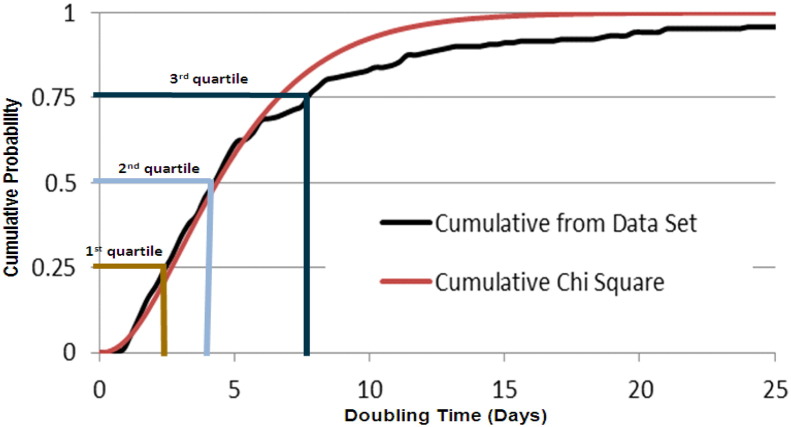
The calculated CMV doubling times of the cohort fitted well to a Chi Square distribution (Fig. 3). The infectious episodes were divided into four quartiles based on the doubling time (Fig. 3 and Table 2). This allowed for careful comparison of the recipients on the basis of the CMV doubling times of each quartile. No significant differences between the quartiles of the infectious episodes were detected when comparing factors such as age, gender, type of transplantation, risk of CMV infection associated with CMV IgG serostatus, and, for proportion of time on anti-CMV treatment during the interval used for calculation of doubling time (p > 0.2). There was no significant difference between the quartiles when assessing the proportion of rejection episodes or graft-versus-host disease (p = 0.4). There was a significantly higher proportion of symptomatic CMV infection when comparing the first quartile to the second, however, this was not persistent when comparing the first quartile to the two other quartiles (p > 0.1).
Fig. 3.
Distribution of the variation in CMV doubling time in the cohort.
Cumulative probability of the unique 193 doubling times of the cohort is plotted and fitted to a Chi Square distribution.
The infectious episodes could based on this plot be divided into four quartiles based on the doubling time, where the 1st quartile represent the fastest doubling times, and the 4th the slowest.
Table 2.
Characteristics of recipients stratified for quartile of CMV doubling time.
| Characteristics |
1st quartile |
2nd quartile |
3rd quartile |
4th quartile |
p-Value |
|---|---|---|---|---|---|
| N = 49 | N = 48 | N = 48 | N = 48 | ||
| Median CMV doubling time (IQR), days | 1.7 (1.4–2.1) | 3.4 (2.9–3.9) | 5.6 (4.7–6.6) | 12.0 (9.2–21.2) | |
| Median age, years (IQR) | 51 (34–62) | 47.5 (33–58) | 47 (24–57) | 45 (36–54.5) | 0.7 |
| Gender (proportion of males) | 36/49 (73%) | 31/48 (65%) | 34/48 (71%) | 32/48 (67%) | 0.3 |
| Median proportion of time during CMV infection on treatment (IQR) | 0.33 (0–0.75) | 0.43 (0–0.81) | 0.62 (0.21-0.85) | 0.61 (0–0.85) | 0.3 |
| Type of transplantation | |||||
| Proportion of SOT recipients | 22/49 (45%) | 15/48 (31%) | 15/48 (31%) | 22/48 (46%) | 0.2 |
| Proportion of HSCT recipients | 27/49 (55%) | 33/48 (69%) | 33/48 (69%) | 26/48 (54%) | 0.2 |
| Risk of CMV infection | |||||
| Proportion at high risk | 28/49 (57%) | 20/48 (42%) | 28/48 (58%) | 20/48 (42%) | 0.2 |
| Proportion at intermediary/low risk | 21/49 (43%) | 28/48 (58%) | 20/48 (42%) | 28/48 (58%) | 0.2 |
| Median V1 (IQR) IU/mL | 1001 (455–3640) | 1147 (546–3076) | 915 (455–4596) | 1911 (814–14,560) | 0.02⁎ |
| Median Vpeak (IQR) IU/mL | 18,200 (4186–58,240) | 5779 (1957–17,290) | 3044 (1456–12,376) | 3504 (1124–31,987) | 0.01 |
| Proportion of recipients with symptomatic CMV infection1 | 22/48 (46%) | 9/46 (20%) | 14/47 (30%) | 14/47 (30%) | 0.01⁎⁎ |
| Proportion of recipients with allograft rejection or GVHD | 7/49 (14%) | 9/48 (19%) | 10/48 (21%) | 10/48 (21%) | 0.4 |
For five HSCT recipients, data regarding CMV disease was unavailable.
The median V1 was not significantly different when comparing the 1st, 2nd and 3rd quartiles. The 4th quartile had a significantly higher V1 sample compared to the 1st quartile, p = 0.02.
There was a significantly higher proportion of symptomatic CMV infection when comparing the 1st quartile to the 2nd, however this was not persistent when comparing the 1st quartile to the two other quartiles (p > 0.1).
3.3. Simulation of CMV Screening Interval
A mathematical model was constructed to determine the proportion of first CMV infections that was diagnosed with an undesirably high viral load. This proportion depended on the assumed doubling time and the test periodicity in the screening interval (Table 3). When applying a fixed doubling time of 1.3 days (Funk et al., 2007), approximately 11% (95% CI 10.1–11.9%) of the recipients would risk being diagnosed at a high viral load if a 7 day screening interval was used and 33% (95% CI 31.7–34.3%) if the screening interval was extended to 10 days, taking into consideration that an additional extra day is needed for laboratory processing. In comparison, using the varied doubling time estimates from our cohort, only 1.4% (95% CI 1.07–1.72%) and 4.3% (95% CI 3.73–4.86%) of the recipients would be at risk of CMV diagnosis at an undesirably high viral load when applying a 7 or 10 day screening interval.
Table 3.
Proportion of recipients with an undesirablya high CMV virus load, when applying a pre-emptive strategy to prevent CMV disease depending on intervals between CMV PCR screening and the assumed doubling time of the CMV infections.
| Intervals between screening with CMV PCR as part of a pre-emptive strategy to detect emerging CMV infection (days)b |
|||
|---|---|---|---|
| 7 |
10 |
14 |
|
| Assumed doubling time: | Estimated % of recipients having undesirably high CMV viral load (IU/mL)a if the infection develops during the screening interval | ||
| 31 h — no variation | 11.1 | 33.3 | 50.0 |
| Varied as observed in our cohort | 1.4 | 4.3 | 8.7 |
Assuming that the doubling time (and possible variation hereof) is as depicted in the left column, infections emerges randomly during the screening interval, and the aim of the pre-emptive strategy is to detect the infection before the virus reach an undesirably high viral load of > 18,200 IU/mL.
7 days is currently the recommended standard; it is assumed that the results of the screening test are available 24 h later (the day displayed + 1).
108 recipients had an infectious episode where the V1 sample was preceded by a previous negative CMV PCR exactly 7 days earlier, and among these, two recipients (1.9%) had an undesirably high viral load upon detection of the infection.
4. Discussion
In this study the overall CMV doubling time was longer and more varied than previously reported (Emery et al., 1999, Emery et al., 2000, Emery et al., 2002, Mattes et al., 2005, Atabani et al., 2012, Buyck et al., 2010). Furthermore, we present a simulation of factors that help determine an optimal interval between CMV PCR screenings as part of a pre-emptive strategy aimed at diagnosing CMV infection while the viral load remains below a defined threshold. Furthermore, one of the model estimates was confirmed to be consistent with observed data. The output from our model question the current recommendation based on previously reported doubling time estimates (Emery et al., 1999, Emery et al., 2000, Emery et al., 2002) of weekly screening (Kotton et al., 2013, Tomblyn et al., 2009), as we project that such a short doubling time would generate an unacceptably high proportion (> 10%) of recipients with an undesirably high viral load at diagnosis. Only intervals shorter than 7 days would lower this proportion to < 5%. Conversely, in cohorts of transplant recipients with doubling times similar to ours, our model suggest that the interval between screening can be extended to 10 days while still preserving a proportion of first infections diagnosed with an undesirably high viral load below 5%.
Several researchers have attempted to estimate the doubling time for CMV infection. Using a similar mathematical approach, our results suggest that the doubling time is substantially longer and more varied. This raises the question of whether the populations or the virus studied in these investigations are intrinsically different to ours. We used a combined cohort of SOT and HSCT recipients, who received different primary CMV chemoprophylaxis and intensity of immunosuppression, but found the doubling time to be comparable. Our hospital doesn't routinely use T-cell depletion through administration of anti-CD52 antibodies. Thus, it may be possible that our cohort on average is less immune deficient than cohorts reported from the United Kingdom where such antibodies are more frequently used (Tauro et al., 2005). Our findings warrant future cross-site comparative studies to further elucidate potential explanations.
Anti-CMV treatment was given to some of the recipients in our cohort during the follow-up time used to estimate the CMV doubling time, but the doubling time estimate was unaffected by this. Most CMV particles are present outside the blood compartment (Plachter et al., 1996, Jarvis and Nelson, 2002), and hence plasma levels of CMV-DNA predominantly reflect wash-out from those compartments. Thus, it is not surprising that use of anti-CMV medication doesn't affect early kinetic changes in plasma CMV-DNA levels after the infection is first diagnosed and treatment is initiated. Previous studies have suggested that blood CMV-DNA may continue to increase the first 2 weeks after initiation of anti-CMV treatment (Nichols et al., 2001, Park et al., 2011).
Most of the previously published work is based on CMV PCR measurements in whole blood (Emery et al., 1999, Emery et al., 2000, Emery et al., 2002, Mattes et al., 2005, Atabani et al., 2012, Buyck et al., 2010), and not plasma as in our study. Whole blood contains more DNA than plasma, and virus loads are typically higher in whole blood compared to plasma (Lisboa et al., 2011). However, the relationship between consecutive CMV PCR samples should be the same regardless of whether they are measured in whole blood or plasma (Lisboa et al., 2011, Razonable et al., 2002), making it less likely that our calculated doubling time is explained by this methodological difference.
Intrinsic variation in the CMV PCR assay could tend to lead to larger variation in estimating the CMV doubling time. We used commercial and standardized assays (the COBAS Amplicor kit and the COBAS AmpliPrep/COBAS TaqMan) (DiDomenico et al., 1996), and our internal calculation suggests that the variation of the PCR kit is maximally 0.5 log10 (data not shown). Thus, it is unlikely that the used CMV PCR would explain the discrepancy between our findings and those reported by others. Future studies focused on estimating CMV doubling time are encouraged to perform daily sampling which would collectively reduce this problem.
We used consecutive CMV infection episodes as basis for estimating the CMV doubling time, although this was only possible for 193 of the 329 first time CMV infections that emerged in our cohort. The excluded episodes typically occurred before the initiation of the MATCH programme. Thus, these episodes were not as rigorously screened, and they contained more SOT recipients. However, key virological parameters such as the Vpeak did not significantly differ, and the included material is considered representable of the cohort.
Conversely, intentional selection has been reported but not quantified in previous studies, where the doubling time for recipients who had < 3 fold increase in CMV virus load or for recipients who had a self-limiting infectious episodes was not calculated (Munoz-Cobo et al., 2011, Gimenez et al., 2014). Such selection may preferentially include recipients experiencing rapid replication, while excluding recipients with a less dramatic increase in virus load.
The cut off for undesirably high virus load was set to > 18,200 IU/mL. This is an arbitrary decision based on both clinical experience and an ongoing study at our hospital on the prevalence of CMV disease at CMV diagnosis. The goal of the pre-emptive strategy at our hospital is to diagnose CMV infections while the viral load is below this threshold, as data from our hospital suggest that the prevalence of CMV disease at diagnosis is substantially higher (> 20 fold) if the infection is diagnosed with a virus load > 18,200 IU/mL (unpublished data; Lodding, I. et al.). This observation is also consistent with that of the previous literature (Gor et al., 1998, Cope et al., 1997, Hassan-Walker et al., 1999). However, when applying the tool for estimation of the optimal screening interval provided in Appendix B, clinicians may choose another threshold for undesirably high virus load depending on their own clinical experiences and preferences.
Our simulation model provides an independent confirmation of the CMV doubling time we estimated. The model derives a proportion of CMV infections diagnosed with an undesirably high CMV viral load when screening the cohort every 7 days to be comparable with what we actually observe in our cohort. With this confirmed fixed point in mind, it is intriguing that our model would predict an unacceptably high proportion of diagnosis being made with an undesirably high CMV viral load had the original estimates of the doubling time in fact been correct. As most transplantation units world-wide are adhering to and are comfortable with the 7 day interval, this also indirectly suggest that previous estimates of the CMV doubling time may be too short. We encourage other transplantation units to present the distribution of their diagnostic viral loads and to consider using our simulation model (enclosed as Appendix A) and possibly engage in collaboration with us to further refine this tool.
In summary, we provide new insight into the estimation of the doubling time of CMV infection in transplant recipients, and its clinical applicability. This report may result in a better understanding of virological and clinical aspects of this frequent and important complication in the immune deficient host.
Contributions
I.P. Lodding was responsible for study design, collection of data, data analysis and interpretation, and, writing of manuscript. C. da Cunha-Bang and J.D. Lundgren was involved in study design, collection of data, data analysis and interpretation, and writing of manuscript. C.M. Frederiksen was involved in creating the algorithm for calculation of doubling time. J.G. Downing and J. Grarup were responsible for the mathematical simulation of CMV screening intervals. A. Mocroft revised the writing of manuscript, and Appendix. H. Sengeløv, S.S. Sørensen, A. Rasmussen, M. Iversen, F. Gustafsson, and N. Kirkby, were involved in the writing of the manuscript and provided scientific input.
Conflicts of Interests
None of the authors have any conflicts of interests to disclose.
Role of the Funding Source
This study is supported by grant [grant number DNRF126] from the Danish National Research Foundation. The funding source had no involvement in any part of the study design, data collection, data analysis, and interpretation of the data or in the writing of this manuscript.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.05.003.
Appendix A and B. Supplementary Data
Supplementary material.
References
- Andrews P.A., Emery V.C., Newstead C. Summary of the British Transplantation Society guidelines for the prevention and management of CMV disease after solid organ transplantation. Transplantation. 2011;92:1181–1187. doi: 10.1097/TP.0b013e318235c7fc. [DOI] [PubMed] [Google Scholar]
- Atabani S.F., Smith C., Atkinson C. Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy. Am. J. Transplant. 2012;12:2457–2464. doi: 10.1111/j.1600-6143.2012.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen E.F., Emery V.C., Wilson P. Cytomegalovirus polymerase chain reaction viraemia in patients receiving ganciclovir maintenance therapy for retinitis. AIDS. 1998;12:605–611. doi: 10.1097/00002030-199806000-00009. [DOI] [PubMed] [Google Scholar]
- Buyck H.C., Griffiths P.D., Emery V.C. Human cytomegalovirus (HCMV) replication kinetics in stem cell transplant recipients following anti-HCMV therapy. J. Clin. Virol. 2010;49:32–36. doi: 10.1016/j.jcv.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Cope A.V., Sabin C., Burroughs A., Rolles K., Griffiths P.D., Emery V.C. Interrelationships among quantity of human cytomegalovirus (HCMV) DNA in blood, donor–recipient serostatus, and administration of methylprednisolone as risk factors for HCMV disease following liver transplantation. J. Infect. Dis. 1997;176:1484–1490. doi: 10.1086/514145. [DOI] [PubMed] [Google Scholar]
- Cunha-Bang C., Kirkby N., Sonderholm M. The time course of development and impact from viral resistance against ganciclovir in cytomegalovirus infection. Am. J. Transplant. 2013;13:458–466. doi: 10.1111/ajt.12042. [DOI] [PubMed] [Google Scholar]
- da Cunha-Bang C., Sorensen S.S., Iversen M. Factors associated with the development of cytomegalovirus infection following solid organ transplantation. Scand. J. Infect. Dis. 2011;43:360–365. doi: 10.3109/00365548.2010.549836. [DOI] [PubMed] [Google Scholar]
- DiDomenico N., Link H., Knobel R. COBAS AMPLICOR: fully automated RNA and DNA amplification and detection system for routine diagnostic PCR. Clin. Chem. 1996;42:1915–1923. [PubMed] [Google Scholar]
- Emery V.C., Cope A.V., Bowen E.F., Gor D., Griffiths P.D. The dynamics of human cytomegalovirus replication in vivo. J. Exp. Med. 1999;190:177–182. doi: 10.1084/jem.190.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery V.C., Sabin C.A., Cope A.V., Gor D., Hassan-Walker A.F., Griffiths P.D. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- Emery V.C., Hassan-Walker A.F., Burroughs A.K., Griffiths P.D. Human cytomegalovirus (HCMV) replication dynamics in HCMV-naive and -experienced immunocompromised hosts. J. Infect. Dis. 2002;185:1723–1728. doi: 10.1086/340653. [DOI] [PubMed] [Google Scholar]
- Funk G.A., Gosert R., Hirsch H.H. Viral dynamics in transplant patients: implications for disease. Lancet Infect. Dis. 2007;7:460–472. doi: 10.1016/S1473-3099(07)70159-7. [DOI] [PubMed] [Google Scholar]
- Ghisetti V., Barbui A., Franchello A. Quantitation of cytomegalovirus DNA by the polymerase chain reaction as a predictor of disease in solid organ transplantation. J. Med. Virol. 2004;73:223–229. doi: 10.1002/jmv.20079. [DOI] [PubMed] [Google Scholar]
- Gimenez E., Munoz-Cobo B., Solano C., Amat P., Navarro D. Early kinetics of plasma cytomegalovirus DNA load in allogeneic stem cell transplant recipients in the era of highly sensitive real-time PCR assays: does it have any clinical value? J. Clin. Microbiol. 2014;52:654–656. doi: 10.1128/JCM.02571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gor D., Sabin C., Prentice H.G. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant. 1998;21:597–605. doi: 10.1038/sj.bmt.1701139. [DOI] [PubMed] [Google Scholar]
- Hassan-Walker A.F., Kidd I.M., Sabin C., Sweny P., Griffiths P.D., Emery V.C. Quantity of human cytomegalovirus (CMV) DNAemia as a risk factor for CMV disease in renal allograft recipients: relationship with donor/recipient CMV serostatus, receipt of augmented methylprednisolone and antithymocyte globulin (ATG) J. Med. Virol. 1999;58:182–187. [PubMed] [Google Scholar]
- Jarvis M.A., Nelson J.A. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr. Opin. Microbiol. 2002;5:403–407. doi: 10.1016/s1369-5274(02)00334-x. [DOI] [PubMed] [Google Scholar]
- Kotton C.N., Kumar D., Caliendo A.M. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- Lisboa L.F., Asberg A., Kumar D. The clinical utility of whole blood versus plasma cytomegalovirus viral load assays for monitoring therapeutic response. Transplantation. 2011;91:231–236. doi: 10.1097/TP.0b013e3181ff8719. [DOI] [PubMed] [Google Scholar]
- Mattes F.M., Hainsworth E.G., Hassan-Walker A.F. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J. Infect. Dis. 2005;191:89–92. doi: 10.1086/425905. [DOI] [PubMed] [Google Scholar]
- Munoz-Cobo B., Solano C., Costa E. Dynamics of cytomegalovirus (CMV) plasma DNAemia in initial and recurrent episodes of active CMV infection in the allogeneic stem cell transplantation setting: implications for designing preemptive antiviral therapy strategies. Biol. Blood Marrow Transplant. 2011;17:1602–1611. doi: 10.1016/j.bbmt.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Nebbia G., Mattes F.M., Cholongitas E. Exploring the bidirectional interactions between human cytomegalovirus and hepatitis C virus replication after liver transplantation. Liver Transpl. 2007;13:130–135. doi: 10.1002/lt.21037. [DOI] [PubMed] [Google Scholar]
- Nichols W.G., Corey L., Gooley T. Rising pp 65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001;97:867–874. doi: 10.1182/blood.v97.4.867. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Lee S.O., Choi S.H. Paradoxical rising cytomegalovirus antigenemia during preemptive ganciclovir therapy in hematopoietic stem cell transplant recipients: incidence, risk factors, and clinical outcomes. J. Clin. Microbiol. 2011;49:4179–4184. doi: 10.1128/JCM.05464-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachter B., Sinzger C., Jahn G. Cell types involved in replication and distribution of human cytomegalovirus. Adv. Virus Res. 1996;46:195–261. doi: 10.1016/s0065-3527(08)60073-1. [DOI] [PubMed] [Google Scholar]
- Razonable R.R., Humar A. Cytomegalovirus in solid organ transplantation. Am. J. Transplant. 2013;13(Suppl. 4):93–106. doi: 10.1111/ajt.12103. [DOI] [PubMed] [Google Scholar]
- Razonable R.R., Brown R.A., Wilson J. The clinical use of various blood compartments for cytomegalovirus (CMV) DNA quantitation in transplant recipients with CMV disease. Transplantation. 2002;73:968–973. doi: 10.1097/00007890-200203270-00025. [DOI] [PubMed] [Google Scholar]
- Tauro S., Craddock C., Peggs K. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J. Clin. Oncol. 2005;23:9387–9393. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- Tomblyn M., Chiller T., Einsele H. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol. Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.