Abstract
Background
Efavirenz (EFV) based antiretroviral therapy is expanding worldwide. However discontinuation of EFV containing regimens is common in some patients, particularly black patients, due most often to neuropsychiatric side effects. These adverse drug effects often result in premature drug discontinuation, as well as considerable morbidity.
Methods
We genotyped CYP2A6, CYP2B6 and CYP3A4, which encode enzymes principally involved in EFV metabolism, from patients enrolled in the multinational SMART, FIRST and ESPRIT studies, for whom outcome data of treatment adherence was available. Patients with loss or decrease of function single nucleotide polymorphisms (SNPs) in the above genes were assigned a risk score based upon the number of SNPs present weighted relative to whether CYP2B6 (main metabolism pathway) and/or CYP2A6 and CYP3A4 (accessory pathways) were involved. Cox regression models were used to study the association between high genetic risk and time from initiation to EFV discontinuation. Failure was defined as discontinuation of an antiretroviral regimen other than for virologic failure or protocol determined discontinuation.
Findings
Patients with highest pharmacogenetic risk, as defined by cumulative SNPs in CYP2A6, CYP2B6 and CYP3A4, have an increased risk of discontinuation of EFV containing therapy compared to patients with lower genetic risk scores (adjusted HR 1.9, 95% CI 1.2, 3.1, P = 0.009). High genetic risk score was not associated with an increased risk of discontinuing atazanavir or nevirapine. High genetic risk was present more often in blacks compared to non-blacks (Adjusted OR 4.5, 95% CI: 1.9,10.5), and treatment discontinuation was also increased in blacks overall (Adjusted HR 1.4, 95% CI 1.0, 1.9). However, high genetic risk was more associated with treatment discontinuation than race alone for both blacks (Adjusted OR 1.9, 95% CI 0.8, 4.8) and non-blacks (Adjusted OR 5.3, 95% CI 1.5, 18.0).
Interpretation
Premature discontinuation of ART delays the time to effective long term viral suppression, and is associated with significant morbidity. Pharmacogenetic testing may predict those with a high risk of EFV discontinuation, and therefore should be considered in patients in whom initiation of EFV based ART is being considered.
Funding
Funded by NIH.
Keywords: HIV, Pharmacogenetics, Efavirenz, Premature discontinuation
Highlights
-
•
Efavirenz containing antiretroviral regimens are frequently complicated by premature discontinuation due to adverse drug effects.
-
•
Elevated pharmacogenetic risk based on genes for efavirenz-metabolizing enzymes is associated with premature discontinuation of efavirenz.
-
•
Pharmacogenetic testing prior to prescribing antiretrovirals may decrease premature discontinuation due to adverse effects.
1. Introduction
Effective combination antiretroviral therapy (cART) has dramatically changed the clinical course of HIV infection. However, this clinical success requires affordable access to medications, and lifelong adherence. Specific antiretroviral agents may have high rates of adverse drug effects (ADEs), which lead to discontinuation of otherwise effective (i.e., virologically suppressive) treatment regimens in up to 40% of patients within 1 year (Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America, 2002–2009, 2013). The World Health Organization, the International AIDS Society and the Department of Health and Human Services (Adolescents PoAGfAa, 2014, Gunthard et al., 2014) recommend efavirenz (EFV) containing regimens as initial first line therapy for HIV, due to once daily dosing which promotes compliance, and favorable long term treatment outcomes. In addition EFV came off patent in November 2013, making generic versions of this drug more affordable particularly in resource poor settings (Walensky et al., 2013). However, treatment discontinuation occurs in up to 20% percent of patients on once daily EFV based therapy (Scourfield et al., 2012, Walmsley et al., 2013), due primarily to the ADE's associated with EFV: central neuro-psychiatric symptoms, dizziness, lightheadedness, confusion and abnormal dreams, and a higher likelihood of committing suicide than patients on other agents (Katie Mollan et al., 2013, Mollan et al., 2014). The WHO estimates that 16.8 million persons living with HIV in low- and middle-income countries will be using cART by 2016, and it is estimated that the global market share of EFV among non-nucleotide reverse transcriptase inhibitor based regimens will increase from 40% to 63% (Organization WH, 2014). Given the effectiveness of EFV, and its increasing affordability, it is of global public health importance to accurately predict those patients who will tolerate EFV, particularly in light of the expanding use of EFV in resource limited settings (Takuva et al., 2013).
EFV undergoes hepatic metabolism catalyzed by cytochromes P450 CYP2B6 (main pathway), and CYP2A6 and CYP3A4 (accessory pathways) (Desta et al., 2007, Telenti and Zanger, 2008). Single nucleotide polymorphisms (SNPs) in CYP2B6, CYP2A6 and CYP3A4 exist that result in loss of function (LOF) or decrease of function (DOF), which associate with slower EFV metabolism and consequently increased drug levels (di Iulio et al., 2009, Arab-Alameddine et al., 2009). Because the risk of EFV associated neuro-psychiatric symptoms is increased in patients with higher plasma concentrations (Marzolini et al., 2001) (Csajka et al., 2003) (Gounden et al., 2010) (Nanzigu et al., 2012, Gutierrez et al., 2005), it is likely that patients who have SNPs in CYP2B6, CYP2A6 and CYP3A4, will have a higher incidence of EFV intolerance. Indeed, in 831 participants from three AIDS Clinical Trials group studies, SNPs in CYP2B6 were associated with an increased likelihood of a CNS adverse effect from EFV (Ribaudo et al., 2010), and in a separate study of 577 patients, SNPs in all three enzymes, CYP2A6, CYP2B6 and CYP3A4, were associated with discontinuation of EFV-based regimens within 12 months of initiation (Lubomirov et al., 2011).
In the studies referenced above, the majority of subjects were non-black (66% in (Ribaudo et al., 2010) and 80% in (Lubomirov et al., 2011)), yet the greatest increase in EFV use is likely in populations that are of broader ethnicity, thus it is of importance to determine whether SNP analysis is associated with EFV discontinuation in mixed ethnicity populations. We therefore sought to determine if pharmacogenetic testing of select SNPs in CYP2B6, CYP2A6, and CYP3A4 would be associated with premature treatment discontinuation of virologically suppressive, EFV-containing ART regimens in a multi-national, mixed ethnicity cohort of patients with a long duration of follow up.
2. Materials and Methods
2.1. Participants
Participants included in this study were HIV-positive patients who participated in studies conducted by INSIGHT (International Network for Strategic Initiatives in Global HIV Trials) and the Community Programs for Clinical Research on AIDS (CPCRA). The INSIGHT SMART (Strategies for Management of Anti-Retroviral Therapy) study opened in January 2002 and enrolled 5472 participants. Patients could be cART-naïve or experienced (El-Sadr et al., 2008). The INSIGHT ESPRIT (Evaluation of Subcutaneous Proleukin® in a Randomized International Trial) study opened in March 2000 and enrolled 4150 patients. ESPRIT enrolled patients who were taking cART at study entry (Abrams et al., 2009). The CPCRA FIRST (Flexible Initial Retrovirus Suppressive Therapies) study enrolled 1397 patients who were ART-naïve from 1999 to 2002 (MacArthur et al., 2006). For the SMART and FIRST studies, ART-naïve patients beginning their first EFV-based or nevirapine (NVP)-based regimen at study entry were included. For SMART and ESPRIT, patients who were cART experienced and who switched to EFV or atazanavir (ATV) during follow-up were included. For the FIRST study, control participants on only 2 classes of drugs were included.
Inclusion criteria for this investigation included 1) signed informed consent for collection and preservation of DNA samples, 2) DNA samples available for testing, and 3) presence of a minimum of 12 months of follow up data concerning antiretroviral therapy adherence, duration, changes, side effects and ADEs. Patients were excluded if virologic failure led to treatment discontinuation or change. Written informed consent was obtained for participation in each trial and for the collection of blood for DNA extraction for the future studies of genetic variants.
Participants were enrolled in 26 countries from 6 different continents. Over half (57%) were enrolled in North America, 21% were enrolled in Europe, 9% in South America, 8% in Asia, 4% in Australia and 1% in Africa.
2.2. Genotyping
Among patients who consented, DNA was extracted and 6 loss of function (LOF) or decrease of function (DOF) SNPs, in CYP2A6 (rs28399433 [risk allele C]), CYP2B6 (rs28399499 [G], rs35303484 [G], rs35979566 [A], rs3745274 [A]), and CYP3A4 (rs4646437 [A]) were assessed using the Illumina VeraCode™ Genotyping Assay. The BeadArrays were scanned in an Illumina BeadXpress™ reader and the fluorescent signals analyzed with Illumina GenomeStudio software, with automated genotype clustering and calling. A genetic risk score from 1 (lowest risk) to 6 (highest risk) based on the presence of LOF and DOF SNPs was defined a priori (Lubomirov et al., 2011) and calculated for each participant (Table 1).
Table 1.
Genetic risk score for premature discontinue of efavirenz.
| Score 1 (reference score): Homozygous for the reference allele in all 3 genes |
| Score 2: Homozygous for the reference allele for CYP2B6, and 1 to 4 LOF/DOF alleles for CYP2A6 and CYP3A4 |
| Score 3: 1 LOF/DOF allele for CYP2B6 but no LOF/DOF allele for CYP2A6 and CYP3A4 |
| Score 4: 1 LOF/DOF allele for CYP2B6 and 1 to 4 LOF/DOF alleles for CYP2A6 and CYP3A4 |
| Score 5: 2 LOF/DOF alleles for CYP2B6 but no LOF/DOF allele for CYP2A6 and CYP3A4 |
| Score 6: 2 LOF/DOF alleles for CYP2B6 and 1 to 4 LOF/DOF alleles for CYP2A6 and CYP3A4 |
2.3. Statistical Analysis
Descriptive statistics were used to describe baseline characteristics of study participants. For ART-naïve patients, characteristics at study entry are given; for those that were cART-experienced, characteristics at the time the EFV or ATV regimen was initiated during follow-up are given. Logistic regression models were used to study factors measured at treatment initiation associated with genetic risk score. Unadjusted and adjusted odds ratios (OR) (genetic score of 6 versus 1–5) are given with 95% confidence intervals (CIs).
Time to event methods (Kaplan–Meier survival scores and Cox regression) were used to compare time from initiation to discontinuing EFV according to genetic risk score. Discontinuation of a single drug for any reason was considered because detailed information regarding reasons for discontinuation was not collected. In the Cox regression models, the following baseline covariates were considered: age, gender, race, CD4 + T cell count, HIV-RNA, BMI and co-infection with hepatitis B or C to estimate adjusted hazard ratios (HRs) of discontinuing EFV (genetic risk score of 6 versus 1–5) (Lubomirov et al., 2011). These models were stratified by study (SMART, ESPRIT, and FIRST). Separate and combined models for ART-naïve and ART-experienced patients were considered. To increase confidence that the associations found were due to EFV, as negative controls, we also estimated the risk of discontinuing nevirapine (NVP) and atazanavir (ATV) associated with the genetic risk score. A priori, no association of the genetic risk score with discontinuing these treatments was expected. For these analyses, expanded Cox models with interaction terms were used to assess whether the risk of discontinuation of EFV associated with the genetic risk score differed from that of NVP and ATV.
We also carried out pooled analyses using individual level data previously published from the Swiss Cohort Study (Lubomirov et al., 2011). For this analysis, a 12 month follow-up period was considered in both studies. Logistic regression was used to study the association of genetic risk score with EFV discontinuation by 12 months. Odds ratios (ORs) and 95% CIs are cited for scores of 1 through 5, each versus a score of 6. The logistic model was stratified by study and included covariates corresponding to age, gender, race, CD4 + T cell count, HIV RNA level, BMI and hepatitis co-infection. The pooled analyses were also carried out for self-identified blacks and non-blacks separately.
Two-sided p-values less than 0.05 was considered statistically significant. Statistical analyses were performed using SAS software (version 9.3).
3. Results
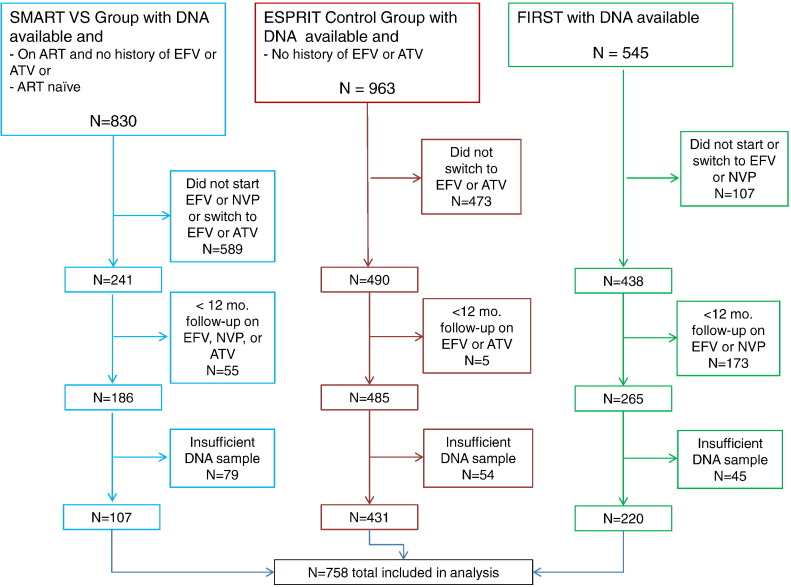
1134 participants were identified as meeting inclusion criteria, whereas 1204 participants did not. Of these, 761 (67%) participants had DNA of sufficient quantity and quality to perform the genotyping assay. After genotyping, 3 participants were excluded due to uninterpretable assay results. Thus, the analyses in this report are based on a total of 758 patients (See Fig. 1 for flow diagram).
Fig. 1.
Participant eligibility and exclusion stratified by study (SMART, ESPRIT and FIRST).
Of the 758 patients, 131 (17%) were ART naïve and initiated EFV as their first regimen and 315 (42%) were ART experienced who switched to EFV from another regimen. Eighty (11%) participants were ART naïve and initiated nevirapine (NVP) and 232 (31%) participants were ART experienced who switched to atazanavir (ATV) from another regimen. The characteristics of these patients at the time their cART regimen was initiated are summarized in Supplemental Tables 1 and 2. Of interest, 24% of all patients were black, which is a population known to have a greater incidence of premature cART discontinuation (Ribaudo et al., 2013).
3.1. Pre-treatment Factors Associated With High Genetic Risk Score
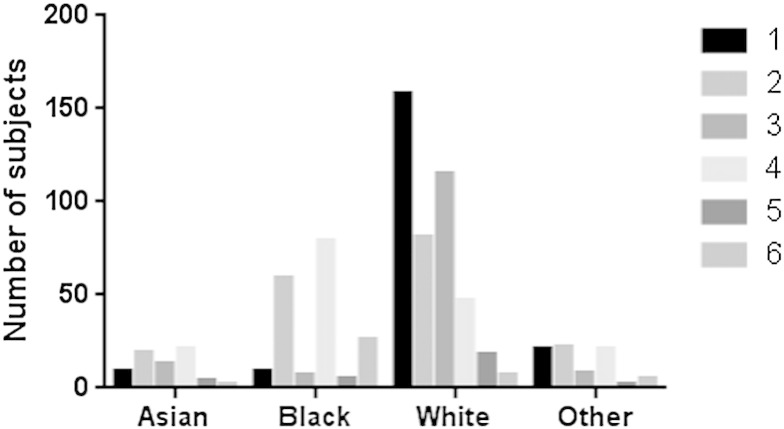
Genetic risk score was calculated based on accumulation of LOF and DOF SNPs in CYP2A6, CYP2B6 and CYP3A4 as previously described (see Table 1 and (Lubomirov et al., 2011)). Overall, 40 patients (5.3%) had a genetic risk score of 6 (Fig. 2), 26 of whom were black. The prevalence of a high genetic risk in blacks was 26/185 (14.1%), whereas for non-blacks it was 14/573 (2.4%) (unadjusted OR (black/non-black) for a genetic risk score of 6 was 5.5; 95% CI:2.6–12.0; p < 0.001 Table 2a, Table 2b). Therefore while EFV toxicity occurs commonly in blacks, the prevalence of high genetic risk remains uncommon in blacks, and even less common in non-blacks. Apart from the black race, and gender (female vs male unadjusted OR 3.3 (1.7–6.4), p < 0.001; adjusted OR 2.1 (1.0, 4.3), p = 0.04) there were not significant associations with the genetic risk score.
Fig. 2.
Number of study participants with each EFV genetic score (from 1 [lowest] to 6 [highest]) is depicted according to race (Asian, black, white or others).
Table 2a.
Baseline characteristics of participants by genetic risk score (INSIGHT).
| Characteristic | Scores 1–5 | Score 6 | OR (95% CI) score 6 vs 1–5a | OR (95% CI) score 6 vs 1–5b |
|---|---|---|---|---|
| No. | 718 | 40 | ||
| Age in years (median, IQR; OR per 10 year increase) | 40 (34, 45) | 37 (34, 41) | 0.8 (0.5, 1.1) | 0.8 (0.5, 1.1) |
| Female (%) | 21.3 | 47.5 | 3.3 (1.7, 6.4) | 2.1 (1.0, 4.3) |
| Black race (%) | 22.1 | 65.0 | 5.5 (2.6, 12.0) | 4.5 (1.9, 10.5) |
| CD4 T cell count in cells/mm3 (median, IQR; OR per 100 cell increase) | 414 (280, 571) | 312 (136, 520) | 0.9 (0.8, 1.1) | 1.0 (0.8, 1.1) |
| HIV RNA (% < 500 copies/) | 44.3 | 22.5 | 0.6 (0.2, 1.4) | 0.7 (0.3, 1.9) |
| BMI kg/m2 (median, IQR; OR per 5 kg/m2 increase) | 24.0 (21.8, 26.4) | 23.9 (22.0, 27.7) | 1.1 (0.8, 1.6) | 1.0 (0.7, 1.3) |
| Hepatitis B or C co-infection (%) | 13.1 | 10.0 | 1.1 (0.4, 3.4) | 1.1 (0.4, 3.6) |
Unadjusted, stratified by study.
Adjusted for age, gender, race, CD4 T cell count, HIV RNA, BMI, and hepatitis co-infection and stratified by study.
Table 2b.
Baseline characteristics of participants by genetic risk score (Swiss Cohort).
| Characteristic | Scores 1–5 | Score 6 | OR (95% CI) score 6 vs 1–5a | OR (95% CI) score 6 vs 1–5b |
|---|---|---|---|---|
| No. | 259 | 13 | ||
| Age in years (median, IQR; OR per 10 year increase) | 39 (34, 47) | 36 (32, 42) | 0.7 (0.4, 1.3) | 1.2 (0.6, 2.5) |
| Female (%) | 25.5 | 61.5 | 4.7 (1.5, 14.8) | 1.8 (0.5, 6.8) |
| Black race (%) | 14.7 | 76.9 | 19.4 (5.1, 73.7) | 17.5 (3.6, 84.4) |
| CD4 T cell count in cells/mm3 (median, IQR; OR per 100 cell increase) | 207 (119, 269) | 204 (152, 248) | 0.9 (0.6, 1.4) | 0.9 (0.4, 1.7) |
| HIV RNA (log10 copies/ml) | 4.9 (4.4, 5.4) | 4.6 (4.4, 4.9) | 0.8 (0.4, 1.5) | 0.8 (0.3, 2.0) |
| BMI kg/m2 (median, IQR; OR per 5 kg/m2 increase) | 22.6 (20.9, 24.6) | 23.0 (21.4, 25.3) | 1.0 (0.5, 2.2) | 0.9 (0.4, 2.3) |
| Hepatitis B or C co-infection (%) | 45.6 | 53.8 | 1.4 (0.5, 4.3) | 0.9 (0.3, 3.1) |
Unadjusted.
Adjusted for age, gender, race, CD4 T cell count, HIV RNA, BMI, and hepatitis co-infection.
3.2. Association of Genetic Risk Score With Treatment Discontinuation
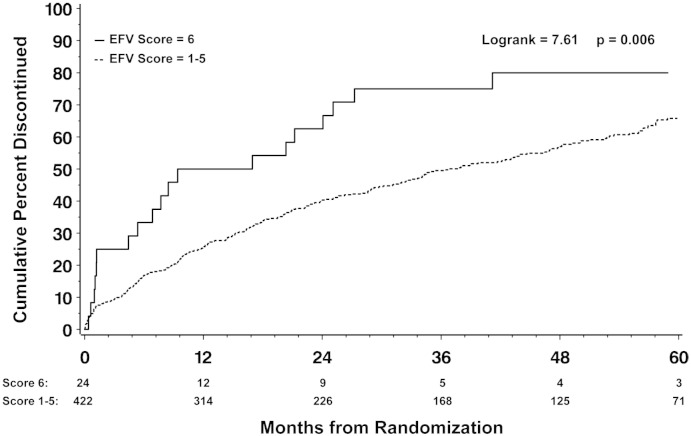
Participants with genetic risk scores of 1–5 had similar rates of discontinuation (range; 14 to 26 per 100 person years, p = 0.10 for difference), thus those groups were combined. Participants with a genetic risk score of 6 had a significantly increased risk of discontinuing effective ART regimens containing EFV compared to participants with risk scores of 1–5 (HR 2.0 (95% CI 1.2, 3.1); P = 0.004). Conversely patients with a high genetic risk were not more likely to discontinue ATV or NVP (HR 0.6 (95% CI 0.1, 4.5) P = 0.64 and HR 1.2 (0.6, 2.6) P = 0.54 respectively). This effect was apparent in both ART naïve participants who initiated EFV based regiments (HR 2.5 (95% CI 1.2, 5.4); P = 0.02) and in ART experienced patients who switched to EFV based regimens (HR 1.7 (95% CI 0.9, 3.0); P = 0.08). The increased risk for EFV discontinuation in participants with risk score of 6 persisted after adjustment for age, gender, race, CD4 T cell count, HIV RNA, body mass index, hepatitis co-infection and stratified by study (HR 1.9 (95% CI 1.2, 3.1); P = 0.009, Table 3). Importantly, black subjects were only slightly more likely to discontinue EFV than non-blacks, (HR 1.4, 95% CI 1.0, 1.9) indicating that genetic risk score was superior to race in associating with which patients discontinued EFV. EFV discontinuation occurred as early as two months after starting EFV containing therapy and continued to occur up until to 60 months of follow up (Fig. 3).
Table 3.
Risk of premature treatment discontinuation of effective antiretroviral therapy according to genetic risk score.
| Patient group | N (ratea) with scores 1–5 who discontinued | N (ratea) with score 6 who discontinued | Unadjusted HR (95% CI) for discontinuation of ART, score 6 vs 1–5 | P value | Adjustedb HR (95% CI) for discontinuation of ART, score 6 vs 1–5 | P value |
|---|---|---|---|---|---|---|
| ART naïve who started EFV (N = 131) | 85 (25.1) | 7 (69.4) | 2.5 (1.2, 5.4) | 0.02 | 2.2 (1.0, 4.9) | 0.05 |
| ART experienced who switched to EFV (N = 315) | 174 (21.4) | 13 (38.9) | 1.7 (0.9, 3.0) | 0.08 | 1.6 (0.9, 3.0) | 0.13 |
| All who started EFV (N = 446) | 259 (22.5) | 20 (45.9) | 2.0 (1.2, 3.1) | 0.004 | 1.9 (1.2, 3.1) | 0.009 |
| ART naïve who started NVP (N = 80) | 56 (34.9) | 9 (37.7) | 1.2 (0.6, 2.6) | 0.54 | 1.5 (0.6, 3.7) | 0.35 |
| ART experienced who switched to ATV (N = 232) | 91 (16.6) | 1 (9.6) | 0.6 (0.1, 4.5) | 0.64 | 0.6 (0.1, 4.8) | 0.65 |
Per 100 person-years.
Adjusted for age, gender, race, CD4 T cell count, HIV RNA, BMI, and hepatitis co-infection and stratified by study.
Fig. 3.
Kaplan–Meier estimates of the cumulative percentage patients on EFV-containing regimens who discontinued the regimen comparing high genetic risk patients (Score 6) to average genetic risk patients (Scores 1 to 5).
3.3. Pooled Analysis With Swiss Cohort
A pooled analysis of the CPCRA and INSIGHT studies together with the Swiss Cohort (Gutierrez et al., 2005) was carried out to increase the power to determine if there was a graded relationship of the score with risk of discontinuation of EFV (Table 4). Each of the lower scores, 1–5, was associated with a reduced risk of EVF discontinuation compared to a score of 6. However, the risk associated with scores 1–5 did not vary (p = 0.15).
Table 4.
Risk of premature treatment discontinuation of effective antiretroviral therapy according to genetic risk score — pooled analysis with Swiss Cohort.
| Genetic risk score | N (%) who discontinued within first year: INSIGHT | N (%) who discontinued within first year: Swiss Cohort | Unadjusteda OR (95% CI) for discontinuation in first year, vs. score of 6 | P value | Adjustedb OR (95% CI) for discontinuation in first year, vs. score of 6 | P value |
|---|---|---|---|---|---|---|
| Score 1 | 31 (28.4) | 22 (30.6) | 0.38 (0.19, 0.79) | 0.009 | 0.41 (0.18, 0.90) | 0.03 |
| Score 2 | 30 (28.6) | 16 (28.6) | 0.38 (0.18, 0.79) | 0.01 | 0.36 (0.17, 0.77) | 0.009 |
| Score 3 | 16 (18.4) | 14 (21.9) | 0.23 (0.11, 0.49) | < 0.001 | 0.24 (0.10, 0.54) | < 0.001 |
| Score 4 | 29 (27.6) | 19 (35.2) | 0.41 (0.20, 0.85) | 0.02 | 0.39 (0.18, 0.82) | 0.01 |
| Score 5 | 2 (12.5) | 3 (23.1) | 0.19 (0.06, 0.60) | 0.005 | 0.20 (0.06, 0.65) | 0.008 |
| Score 6 | 12 (50.0) | 7 (53.8) | 1.0 | – | 1.0 | – |
| Total | 120 (26.9) | 81 (29.8) |
Stratified by study.
Adjusted for age, gender, race, CD4 T cell count, HIV RNA, BMI, and hepatitis co-infection and stratified by study.
3.4. Analysis by Population and Study Stratification
Because high genetic risk score was over-represented in black participants, we conducted population stratification analyses based on race and study (Table 5a, Table 5b). The risk of discontinuation of EFV was increased for those with high genetic risk score for both blacks (Adjusted OR 1.9 (p = 0.18)) and non-blacks (Adjusted OR 5.3 (p = 0.008)), indicating that pharmacogenetic prediction was associated with increased risk of EFV discontinuation independent of self-identified race.
Table 5a.
Risk of premature treatment discontinuation of effective antiretroviral therapy according to genetic risk score – Pooled analysis with Swiss Cohort – Black race (N = 166).
| Genetic Risk Score | N (%) who discontinued within first year: INSIGHT | N (%) who discontinued within first year: Swiss Cohort | Unadjusteda OR (95% CI) for discontinuation in first year, vs. score of 6 | P value | Adjustedb OR (95% CI) for discontinuation in first year, vs. score of 6 | P value |
|---|---|---|---|---|---|---|
| Score 1 | 2 (28.6) | 1 (100.0) | 0.96 (0.18, 5.04) | 0.96 | 0.60 (0.08, 4.47) | 0.62 |
| Score 2 | 11 (29.7) | 1 (9.1) | 0.44 (0.16, 1.24) | 0.12 | 0.37 (0.12, 1.14) | 0.08 |
| Score 3 | 2 (50.0) | 0 (0) | 1.44 (1.17, 12.4) | 0.74 | 2.46 (0.24, 25.1) | 0.45 |
| Score 4 | 14 (25.9) | 10 (41.7) | 0.58 (0.23, 1.47) | 0.25 | 0.54 (0.20, 1.46) | 0.23 |
| Score 5 | 1 (100.0) | 1 (50.0) | 1.97 (0.16, 24.7) | 0.60 | 2.87 (0.17, 48.1) | 0.46 |
| Score 6 | 7 (46.7) | 7 (40.0) | 1.0 | – | 1.0 | – |
| Total | 37 (31.4) | 17 (35.4) | ||||
| Score 6 vs. scores 1–5 | 1.71 (0.72, 4.08) | 0.23 | 1.89 (0.75, 4.78) | 0.18 |
Stratified by study.
Adjusted for age, gender, CD4 T cell count, HIV RNA, BMI, and hepatitis co-infection and stratified by study.
Table 5b.
Risk of premature treatment discontinuation of effective antiretroviral therapy according to genetic risk score – pooled analysis with Swiss Cohort – non-black race (N = 552).
| Genetic risk score | N (%) who discontinued within first year: INSIGHT | N (%) who discontinued within first year: Swiss Cohort | Unadjusteda OR (95% CI) for discontinuation in first year, vs. score of 6 | P value | Adjustedb OR (95% CI) for discontinuation in first year, vs. score of 6 | P value |
|---|---|---|---|---|---|---|
| Score 1 | 29 (28.4) | 21 (29.6) | 0.21 (0.06, 0.72) | 0.01 | 0.24 (0.07, 0.84) | 0.03 |
| Score 2 | 19 (27.9) | 15 (33.3) | 0.23 (0.06, 0.81) | 0.02 | 0.24 (0.07, 0.85) | 0.03 |
| Score 3 | 14 (16.9) | 14 (21.9) | 0.12 (0.03, 0.43) | 0.001 | 0.13 (0.04, 0.47) | 0.002 |
| Score 4 | 15 (29.4) | 9 (30.0) | 0.23 (0.06, 0.82) | 0.02 | 0.23 (0.06, 0.85) | 0.03 |
| Score 5 | 1 (6.7) | 2 (18.2) | 0.07 (0.01, 0.36) | 0.002 | 0.07 (0.01, 0.38) | 0.002 |
| Score 6 | 5 (55.6) | 3 (100.0) | 1.0 | – | 1.0 | – |
| Total | 83 (25.3) | 64 (28.6) | ||||
| Score 6 vs. scores 1–5 | 5.59 (1.66, 18.8) | 0.006 | 5.27 (1.54, 18.0) | 0.008 |
Stratified by study.
Adjusted for age, gender, CD4 T cell count, HIV RNA, BMI, and hepatitis co-infection and stratified by study.
4. Discussion
EFV-based regimens are recommended as first-line and command a substantial market share of current ART use; yet as EFV is now off patent and generic versions are available at lower cost, it is likely that the use of EFV containing regimens will increase in resource limited settings where cost of medications has prohibited widespread access to therapy. However EFV discontinuation rates for non-virologic reasons (i.e., for reasons other than viral resistance) can reach as high as 20% and therefore being able to a priori discriminate those who are more likely to tolerate EFV based therapy is of great global health importance. Herein we demonstrate in a multiethnic population, individuals with loss or decrease of function alleles for 2 or more EFV metabolizing enzymes, including CYP2B6, are at ~ 2-fold increased risk of premature discontinuation of EFV, irrespective of race or any other factor.
As EFV becomes a more affordable and widespread option for HIV care, a pharmacogenetic test that accurately discriminates those patients who will discontinue EFV for ADE reasons could be an important addition to the clinician's decision tools for selecting cART regimens for individual patients. HLA-B*5701 testing to predict abacavir hypersensitivity significantly influenced the care of HIV, by allowing the safe use of a medication that had life threatening ADE potential. Notably, the frequency of the highest genetic risk score in the present study (5.3%) is similar to the reported frequency of HLA-B*5701 (Orkin et al., 2010). Being able to determine who will tolerate EFV may enhance the ability of patients who take EFV to tolerate EFV, lower risk of developing NNRTI resistance due to missed doses, and reduced morbidity due to ADEs. Each of these predictions is testable and warrants further study. This may lead to cost savings through less work and school absences, less need to switch cART regimens and incur the additional expense and inconvenience of doctor visits and lab tests needed to initiate and monitor a therapy change. This may not necessarily be the case though; the rate per 100 person years for stopping EFV (including ART experienced and naïve combined) in our study was 23.3. The corresponding rate for stopping NVP was 35.3; however, better tolerated agents are available now, including integrase inhibitors, which could be used instead of EFV or NVP. An alternative strategy could be reducing EFV dose. Evidence supporting reduced dosing for EFV is found in the ENCORE1 trial where reduced dose EFV (400 mg) was non-inferior to 600 mg EFV for virologic suppression, and study-drug related ADEs (Puls et al., 2014); it would be of great interest to stratify such an analysis by genetic risk score.
In our study, those participants with the highest genetic risk score of 6 had an increased risk for EFV discontinuation compared to those participants with risk scores 1–5. We therefore performed a post hoc analysis of pooled data of 781 patients starting EFV-based regimens from our cohort and the patients in the Swiss Cohort (Lubomirov et al., 2011) and that showed the risk of EFV discontinuation did not change in patients with genetic risk scores of 1–5, likely reflecting the known redundancy in EFV metabolizing pathways, and is consistent with data in patients with CYP2B6 polymorphisms 516G > T and 983 T > C where plasma EFV plasma concentrations were elevated only in those patients who also had other accessory pathway mutations (Haas et al., 2014).
Black participants in our cohort had a higher prevalence (14.1%) of high genetic risk compared to other races (2.4%), consistent with reported population allele frequencies for variants in those genes (http://www.ncbi.nlm.nih.gov/snp/). A high prevalence of CYP2B6 516G > T (rs3745274) has been previously reported in Ghanaian patients (Sarfo et al., 2014), and racial differences in the prevalence are evident in HapMap and 1000 Genomes data (35–42% in Sub-Saharan Africans, 23–27% in Europeans, 15–18% in Asians). Thus if an individual with this allele also happens to have other LOF/DOF alleles in EFV metabolizing enzymes, it is logical that they might be intolerant to EFV. It is noteworthy however that race alone did not predict EFV discontinuation in our analyses — indicating that the pharmacogenetic risk stratification employed in our study is more discriminatory than determination of race alone. In addition, it is likely that factors other than genetic risk contribute to disparities in treatment discontinuation which could contribute to an attenuation of observed genetic risk.
There are potential limitations of our study. Our study focused on three CYP enzymes, and thus on pharmacokinetic pharmacogenetics. There might be other pharmacokinetic factors, e.g., genetically polymorphic transporters, that contribute to premature discontinuation of EFV that were not assessed here. It is also conceivable that some of the side effects might also be influenced by pharmacodynamic pharmacogenetics — i.e., genetic variation in the targets for the drug. In addition, since we included only those participants with 12 months of follow up data, there is the potential for survivorship bias. Finally, data was not available in all studies regarding pregnancy as a potential cause for premature treatment discontinuation. Future studies should take these potential factors into account.
In conclusion, given the significant association of high genetic risk score with EFV but not NVP or ATZ discontinuation in a multiethnic cohort, assessment of this score is warranted in large prospective international cohorts. The cost effectiveness of this strategy would need to be determined, particularly in resource-limited settings.
Conflict of Interest
JKR has received honoraria for speaking at educational events or consulting from AbbVie, Bionor, Boehringer-Ingelheim, BMS, Gilead, Janssen, Merck, Tibotec, and ViiV. MB has received honoraria from AbbVie, Boehringer-Ingelheim, Bristol Myers Squibb, Gilead, Janssen-Cilag and Merck and grant funding from Gilead and Merck. All other authors declare no conflict of interests.
Funding
This publication was made possible in part by through the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health through grants U01AI042170, U01AI46957, U01AI046362, U01AI068641, R01AI110173 and R56AI102959; CTSA Grant Number UL1 TR000135 and 8KL2TR000136-08 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH; as well as NIH grants R01 GM28157 and U19GM61388 (Pharmacogenomics Research Network); and the Swiss National Science Foundation (no. 141234). It was also supported by the Pharmacogenomics Translational Program of the Mayo Center for Individualized Medicine. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. We thank the staff of the Medical Genome Facility Genotyping Core (GTC) at the Mayo Clinic for carrying out the genotyping analyses for this study. The GTC is supported in part by the NCI Cancer Center Support Grant P30 CA 15083.
Acknowledgments
We thank the participants of the studies. See N Engl J Med 2006; 355:2283-96 for the complete list of SMART investigators, N Engl J Med 2009; 361:1548-59 for the complete list of ESPRIT investigators and Lancet 2006; 368:2125-35 for the complete list of FIRST investigators.
Portions of the data were previously presented at the Individualizing Medicine Conference 2013 in Rochester, MN, USA.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.05.012.
Appendix A. Supplementary Data
Supplementary Tables.
References
- Abrams D., Levy Y., Losso M.H. Interleukin-2 therapy in patients with HIV infection. N. Engl. J. Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolescents PoAGfAa . 2014. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [Google Scholar]
- Arab-Alameddine M., Di Iulio J., Buclin T. Pharmacogenetics-based population pharmacokinetic analysis of efavirenz in HIV-1-infected individuals. Clin. Pharmacol. Ther. 2009;85:485–494. doi: 10.1038/clpt.2008.271. [DOI] [PubMed] [Google Scholar]
- Csajka C., Marzolini C., Fattinger K. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin. Pharmacol. Ther. 2003;73:20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- Desta Z., Saussele T., Ward B. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics. 2007;8:547–558. doi: 10.2217/14622416.8.6.547. [DOI] [PubMed] [Google Scholar]
- di Iulio J., Fayet A., Arab-Alameddine M. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet. Genomics. 2009;19:300–309. doi: 10.1097/FPC.0b013e328328d577. [DOI] [PubMed] [Google Scholar]
- Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002–2009. AIDS. 2013;27:803–813. doi: 10.1097/QAD.0b013e32835cb997. [DOI] [PubMed] [Google Scholar]
- El-Sadr W.M., Grund B., Neuhaus J. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann. Intern. Med. 2008;149:289–299. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- Gounden V., van Niekerk C., Snyman T., George J.A. Presence of the CYP2B6 516G > T polymorphism, increased plasma efavirenz concentrations and early neuropsychiatric side effects in South African HIV-infected patients. AIDS Res. Ther. 2010;7:32. doi: 10.1186/1742-6405-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthard H.F., Aberg J.A., Eron J.J. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA panel. JAMA. 2014;312:410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- Gutierrez F., Navarro A., Padilla S. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin. Infect. Dis. 2005;41:1648–1653. doi: 10.1086/497835. [DOI] [PubMed] [Google Scholar]
- Haas D.W., Kwara A., Richardson D.M. Secondary metabolism pathway polymorphisms and plasma efavirenz concentrations in HIV-infected adults with CYP2B6 slow metabolizer genotypes. J. Antimicrob. Chemother. 2014;69(8):2175–2182. doi: 10.1093/jac/dku110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katie Mollan M., Smurzynski Marlene, Na Lumine, Robertson Kevin, Campbell Thomas, Sax Paul, Daar Eric, Eron Joseph, Gulick Roy, O'keefe Lauren, Tierney Camlin. ID Week 2013. 2013. Hazard of suicidality in patients randomly assigned to efavirenz for initial treatment of HIV-1: a cross-study analysis conducted by the AIDS Clinical Trials Group (ACTG) [Google Scholar]
- Lubomirov R., Colombo S., di Iulio J. Association of pharmacogenetic markers with premature discontinuation of first-line anti-HIV therapy: an observational cohort study. J. Infect. Dis. 2011;203:246–257. doi: 10.1093/infdis/jiq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur R.D., Novak R.M., Peng G. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST study): a long-term randomised trial. Lancet. 2006;368:2125–2135. doi: 10.1016/S0140-6736(06)69861-9. [DOI] [PubMed] [Google Scholar]
- Marzolini C., Telenti A., Decosterd L.A., Greub G., Biollaz J., Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- Mollan K.R., Smurzynski M., Eron J.J. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann. Intern. Med. 2014;161:1–10. doi: 10.7326/M14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanzigu S., Eriksen J., Makumbi F. Pharmacokinetics of the nonnucleoside reverse transcriptase inhibitor efavirenz among HIV-infected Ugandans. HIV Med. 2012;13:193–201. doi: 10.1111/j.1468-1293.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- Organization WH . 2014. Antiretroviral Medicines in Low-and Middle-Income Countries: Forecasts of Global and Regional Demand for 2013–2016. [Google Scholar]
- Orkin C., Wang J., Bergin C. An epidemiologic study to determine the prevalence of the HLA-B*5701 allele among HIV-positive patients in Europe. Pharmacogenet. Genomics. 2010;20:307–314. doi: 10.1097/FPC.0b013e3283390666. [DOI] [PubMed] [Google Scholar]
- Puls R., Amin J., Losso M. Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet. 2014;383:1474–1482. doi: 10.1016/S0140-6736(13)62187-X. [DOI] [PubMed] [Google Scholar]
- Ribaudo H.J., Liu H., Schwab M. Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS clinical trials group study. J. Infect. Dis. 2010;202:717–722. doi: 10.1086/655470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribaudo H.J., Smith K.Y., Robbins G.K. Racial differences in response to antiretroviral therapy for HIV infection: an AIDS clinical trials group (ACTG) study analysis. Clin. Infect. Dis. 2013;57:1607–1617. doi: 10.1093/cid/cit595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfo F.S., Zhang Y., Egan D. Pharmacogenetic associations with plasma efavirenz concentrations and clinical correlates in a retrospective cohort of Ghanaian HIV-infected patients. J. Antimicrob. Chemother. 2014;69:491–499. doi: 10.1093/jac/dkt372. [DOI] [PubMed] [Google Scholar]
- Scourfield A., Zheng J., Chinthapalli S. Discontinuation of Atripla as first-line therapy in HIV-1 infected individuals. AIDS. 2012;26:1399–1401. doi: 10.1097/QAD.0b013e328353b047. [DOI] [PubMed] [Google Scholar]
- Takuva S., Evans D., Zuma K., Okello V., Louwagie G. Comparative durability of nevirapine versus efavirenz in first-line regimens during the first year of initiating antiretroviral therapy among Swaziland HIV-infected adults. Pan Afr. Med. J. 2013;15:5. doi: 10.11604/pamj.2013.15.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A., Zanger U.M. Pharmacogenetics of anti-HIV drugs. Annu. Rev. Pharmacol. Toxicol. 2008;48:227–256. doi: 10.1146/annurev.pharmtox.48.113006.094753. [DOI] [PubMed] [Google Scholar]
- Walensky R.P., Sax P.E., Nakamura Y.M. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann. Intern. Med. 2013;158:84–92. doi: 10.7326/0003-4819-158-2-201301150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley S.L., Antela A., Clumeck N. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N. Engl. J. Med. 2013;369:1807–1818. doi: 10.1056/NEJMoa1215541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables.