Abstract
The overall 5-year survival for melanoma is 91%. However, if distant metastasis occurs (stage IV), cure rates are < 15%. Hence, melanoma detection in earlier stages (stages I–III) maximises the chances of patient survival. We measured the expression of a panel of 17 microRNAs (miRNAs) (MELmiR-17) in melanoma tissues (stage III; n = 76 and IV; n = 10) and serum samples (collected from controls with no melanoma, n = 130; and patients with melanoma (stages I/II, n = 86; III, n = 50; and IV, n = 119)) obtained from biobanks in Australia and Germany. In melanoma tissues, members of the ‘MELmiR-17’ panel were found to be predictors of stage, recurrence, and survival. Additionally, in a minimally-invasive blood test, a seven-miRNA panel (MELmiR-7) detected the presence of melanoma (relative to controls) with high sensitivity (93%) and specificity (≥ 82%) when ≥ 4 miRNAs were expressed. Moreover, the ‘MELmiR-7’ panel characterised overall survival of melanoma patients better than both serum LDH and S100B (delta log likelihood = 11, p < 0.001). This panel was found to be superior to currently used serological markers for melanoma progression, recurrence, and survival; and would be ideally suited to monitor tumour progression in patients diagnosed with early metastatic disease (stages IIIa–c/IV M1a–b) to detect relapse following surgical or adjuvant treatment.
Abbreviations: AGO2, argonaute RISC catalytic component 2; AJCC, American Joint Committee on Cancer; AUC, area under the curve; AUROC, area under the receiver operator curve; CI, confidence interval; Ct, threshold cycle; DOR, diagnostic odds ratio; FFPE, formalin-fixed paraffin-embedded; HR, hazard ratio; LDH, lactate dehydrogenase; M1a, metastasis to skin, subcutaneous (below the skin) tissue, or lymph nodes in distant parts of the body, with a normal blood LDH level; M1b, metastasis to the lungs, with a normal blood LDH level; M1c, metastasis to any other organs, OR distant spread to any site along with an elevated blood LDH level; MIA, Melanoma Institute of Australia; miR, microRNA; miRNA, microRNA; N stage, nodal or number of lymph nodes stage; NA, not applicable; NM, nodular melanoma; OR, odds ratio; PD1, programmed cell death protein; RNA, ribonucleic acid; S100B, S100 calcium-binding protein B; USA, United States of America; SMM, superficial spreading melanoma
Keywords: Melanoma, MiRNA, MicroRNA, Biomarker, Diagnostic, Prognostic
Highlights
-
•
A seven-miRNA panel (MELmiR-7) detected the presence of melanoma with high sensitivity (93%) and specificity (≥ 82%).
-
•
In serially collected stage IV specimens, members of the ‘MELmiR-7’ panel confirmed tumour progression in 100% of cases.
-
•
The ‘MELmiR-7’ panel is superior to currently used serological markers for melanoma progression, recurrence, and survival.
1. Introduction
Melanomas are among the most commonly occurring cancers. Crude incidence rates in Australia (AIHW, 2014) and the USA (SEER, 2014) were approximately 50 cases (in 2010) and 20 cases (in 2011) per 100,000 respectively. With the number of new cases rising each year, melanoma is currently is listed as the 4th and 6th most common cancer in Australia and the USA respectively (AIHW, 2014, SEER, 2014). Current clinical staging criteria classify melanoma progression from a pre-invasive lesion, confined to the epidermis (stage 0), a series of early stages of local invasion (I and II), a stage involving regional lymph nodes (stage III) and finally metastasis to distant sites (stage IV). The overall 5-year survival for melanoma is 91%, which is largely due to curative surgery for early stage disease. However, cure rates are < 15% (Balch et al., 2009) if distant metastasis occurs (stage IV; AJCC 7th edition). We now have evidence that current therapeutic options for late stage disease are more effective if the disease is treated with a lower disease burden (Sosman et al., 2012, Hodi et al., 2010). Hence, melanoma must be treated in earlier stages to maximise the chances of patient survival. Therefore, the ability to identify signs of melanoma progression sooner would be a valuable clinical tool.
Melanoma progression biomarkers have been studied intensively with varying levels of success. Serum lactate dehydrogenase (LDH) levels have been integrated into current staging regimens (Balch et al., 2009) and elevation of LDH levels increases in specificity as disease progresses (stages II (83%), III (87%), and IV (92%)). However the sensitivity of this marker is reduced during progression (stages II (95%), III (57%), and IV (79%)) (Brochez and Naeyaert, 2000, Finck et al., 1983, Karakousis et al., 1996, Sirott et al., 1993, Weide et al., 2012, Deichmann et al., 1999). S100B, a calcium binding protein, is raised in serum of stages III and IV melanoma patients (Guo et al., 1995, Smit et al., 2005). However, the proportion of patients with elevated S100B levels varies by stage: 0–9% in stages I/II, 5–98% in stage III, and 40–100% in stage IV (reviewed in Kruijff et al., 2009). As such, serum S100B is not routinely used in the clinic (Leiter et al., 2014), highlighting the fact that the current serological methods of progression detection, whilst relatively specific, are inadequate due to variability in sensitivity across all stages of disease. To date, there are no biomarkers that are sensitive or specific enough to be beneficial for early detection of melanoma (all stages). A blood test (‘circulating’ biomarkers) that detected melanoma with regional spread, prior to clinically evident distant metastasis, could improve treatment and outcomes for melanoma patients.
For a circulating biomarker to be effective, not only must it be sufficiently sensitive and specific, but it must also be highly stable and resistant to degradation. In recent years, circulating microRNAs (miRNAs) have been studied for their utility as biomarkers in a wide range of malignancies and disorders (Allegra et al., 2012, De Guire et al., 2013). miRNAs are small (20–22 nt) non-coding RNAs which function to regulate gene expression in the cell. Recently, tumour cells have been shown to release miRNAs into the circulation (Mitchell et al., 2008), contained primarily in micro-vesicles or exosomes (extracellular vesicles), or bound to AGO2 — a part of the miRNA-mediated silencing complex (Allegra et al., 2012, De Guire et al., 2013). Due to the ‘encapsulation’ of these miRNAs in serum or plasma they are highly resistant to degradation by RNases (highly concentrated in the blood), thus their potential usefulness as a ‘biomarker’ is relatively high. To date, circulating melanoma-related miRNAs have been rarely studied (Fleming et al., 2015, Friedman et al., 2012).
Herein we report a multi-centre study that identifies a panel of ‘melanoma-related’ miRNAs that offer superior sensitivity to currently used serological markers for melanoma progression, recurrence, and survival.
2. Materials and Methods
2.1. Patient Specimen Details
Formalin-fixed paraffin-embedded (FFPE) melanoma tissues and serum (melanoma and control patients) were obtained from prospectively collected biobanks in Australia and Germany.
2.2. Tissue Validation Cohort
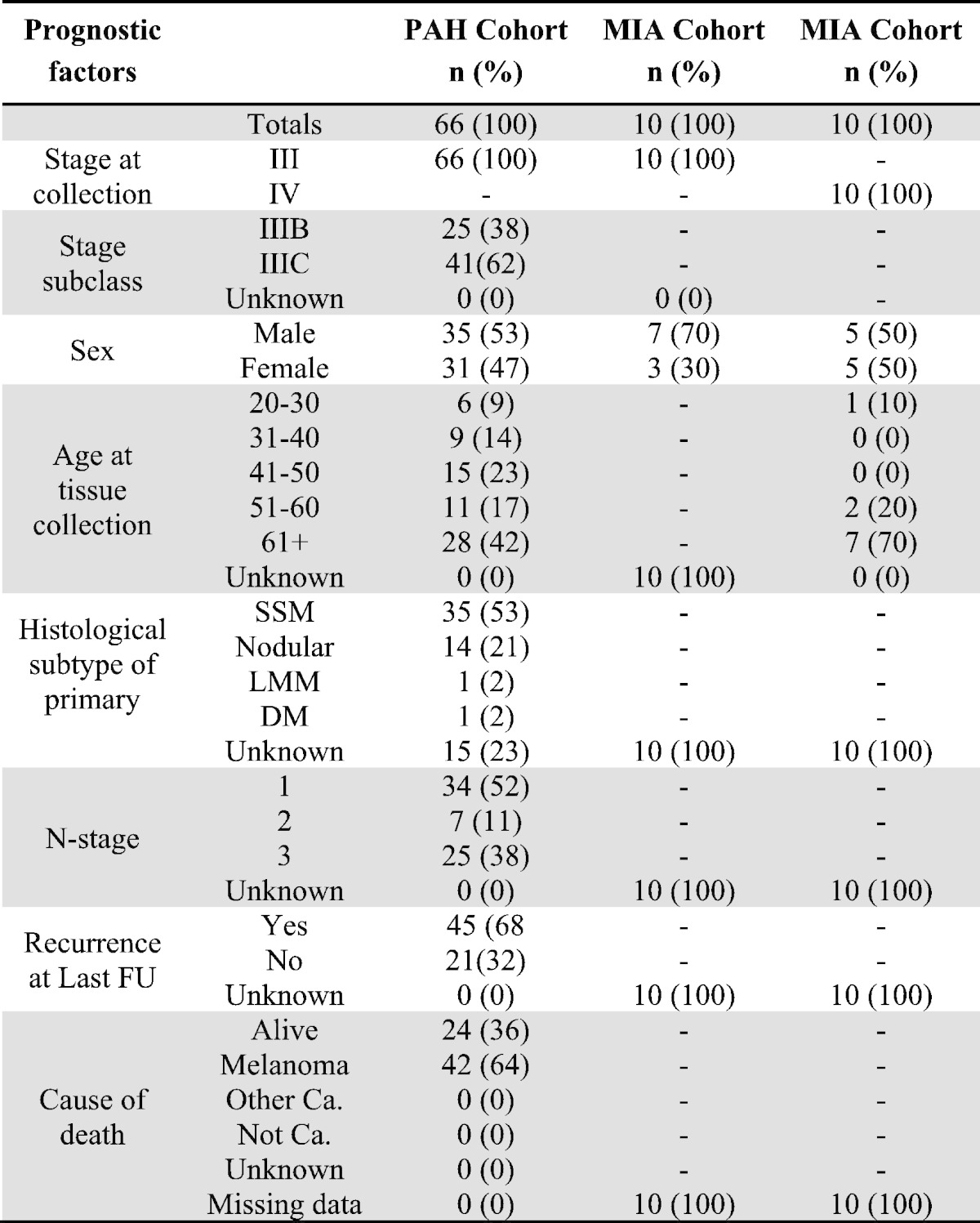
FFPE melanoma tissues, collected at diagnosis of stage III (PAH-tissue) were obtained via a database of prospective stage IIIA–C cutaneous melanoma cases, presenting to the Princess Alexandra Hospital (PAH) Melanoma Unit and affiliated private hospitals, which has been maintained since 1997. Permission to collect and use information was approved by the hospital ethics committee (HREC Reference number: HREC/11/QPAH/650; SSA reference number: SSA/11/QPAH/694). Inclusion criteria were the same as those previously presented (Tembe et al., 2015). An additional collection (collected at diagnosis) of stages III and IV melanoma tissues (MIA-tissue) were obtained via a database of prospectively recruited melanoma cases, presenting to the Melanoma Institute Australia (MIA) and affiliated private hospitals, which has been maintained since 1967. Informed written consent was obtained for each patient under approved protocols (Protocol No. X10-0305 &HREC/10/RPAH/539 and Protocol No. X10-0300 HREC/10/RPAH/530) governed by the Human Research Ethics Committee of the Royal Prince Alfred Hospital (Sydney NSW, Australia). Inclusion criteria were the same as those previously presented (Tembe et al., 2015). See Table 1 for participant descriptive statistics. Supplementary Table 2 shows the mean, median, and range of follow-up times.
Table 1.
Descriptive statistics for tissue cohorts.
2.3. Serum Validation Cohorts: Control Sera
Sera from ‘healthy co7ntrols’ were ascertained from a cohort of participants collected as part of the Australian Cancer Study (ACS) (QIMR Berghofer HREC approved project no. P399). As part of the ACS, potential controls were randomly selected from the Australian Electoral Roll (enrolment is compulsory). Controls were prospectively sampled from within strata of age (in 5 year age-groups) and state of residence. Of the 3258 potentially eligible control participants, 41 could not be contacted and 175 were excluded because they were deceased (16), too ill (61), or unable to read or write in English (98). Of 3042 controls meeting the inclusion criteria, 1680 (55%) gave their consent to take part. Completed questionnaires were returned by 1580 controls (48% of all potentially eligible controls selected from the roll). See Table 2 for participant descriptive statistics.
Table 2.
Descriptive statistics for serum cohorts.
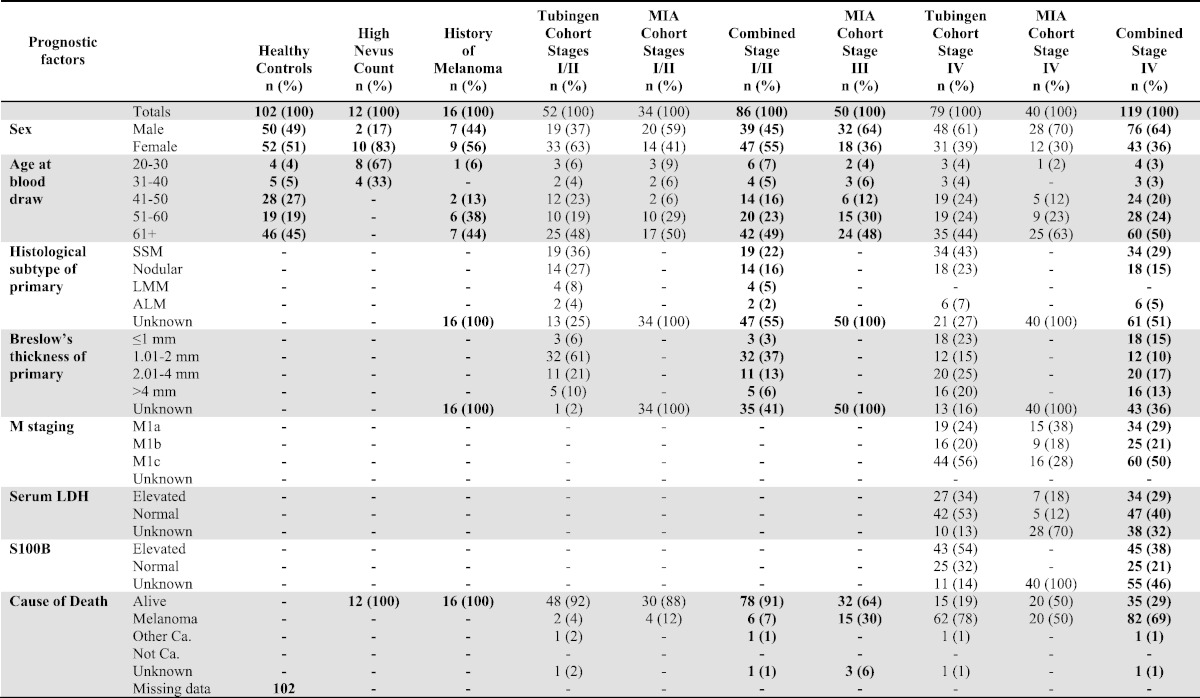
Sera from ‘high naevus count’ and ‘history of melanoma, disease-free’ participants were prospectively collected from cohorts who were enrolled in the study: ‘Pigmentation genotypes and phenotypic correlations with dermoscopic naevus types and distribution’. These samples were included as ‘controls’ to determine the level of expression measurable in sera derived from patients with a high melanocyte burden. All study participants were enrolled in the following human ethics approved projects: QIMR HREC/P1237, The Metro South Health District HREC/09/QPAH/162, and UQ HREC approval number is 2009001590. Participants with a history of melanoma (clinically free of disease at time of blood draw) were recruited through the Melanoma Unit and Dermatology Department of the Princess Alexandra Hospital, Brisbane, Queensland, Australia, between May 2012 and November 2012. Control participants, with no personal history of melanoma, were recruited from the Brisbane Twin Naevus Study between August 2012 and November 2012. All participants had 16-panel full-body images and dermoscopic images of significant naevi recorded. Significant naevi were defined as naevi greater than or equal to 5 mm on all body sites except the scalp, buttocks, mucosal surfaces and genitals, and greater than or equal to 2 mm on the back of both males and females and on the legs of females. All significant naevi were classified by the predominant dermoscopic pattern (reticular, globular, or non-specific), colour, and profile (flat, raised, domed or papillomatous). See Table 2 for participant descriptive statistics.
The description of ‘controls’ used in the analyses refers to a combined cohort of ‘healthy controls’, ‘high naevus count’, and ‘history of melanoma, disease-free’ participants.
2.4. Serum Validation Cohorts: Melanoma Patient Sera
Sera from stages I–IV melanoma patients (at time of blood draw and staged according to the current AJCC staging manual 3 (Balch et al., 2009)) had blood drawn and serum stored as part of a large prospectively collected cohorts from the university department of dermatology in Tubingen, Germany (‘Tubingen’ cohort) and Melanoma Institute of Australia, Sydney (‘MIA’ cohort). Usage of the ‘Tubingen’ bio-bank with corresponding patient data was approved by the Ethics Committee, University of Tübingen (approvals 657/2012BO2). Serially-collected stage IV patients (‘MIA’ cohort only) had blood drawn at time of diagnosis or at lower disease burden and then at higher disease burden (determined by routine diagnostic tests). All samples from the ‘MIA’ cohort had informed written consent obtained from each patient under approved protocols (Protocol No. X10-0305 &HREC/10/RPAH/539 and Protocol No. X10-0300 HREC/10/RPAH/530) governed by the Human Research Ethics Committee of the Royal Prince Alfred Hospital (Sydney NSW, Australia). See Table 2 for participant descriptive statistics. Supplementary Table 2 shows the mean, median, and range of follow-up times.
All serum samples were collected in 10-mL BD serum tubes then centrifuged for 10 min at 1500 ×g. The supernatant serum was then aliquoted into 1.5-mL cryovials and stored at − 80 °C until further use.
2.5. Total RNA Extraction From Validation Cohorts
A sterile disposable biopsy punch (Kai Medical, Japan) was used to retrieve tumour content from blocks that had been scored and marked for content via H&E histological staining. The extraction of total RNA from FFPE tissue and serum was performed respectively using miRNeasy FFPE Kits (Qiagen) as per manufacturer's instructions or as previously described (Tembe et al., 2015).
2.6. Selection Criteria for ‘Melanoma-related’ miRNAs
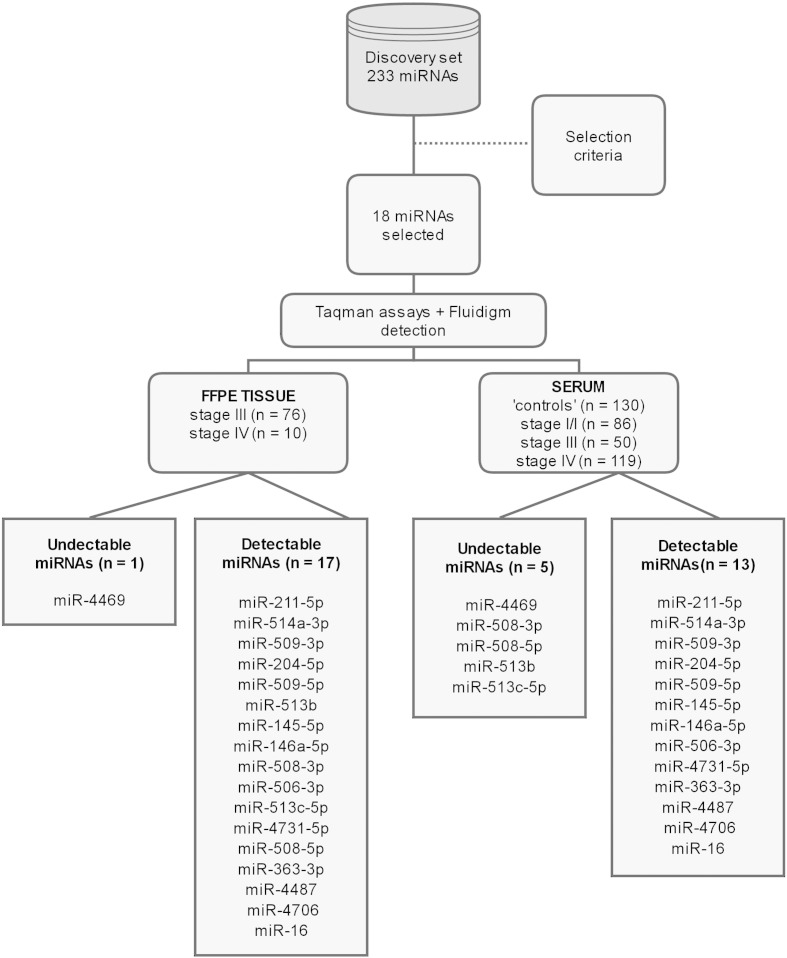
In our previously published miRNA microarray dataset (Stark et al., 2015) we found a total of 233/1898 miRNAs (‘Discovery’ set) (Fig. 1) that were differentially expressed (DE; corrected p ≤ 0.05 and ≥ 2-fold) between the melanoma cell lines (n = 55) and the ‘other’ solid cancers (n = 34). We applied filtering criteria to the 233 DE miRNAs to identify which miRNAs would be suitable to measure in patient derived serum. The following strict criteria were used to filter the ‘Discovery’ set: ≥ 15-fold higher expression in cutaneous melanoma vs. ‘other’ solid malignancies (n = 14/14), or ≥ 2-fold higher expression in cutaneous melanoma vs ‘other’ solid malignancies with no detectable expression in melanocytes or melanoblasts (n = 3/6). In addition, miR-16, which is known to be highly expressed in blood, was assessed for its suitability as an endogenous control. The 18 miRNA panel (MELmiR-18) comprising: miR-211-5p, miR-514a-3p, miR-509-3p, miR-204-5p, miR-509-5p, miR-513b, miR-145-5p, miR-146a-5p, miR-508-3p, miR-506-3p, miR-513c-5p, miR-4731-5p, miR-508-5p, miR-363-3p, miR-4487, miR-4469, miR-4706, and miR-16. This panel was carried forward for testing in independent cohorts of FFPE melanoma tumours and patient derived sera.
Fig. 1.
Study summary.
2.7. Reverse Transcription, Pre-amplification, Taqman Assays and Fluidigm Real-time PCR
We performed a custom Taqman assay combined with a sensitive method of detection (Fluidigm, HD Biomark) as previously described (Tembe et al., 2015). Briefly, a custom reverse transcription (RT) primer pool consisting of equal amounts of miRNA-specific RT primers contained within each TaqMan® Assay (Life Technologies, Carlsbad, USA; miR-211-5p (000514), miR-514a-3p (001147), miR-509-3p (002236), miR-204-5p (000508), miR-509-5p (002235), miR-513b (002757), miR-145-5p (002278), miR-146a-5p (000468), miR-508-3p (001052), miR-506-3p (001050), miR-513c-5p (002756), miR-4731-5p (464084_mat), miR-508-5p (002092), miR-363-3p (001271), miR-4487 (462492_mat), miR-4469 (465059_mat), miR-4706 (464518_mat), and miR-161 (000391) along with cel-miR-39 (000200; serum spiked-in control) and RNU-6 (001973; FFPE endogenous control)) plus an additional pool of the corresponding TaqMan® MicroRNA Assay (Pre-Amp Primer Pool) were used to pre-amplify the RT reaction. Each assay had a serial dilution of a positive control sample (known expression for all miRNAs in panel) that had a total input of 1, 3, 15, and 45 ng in the original cDNA reaction.
2.8. qRT-PCR Analysis
The expression of the ‘MELmiR-18’ panel (Fig. 1) was assayed in each sample with at least 4 technical replicate Taqman assays to determine their expression. Real-time expression data was extracted and analysed as previously described (Tembe et al., 2015).
2.9. Statistical Methods
The marker level differences (e.g., univariate analysis of each miRNA in each cohort comparison represented in Table 3) were assessed using the Mann–Whitney U-test and adjusted for multiple comparisons using the Benjamini & Hochberg method. Significant markers' predictive ability was evaluated using receiver operating characteristic (ROC curve) and area under the curve (AUC) or AUROC. Univariate and multivariate logistic regressions with backward covariate search based on AIC (Sakamoto et al., 1986, Vermont et al., 1991) were performed to identify significant markers which were associated with melanoma status/disease stages, when time to event information was missing. For survival and recurrence analyses, univariate and multivariate Cox proportional hazard model with backward covariate search based on AIC (Sakamoto et al., 1986, Vermont et al., 1991) was performed. Time to follow-up was measured from date of blood collection which was ≤ 1 month of staging. The proportional hazards assumption was also evaluated for each Cox regression (Grambsch and Therneau, 1994). The model fits were compared using likelihood ratio test. The predictive abilities of the selected significant markers were evaluated using AUROC. The selected markers were then used to classify patients using conditional inference tree analysis (Hothorn et al., 2006). For serum markers, the cutoff point of each marker that characterised the melanoma status was determined to maximise AUROC statistics.
Table 3.
Univariate analysis in serum cohorts for detectable miRNA.
| Comparison | Test | miR-145 | miR-146a | miR-16 | miR-204 | miR-211 | miR-363-3p | miR-4487 | miR-4706 | miR-4731 | miR-506-3p | miR-509-3p | miR-509-5p | miR-514a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n = 130) vs. Stages I/II (n = 86) | Mann–Whitney U test | 0.026 | < 0.0001 | < 0.0001 | ns | ns | 0.0088 | < 0.0001 | < 0.0001 | < 0.0001 | ns | < 0.0001 | ns | ns |
| AUROC score | 0.64 (0.56, 0.7) | 0.73 (0.67, 0.80) | 0.85 (0.79, 0.90) | 0.59 (0.48, 0.67) | 0.53 (0.47, 0.59) | 0.66 (0.58, 0.72) | 0.95 (0.91, 0.98) | 0.88 (0.83, 0.93) | 0.89 (0.85, 0.94) | 0.53 (0.48, 0.59) | 0.76 (0.69, 0.82) | 0.95 (0.92, 0.98) | 0.53 (0.47, 0.59) | |
| Controls (n = 130) vs. Stage III (n = 50) | Mann–Whitney U test | ns | 0.039 | < 0.0001 | ns | ns | ns | < 0.0001 | < 0.0001 | < 0.0001 | ns | < 0.0001 | < 0.0001 | ns |
| AUROC score | 0.57 (0.48, 0.66) | 0.65 (0.57, 0.73) | 0.87 (0.81, 0.91) | 0.54 (0.46, 0.62) | 0.55 (0.46, 0.65) | 0.62 (0.49, 0.70) | 0.93 (0.87, 0.98) | 0.85 (0.79, 0.91) | 0.85 (0.78, 0.90) | 0.54 (0.47, 0.62) | 0.72 (0.64, 0.79) | 0.93 (0.89, 0.96) | 0.53 (0.48, 0.61) | |
| Controls (n = 130) vs. Stage IV (n = 119) | Mann–Whitney U test | ns | ns | 0.0001 | 0.008 | < 0.0001 | ns | < 0.0001 | < 0.0001 | < 0.0001 | ns | < 0.0001 | < 0.0001 | ns |
| AUROC score | 0.53 (0.47, 0.59) | 0.60 (0.53, 0.66) | 0.70 (0.64, 0.77) | 0.65 (0.57, 0.71) | 0.72 (0.65, 0.78) | 0.55 (0.48, 0.61) | 0.89 (0.84, 0.93) | 0.85 (0.80, 0.90) | 0.93 (0.89, 0.96) | 0.53 (0.47, 0.59) | 0.74 (0.67, 0.80) | 0.91 (0.87, 0.94) | 0.57 (0.47, 0.64) | |
| Stage III (n = 50) vs. Stage IV (n = 119) | Mann–Whitney U test | ns | ns | ns | 0.025 | 0.025 | 0.025 | 0.0099 | ns | 0.025 | ns | < 0.0001 | ns | ns |
| AUROC score | 0.55 (0.47, 0.64) | 0.54 (0.47, 0.61) | 0.64 (0.55, 0.72) | 0.67 (0.59, 0.75) | 0.66 (0.58, 0.74) | 0.66 (0.58, 0.74) | 0.73 (0.62, 0.82) | 0.54 (0.47, 0.62) | 0.67 (0.58, 0.76) | 0.55 (0.47, 0.63) | 0.78 (0.71, 0.84) | 0.53 (0.47, 0.61) | 0.57 (0.46, 0.66) | |
| Stages I/II (n = 86) vs. Stage IV (n = 119) | Mann–Whitney U test | 0.026 | 0.043 | 0.031 | 0.0002 | 0.0014 | 0.0029 | 0.0082 | ns | ns | ns | < 0.0001 | ns | ns |
| AUROC score | 0.64 (0.58, 0.72) | 0.62 (0.55, 0.70) | 0.63 (0.56, 0.70) | 0.72 (0.66, 0.79) | 0.71 (0.65, 0.78) | 0.68 (0.61, 0.75) | 0.67 (0.59, 0.74) | 0.58 (0.49, 0.66) | 0.57 (0.48, 0.65) | 0.51 (0.48, 0.59) | 0.79 (0.73, 0.86) | 0.56 (0.44, 0.65) | 0.58 (0.47, 0.66) |
5000 nonparametric bootstraps were performed, per cohort (e.g., controls versus stage IV) and miRNA analysis pairs, to obtain robust effect size estimates, p values (for univariate analysis) and AUROC. To reflect the uncertainties of the values greater than Ct 36, the values above 36 were replaced by random values from 37 to 40 during the bootstrap. The original data was analysed without this consideration and the final models were rerun using 5000 bootstrap runs to generate robust outcomes.
For the analyses, OptimalCutpoints (v1.1–3), boot, and party packages on R version 3.0.2 were used to find cutpoints in univariate analyses (cohort vs. markers), bootstrapping and tree analyses respectively.
ROC curves and scatter plots were drawn using GraphPad Prism 6. Survival analysis was performed using R version 3.0.2.
2.10. Diagnostic Inclusion Criteria, Score Assignment, and Test Evaluation
To maximise the chances of having a positive signal in the patients serum, a combined stage IV cohort (n = 119; ‘Tubingen’ and ‘MIA’) was compared with disease-free ‘controls’ (n = 130; no history of melanoma or nevi, prior history of melanoma but disease-free, high nevus count with no melanoma). Initially, all members of the ‘MELmiR-17’ (miR-4469 was excluded due to assay failure) panel underwent a simple Mann–Whitney U test to identify the highly significant (p < 0.0001) miRNAs to be included in the next step (Fig. 1). Those miRNAs that met these criteria then underwent AUROC analysis to determine their area under the curve (AUC) (Fig. 1, Fig. 2). AUC scores of ≥ 0.70 were deemed to be diagnostically useful (Wians, 2009). The miRNAs that had an AUC of ≥ 0.70 were interrogated further to classify the median-normalised Ct values as ‘high’ or ‘low’ expression (interpretation of the median normalised Ct expression values used to determine ROC curves were evaluated with the Optimal Cutoff algorithm (‘OptimalCutpoints’ R package v1.1–3)). For those miRNAs that met the criteria for inclusion in the diagnostic panel, the patient was given a diagnostic score (ranging from 0 to 7) determined by the number of miRNAs that were present as ‘high’ and ‘low’ or ‘normal’ (most like the ‘control’ cohort). To be deemed positive for melanoma, the patients sample must have had a score of ≥ 4 (max 7). A negative test was a score of 0–3.
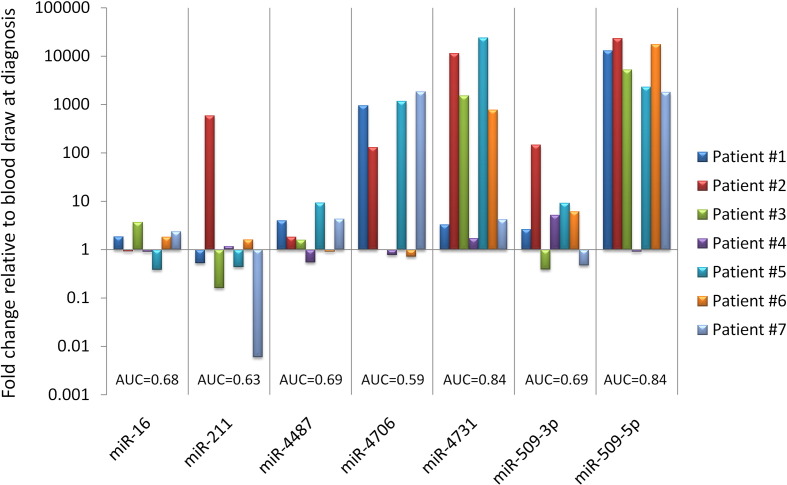
Fig. 2.
Expression of MELmiR-7 in stage IV progression patients.
The following formulas were used to determine diagnostic test ability: Positive Predictive Value (PPV) or Precision = True Positive (TP) / (TP + False Positive (FP)); Negative Predictive Value (NPV) = True Negative (TN) / (False Negative (FN) + TN); Sensitivity = TP / (TP + FN); Specificity = TN / (FP + TN) False Positive Rate = 1 − Specificity; False Negative Rate = 1 − Sensitivity; Likelihood Ratio Positive = Sensitivity / 1 − Specificity; Likelihood Ratio Negative = 1 − Sensitivity / Specificity; Diagnostic Odds Ratio (DOR) = (TP / FN) / (FP / TN).
2.11. Funding
This project was funded by the National Health and Medical Research Council (NHMRC) of Australia.
3. Results
3.1. Members of the ‘MELmiR-17’ Panel are Predictors of Stage, Recurrence, and Survival in Patient Tissue
To confirm that miRNA expression was detectable in melanoma tissues prior to the serum assessment, we first measured an 18-miRNA panel (MELmiR-18) in a prospective collection (Tissue Validation Cohort, see Materials and Methods) of melanoma tissues derived from stage III (n = 76) and stage IV (n = 10) melanoma patients (Table 1 and Fig. 1). Expression was detected in all dilutions of a positive control (except miR-4469 which had assay failure thus the panel herein will be referred to as ‘MELmiR-17’) and in tissue samples (Fig. 1), which indicated that even at low input levels, the assay and detection method was adequate (data not shown). We observed that thirteen miRNAs were differentially expressed when stage III tissues were compared with stage IV tissues (logistic regression, p < 0.05; Supplementary Table 1 and Supplementary Fig. 1). All but one of these miRNAs (miR-204) showed higher levels in stage III compared with stage IV tissues (Supplementary Table 1 and Supplementary Fig. 1). Of these miRNAs, seven (miR-506-3p, miR-508-3p, miR-508-5p, miR-509-3p, miR-509-5p, miR-513c, and miR-514a) were members of the miR-506–514a cluster (Streicher et al., 2012). Supplementary Table 1 summarises the associated AUROC analyses. Members of the miR-506–514 cluster, had AUC scores ranging from 0.65 to 0.79 with the highest scores being shared by miR-506-3p and miR-509-5p. To determine the minimum number of miRNAs required to discriminate stage III from stage IV, we next performed a multivariate logistic regression and illustrated this using a conditional inference tree (Supplementary Fig. 2). These analyses revealed that only miR-4731 (p = 0.003, OR = 3.0, CI 1.45–6.2) and miR-204 (p = 0.015, OR = 0.63, CI 0.43–0.92) were required to discriminate the tissue stage (in general, higher Ct values = lower expression). Subsequent AUROC analysis (AUC = 0.89) showed an improved score than individual miRNAs (Supplementary Table 1).
We next used multivariate Cox regression modelling using the ‘MELmiR-17’ panel together with available records of pathology of the primary melanoma (SMM, NM) and number of involved nodes (N stage) to determine its value as a prognostic marker at stage III in the PAH tissue cohort (Table 1). These analyses showed that only nodular histotype (NM; p = 0.002; HR = 3.5; CI 1.57–7.81) and expression of miR-509-5p (p = 0.015; HR = 0.85; CI 0.75–0.97) were associated with overall survival. Expression levels of the ‘MELmiR-17’ panel were not significantly different between the two largest pathology classes (SMM and NM) (data not shown). Furthermore, using the same multivariate analysis, N-stage (p = 0.014; HR = 1.52; CI 1.09–2.12) and lower expression of miR-513b (p = 0.038; HR = 1.08; CI 1.00–1.17) and higher miR-513c expression (p = 0.020; HR = 0.92; CI 0.86–0.99) were related to recurrence.
3.2. A Seven-miRNA Panel Identifies Melanoma with High Sensitivity and Specificity Using Patient Sera
The ‘MELmiR-17’ panel was next assessed in independent cohorts of patient sera (serum validation cohorts, see Materials and Methods) with different stages of disease at time of blood collection (from no melanoma to stages I–IV) (Table 2 and Fig. 1). All expression values (Ct) were normalised to cel-miR-39 (synthetic ‘spike-in’ control) according to previously published methods (Tembe et al., 2015) prior to statistical analysis. In miRNA derived from serum, expression of 13 miRNAs was detected (Fig. 1 and Table 3). Notably, the expression of the miR-506–514a cluster was generally quite low (miR-506-3p and miR-514a) or not detected (miR-508-3p, miR-508-5p, miR-513b, and miR-513c (data not shown)). In the 13 detected miRNAs, seven (miR-16, miR-211-5p, miR-4487, miR-4706, miR-4731, miR-509-3p, and miR-509-5p) showed highly significant differences (Mann–Whitney; corrected p < 0.0001) between ‘controls’ (no melanoma) and patients with stage IV disease (Table 3 and Supplementary Figs. 3 and 4). The same miRNAs were also differentially detectable in stages I/II and stage III, compared with ‘controls’, with the exception of miR-211 (Table 3 and Supplementary Figs. 3 and 4). Whilst miR-16 was originally included as a blood control, our data show that it is significantly associated with disease in both tissue and serum, as observed previously in a study of colorectal cancer (Ristau et al., 2014). Intriguingly, levels of expression of most of these differentially expressed miRNAs were lower in melanoma patients compared to patients without disease (controls). The miRNAs − 4487, − 4706, − 4731, 509-3p, and 509-5p all showed lower expression (on average) and miRNAs − 16 and − 211 (stage IV only) had higher expression (on average) in melanoma patients (Supplementary Fig. 3). The observed lower serum expression of miR-4487, miR-4706, miR-4731, miR-509-3p, and miR-509-5p in the melanoma cases was associated with melanoma (presence of or recently removed tumour; see Discussion), and in the case of miR-509-3p, has been noted previously by Leidinger et al. (2010).
AUROC analysis revealed which of the differentially detected miRNAs (Table 3 and Supplementary Fig. 5) have the potential to be used for diagnostic purposes. Particular attention is paid to those miRNAs that were able to discriminate stage IV disease (i.e., distal metastatic deposits) from disease-free controls (Table 3). These were: miR-16, miR-211-5p, miR-4487, miR-4706, miR-4731, miR-509-3p, and miR-509-5p (herein referred to as ‘MELmiR-7’). Multivariate logistic regression identified the five most robust markers of the ‘MELmiR-7’ panel (miR-211, p < 0.0001; miR-509-3p, p = 0.0014; miR-509-5p, p < 0.0001; miR-4706, p = 0.028; and miR-4731, p < 0.0001). Subsequent AUROC analysis revealed these five markers produced a near perfect AUC score of 0.9907 (cf. ‘MELmiR-7’ = 0.9911). Furthermore, a conditional inference tree analysis highlighted that patients could be discriminated into categories based on combinations of expression levels by members of the MELmiR-7 panel. Supplementary Fig. 6 illustrates that only four miRNAs (miR-509-5p, miR-miR-4731, miR-211, and miR-509-3p) were required to discriminate the stage IV samples (AUC = 0.9738) from controls. Further comparisons showed that ‘MELmiR-7’ panel members can also discriminate stages I/II (AUC = 0.991) and stage III (AUC = 0.9722) from ‘controls’ (Supplementary Figs. 7 and 8).
The sensitivity and specificity of the ‘MELmiR-7’ was then assessed by assigning a diagnostic score to the data. The expression values graphed in the Supplementary Fig. 3 were used to observe the direction of the data (i.e., higher or lower expression in ‘controls’ vs. all stages). The optimal cut points (Vermont et al., 1991) in the AUROC datasets were identified which allowed the expression values to be categorised as positive or negative for melanoma (see Materials and Methods). A diagnostic score (see Materials and Methods) was then applied to each sample which ranged from 0 to 3 (low likelihood of melanoma) and 4 to 7 (high likelihood of melanoma). Upon applying the derived diagnostic score, the ‘MELmiR-7’ panel was evaluated as a group. We found that it had the ability to identify melanoma (independent of stage), when ≥ 4 miRNAs (93% sensitivity and ≥ 82% specificity) reached or exceeded their optimal cut point (Table 4). The sensitivity of the ‘MELmiR-7’ panel increased to 95% in the stage IV cohort. Table 4 provides a summary of the effectiveness of the ‘MELmiR-7’ panel in relation to other stages. The diagnostic odds ratio (DOR) was used to determine the lowest diagnostic score possible for the ‘MELmiR-7’ panel whilst still maintaining very high sensitivity and specificity. Moreover, upon comparison with currently used serological tests (LDH and S100B), we found that the ‘MELmiR-7’ panel was more sensitive than the combined power of both tests. Using the available data (Tubingen cohort), elevated levels of LDH and S100B were found in 40% (27/67) and 63% (42/67) of these patients (Table 2). In the same patients, the ‘MELmiR-7’ panel achieved 91% (63/67) (when ≥ 4 miRNAs reached or exceeded their optimal cut-points) sensitivity and ≥ 82% specificity (specificity could not be determined for serum LDH and S100B as ‘controls’ were not assayed). The sensitivity of the ‘MELmiR-7’ panel was confirmed in an independent serial collection of stage IV patients (initial blood draw at lower disease burden and one at a higher disease burden) (Fig. 2). Fig. 2 highlights that the ‘MELmiR-7’ panel can be used to monitor tumour progression in 100% of the patients assessed (≥ 2 miRNAs with ≥ 1.5-fold relative expression). Subsequent AUROC analysis (Fig. 2) highlights that if measured in isolation, the most informative markers would be miR-509-5p and miR-4731 (AUC = 0.84) respectively.
Table 4.
Sensitivity and specificity summary.
| MELmiR-7-panel | Melanoma vs. controls | Stages I/II vs. controls | Stage III vs. controls | Stage IV vs. controls |
|---|---|---|---|---|
| Sensitivity | 93% | 93% | 86% | 95% |
| Specificity | ≥ 82% | ≥ 82% | ≥ 82% | ≥ 82% |
| False Positive Rate | 18% | 18% | 18% | 18% |
| False Negative Rate | 7% | 7% | 14% | 5% |
| Positive Predictive Value (PPV) | 91% | 77% | 64% | 82% |
| Negative Predictive Value (PPV) | 85% | 95% | 94% | 95% |
| Likelihood Ratio Positive | 5.01 | 5.04 | 4.66 | 5.14 |
| Likelihood Ratio Negative | 0.09 | 0.09 | 0.17 | 0.06 |
| Diagnostic Odds Ratio (DOR) | 54.86 | 58.89 | 27.13 | 83.18 |
3.3. Members of the ‘MELmiR-17’ Differentiate Stage and are Associated With Survival in Patient Sera
To discern whether significant differences in stage could be found, we first assessed the ‘MELmiR-17’ panel using Mann–Whitney tests (with corrected p values) combined with AUROC analysis for stages I/II vs. IV and stages III vs. IV. Table 3 summarises the associated corrected p values and AUROC scores. Next, multivariate logistic regression was used to identify the minimum miRNAs required to predict differences in the melanoma stages which was illustrated using conditional inference trees (Supplementary Figs. 9 and 10) and AUROC analysis (AUC scores for stages I/II vs. IV and stages III vs. IV were 0.989 and 0.9945 respectively). There were no markers, however, that were significantly associated with time to recurrence when Cox regression was performed.
The ‘MELmiR-17’ panel was next assessed to identify miRNAs related to OS in the serum cohorts from Tubingen (n = 131) and Melanoma Institute of Australia (MIA; n = 124) first separately and then jointly (n = 255) (Table 2). The predictive performance of the joint model on each cohort was statistically equivalent to that of the best separate analysis on each cohort (likelihood ratio test; p = 0.34 for MIA cohort; p = 0.22 for Tubingen cohort), hence the joint model was used to analyse the combined Tubingen and MIA cohorts. Furthermore, the miR-4706 marker was dichotomised at Ct 37 to meet the proportionality assumption. The outcome from the combined analysis is summarised in Table 5.
Table 5.
Multivariate survival analysis of serum miRNAs and melanoma stage.
| Covariate | HR | Lower 95% CI | Upper 95% CI | p value |
|---|---|---|---|---|
| Stage III | 6.03 | 2.33 | 15.56 | 0.0002 |
| Stage IV | 8.48 | 3.12 | 23.01 | < 0.0001 |
| hsa-miR-16 | 1.04 | 0.88 | 1.23 | ns |
| hsa-miR-211 | 0.87 | 0.82 | 0.91 | < 0.0001 |
| hsa-miR-4487 | 0.88 | 0.79 | 0.98 | 0.02 |
| hsa-miR-4706 | 0.45 | 0.29 | 0.69 | 0.0002 |
| hsa-miR-4731 | 0.98 | 0.90 | 1.06 | ns |
| hsa-miR-509-3p | 1.04 | 1.00 | 1.09 | ns |
| hsa-miR-514a | 0.92 | 0.85 | 0.99 | 0.02 |
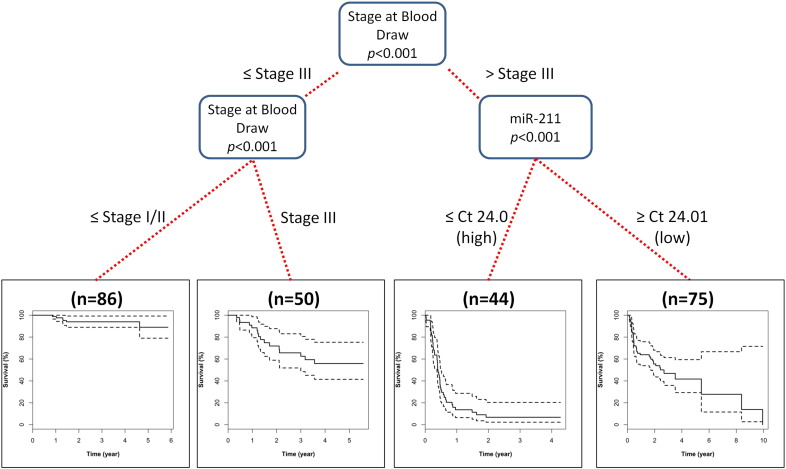
A conditional inference tree analysis for the survival data was then performed which showed that stage at blood draw together with miR-211 expression could be used to triage patients based on overall survival (OS) status (Fig. 3 with Kaplan–Meier plots per each classification). Importantly, upon diagnosis with stage IV, miR-211 expression was able to discern survival based on high (Ct ≤ 24; median survival = 4.8 months, CI 4.5–5.9) and low expression (Ct ≥ 24.01; median survival = 2.7 years, CI 1.7–NA).
Fig. 3.
Conditional inference tree predicting survival outcomes.
Finally, the MELmiR-7 panel was further assessed for its utility in terms of predicting OS in serum cohorts having LDH and S100B status available (Tubingen) (Table 2). The ‘MELmiR-7’ panel performed significantly better than both serum LDH and S100B (delta log likelihood = 11, p < 0.001).
4. Discussion
Five-year survival proportions for melanoma are poor for patients with metastatic disease, however if disease is detected in its early stages, then survival is one of the highest for all cancers. Even for those with metastases, survival differs depending on the extent of disease spread. Patients with metastases confined to regional lymph nodes (stage III disease) have 5-year survival of ~ 50%, whereas patients with widely disseminated metastases (stage IV disease) have 5-year survival of < 15%. Thus better monitoring of a patient's tumour burden may improve survival by precipitating earlier therapeutic interventions. In support of this, clinical trials in stage III unresectable and stage IV melanoma patients, treated with ipilimumab (Hodi et al., 2010), vemurafenib (Sosman et al., 2012), combined dabrafenib and trametinib (Flaherty et al., 2014) or anti-PD1 pembrolizumab (Joseph et al., 2014), have observed improved overall survival and response rate in patients with lower disease volume (M1a/M1b) as compared to those with distal disease (M1c). Moreover, it is believed that there is potential for long-term survival if relapses are identified promptly with treatment initiated without delay (Davidson et al., 2014). In clinical practice, there is currently a lack of reliable, sensitive and specific predictive biomarkers for detecting early melanoma progression. This study aimed to identify a more effective biomarker that was sensitive and specific enough to identify early metastatic disease. Since commencement of this study there have been a number of studies investigating the utility of miRNAs to serve as melanoma blood and tissue biomarkers (Fleming et al., 2015, Friedman et al., 2012, Tembe et al., 2015, Leidinger et al., 2010, Greenberg et al., 2013, Margue et al., 2015, Bonazzi et al., 2012). For example, a study by Friedman et al. (2012) screened 355 miRNAs in sera from 80 melanoma patients using a previously characterised panel of serum-expressed miRNAs. The authors found detectable expression for 170 miRNAs and a panel of five miRNAs (miR-150, miR-15b, miR-199a-5p, miR-33a, and miR-424) showed a significant association with recurrence-free survival. This five-miRNA signature was able to classify the patients into high and low recurrence risk. Our approach was to identify a panel of melanoma-related miRNAs that involved first screening a panel of melanoma cell lines (n = 55) in comparison with a group of other solid malignancies (cell lines were derived from breast, ovarian, colorectal, prostate, etc.) (Stark et al., 2015). Interestingly, the five-miRNA panel indentified by Friedman et al. (2012) was not present in our dataset which may indicate that this panel is not specifically melanoma-related but instead related to the tumourigenic process. We focused on miRNAs that were highly expressed or more predominantly expressed in melanoma with the premise that these may be both ‘diagnostic’ for melanoma and/or more easily detectable in patient serum. Our current approach differed from the aforementioned studies as: 1) this study harnessed the power of our previous comprehensive analysis of known miRNAs (n = 1898) in relation to melanoma (Stark et al., 2015); 2) this study validated the cell-line derived miRNA panel (MELmiR-17) in a large of panel of stage III and IV melanoma tissues prior to serum analysis to confirm they were expressed; and 3) this study used an ultra-sensitive method of detection (see Materials and Methods) to ensure that lowly expressed miRNAs could be detected. We have successfully used these approaches in a previous study where a panel of miRNAs were identified that was related to good and poor prognosis in stage III melanoma patients (Tembe et al., 2015). However, our current study was limited by the lack of available serially collected specimens (to detect recurrence as in Friedman et al., 2012) at time of study design. To address this limitation, further studies in larger, independent, prospectively collected melanoma cohorts will be required to strengthen these data.
In sum, we found that a ‘melanoma-related’ panel of miRNAs was expressed in metastatic melanoma in a stage-specific manner and, together with the tissue pathology and nodal status, was prognostic for recurrence and OS. These markers may therefore also be useful to support histopathologic diagnosis of metastatic deposits suspected of being melanoma. We further observed that expression of the various miRNAs from the MELmiR-17 panel in stage IV tissues was often lower than in stage III tissue, which is in keeping with previous studies. For example, miR-211 expression is commonly lost in subsets of melanoma cell lines (Stark et al., 2015, Boyle et al., 2011), and miR-506, a member of the miR-506–514 cluster, has been shown to be lost during metastatic colonisation despite being up-regulated in early melanoma progression (Streicher et al., 2012, Mueller et al., 2009). We have also recently reported that inhibition of miR-514a leads to increased cell proliferation (Stark et al., 2015). These data indicate that expression of this cluster reduces during melanoma progression.
Currently there is an unmet need for a minimally invasive, highly specific, and predictive serum biomarker of melanoma burden. For many years the use of the seroprotein markers S100B and LDH has been disputed, due to reported inconsistencies in sensitivity and specificity (Brochez and Naeyaert, 2000, Finck et al., 1983, Karakousis et al., 1996, Sirott et al., 1993, Weide et al., 2012, Guo et al., 1995, Smit et al., 2005, Kruijff et al., 2009). Despite this lack of consensus, a recent study did find that elevated levels of S100B were prognostic of survival times in patients with unresectable melanoma (Weide et al., 2013).
Here, we present data that shows that our ‘MELmiR-7’ panel has the potential to be used as a primary screening tool for clinically undetected metastatic melanoma due to its high sensitivity (93%) and specificity (≥ 82%). However, detection of early melanoma lesions (in situ and stages I/II melanoma) is currently being adequately achieved (as evident by high survival rates) via clinical strategies. The ‘MELmiR-7’ panel could be utilised during routine follow-up (i.e., post-primary excision of melanoma and later in advanced disease) of melanoma patients. In comparison with serum LDH and S100B, expression levels of the ‘MELmiR-7’ panel performed better than both markers in predicting overall survival. We have shown that the ‘MELmiR-7’ panel was measurable at time of progression in 100% of stage IV melanoma patients. These data suggest that this panel would therefore be suited to monitor tumour burden.
According to the AJCC Staging committee, stage III melanoma patients have a 50% chance of survival beyond 5 years (Balch et al., 2009); these patients also remain the most difficult for whom to provide effective treatments/surveillance regimens and accurate survival estimates. Following treatment, stage III patients are subjected to a series of physical examinations, scans and serology at regular intervals. The frequency of these tests is deemed necessary for early detection of recurrence; however this causes a burden to both the patient and the healthcare system. It is important to note that these guidelines are not universally accepted and differ from centre to centre (Leiter et al., 2014). We foresee that the ‘MELmiR-7’ panel could be offered to patients to complement physical examination. If the diagnostic score for melanoma positivity has changed from earlier measurements, then this may indicate the presence of disease recurrence and as such, these patients may qualify earlier for adjuvant, systemic, or targeted therapies that would otherwise be only offered to stage IV patients. As previously discussed, due to the panel's high sensitivity and specificity, the use of this miRNA panel in this manner has the potential to increase the chances of survival, by earlier and more precise detection of the presence of metastases.
In terms of prognosis, elevated miR-211 expression levels were associated with poorer survival in stage IV patients. Therefore, miR-211 measurement might allow better triaging of patients diagnosed with stage IV disease, into good and poor prognosis which would be highly informative for not only the treating clinician but also for the quality of life of the patients.
The original premise of this study was that the melanoma-enriched miRNAs identified in our previous study (Stark et al., 2015) would be translated directly to the expression observed in melanoma patient-derived serum. Evidence for this notion is apparent in the serially collected stage IV melanoma patients, when, at progression (or recurrence), the MELmiR-7 panel increases, which is reflective of increased tumour burden (i.e., the detectable miRNA expression was from the presence of tumour cells and/or tumour derived extracellular vesicles (e.g., exosomes) in the circulation). These data strongly suggest that the expression is tumour derived and as such this panel could be considered melanoma-related. However, as we have noted, we observe a paradoxical decrease in the expression of the significantly expressed (miR-509-5p, miR-509-3p, miR-4731-5p, miR-4487, miR-4706) miRNAs when melanoma serum cohorts were compared with control cohorts. These data thus provide evidence that the assessed miRNAs (detectable in serum) are not restricted to the melanocytic lineage as initially thought. The source of this miRNA expression is currently unknown but could include cells of the haematopoietic lineage including T-cells, B-cells and NK cells. This loss of expression from a ‘non-tumour’ source has not been elucidated but warrants further investigation. An observed loss of expression of serum-derived miRNAs has been noted previously by Friedman et al. (2012) in post-operative specimens as compared to specimens collected at disease relapse. A plausible reason for a loss of expression observed in the serum may be due to a cytokine-driven systemic response. For example, pro-inflammatory cytokines have been shown to down-regulate miRNAs present in the circulation (Noren Hooten et al., 2013). Specifically, in a study by Noren Hooten et al. (2013) the serum expression of miR-181a was found to be negatively correlated with pro-inflammatory cytokines IL-6 and TNFα and positively correlated with the anti-inflammatory cytokines TGFβ and IL-10 (Noren Hooten et al., 2013). Recently, it has been confirmed that IL-6 expression is induced in melanoma cells with mutant BRAF (V600E). Therefore a possible explanation for what we have observed is that the miRNAs of interest could be expressed by non-melanocyte derived cells where expression is down-regulated in patients with melanoma due to melanoma-related cytokines (e.g. IL-6) (Whipple and Brinckerhoff, 2014).
In conclusion, we envisage that as a growing number of miRNA-panels have been identified as potential prognostic indicators for melanoma (Fleming et al., 2015, Friedman et al., 2012, Tembe et al., 2015), it will eminently feasible to quantify circulating cell-free miRNAs directly (Ono et al., 2015), paving the way for rapid measurements to occur in a diagnostic laboratory. Given these advances, combined with the data presented herein, future melanoma treatment regimens should consider the utility of miRNAs as a prognostic aid in the clinical setting. Our sensitive and specific miRNA panel, in combination with newly identified panels, may enable more precise measurement of disease progression, and in conjunction with current therapy options, may herald an increase in overall survival.
Conflict of Interest
The authors state no conflict of interest except for GVL who reports personal fees from Amgen, BMS, GSK, Merck, Novartis, Provectus, and Roche, which were outside the submitted work; JFT who reports personal fees and other from Provectus, Bristol Myers Squibb, and other from GlaxoSmithKline, which were outside the submitted work; CG who reports personal fees from Amgen, MSD, Novartis, and grants and personal fees from BMS, Roche, and GSK, outside the submitted work; and PMP who reports personal fees from Novartis, outside the submitted work.
Authors' Contributions
MSS designed the study, performed the experiments, data analysis and interpretation, and wrote the manuscript. KK performed the data analysis and interpretation and wrote the manuscript. BW performed the experiments, data collection, contributed specimens, and wrote the manuscript. LEH performed the data analysis and interpretation. AP, YHT, DCW, and GVL performed the data collection, contributed specimens, and wrote the manuscript. JMP performed the data collection. RS, JFT, APB, HPS, and CG contributed specimens and wrote the manuscript. GJM contributed specimens and data, performed data interpretation and wrote the manuscript. AH and PMP designed the study and wrote the manuscript. NKH designed the study, performed data interpretation, and wrote the manuscript.
Acknowledgements
The authors would like to thank the participants, and are grateful for the support of their colleagues: in particular Jessica Hayman, Dr. James Wilmott, and Valerie Jakrot. GJM, RAS and JFT are grateful for financial support from the National Health and Medical Research Council (NHMRC) of Australia (633004) and the Cancer Institute New South Wales (10TPG/1/02); MSS received scholarships from the NHMRC and the Queensland Government Smart Futures Fund. PMP is supported by a CDF2 fellowship from the NHMRC. NKH holds a fellowship from the NHMRC. The funders had no role in the study design, data collection, data analysis, interpretation, or writing of the report.
Footnotes
Research in context: The use of melanoma progression markers have been used for many years however it is clear from the survival rates (5-year survival of Stage IV patients is < 15%) that melanoma must be detected before disease progresses thus highlighting that the current methods of progression detection are inadequate. We have identified a seven-miRNA panel (MELmiR-7) that has the ability to detect the presence of melanoma with high sensitivity and specificity which is superior to currently used markers for melanoma progression, recurrence, and survival. This panel may enable more precise measurement of disease progression and may herald an increase in overall survival.
The miR-16 Taqman primer assay (00039) is specifically designed to bind to mature miRNA sequence of miR-16-5p which is derived from hsa-miR-16-1 and hsa-miR-16-2 stem–loop sequences. The alias for miR-16-5p is miR-16 hence the reasoning for the shortened name.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.05.011.
Appendix A. Supplementary Data
Supplementary Tables 1 and 2. Supplementary Figures 1–10.
References
- AIHW . AIHW; Canberra: 2014. Australian Cancer Incidence and Mortality (ACIM) Books: Melanoma of the Skin. [Google Scholar]
- Allegra A., Alonci A., Campo S. Circulating microRNAs: new biomarkers in diagnosis, prognosis and treatment of cancer (review) Int. J. Oncol. 2012;41(6):1897–1912. doi: 10.3892/ijo.2012.1647. [DOI] [PubMed] [Google Scholar]
- Balch C.M., Gershenwald J.E., Soong S.J. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi V.F., Stark M.S., Hayward N.K. MicroRNA regulation of melanoma progression. Melanoma Res. 2012;22(2):101–113. doi: 10.1097/CMR.0b013e32834f6fbb. [DOI] [PubMed] [Google Scholar]
- Boyle G.M., Woods S.L., Bonazzi V.F. Melanoma cell invasiveness is regulated by miR-211 suppression of the BRN2 transcription factor. Pigment Cell Melanoma Res. 2011;24(3):525–537. doi: 10.1111/j.1755-148X.2011.00849.x. [DOI] [PubMed] [Google Scholar]
- Brochez L., Naeyaert J.M. Serological markers for melanoma. Br. J. Dermatol. 2000;143(2):256–268. doi: 10.1046/j.1365-2133.2000.03649.x. [DOI] [PubMed] [Google Scholar]
- Davidson M., Lorigan P., Larkin J. High-risk cutaneous melanoma follow-up: time for more intensive surveillance? Melanoma Manag. 2014;1(1):7–10. doi: 10.2217/mmt.14.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guire V., Robitaille R., Tetreault N. Circulating miRNAs as sensitive and specific biomarkers for the diagnosis and monitoring of human diseases: promises and challenges. Clin. Biochem. 2013;46(10–11):846–860. doi: 10.1016/j.clinbiochem.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Deichmann M., Benner A., Bock M. S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J. Clin. Oncol. 1999;17(6):1891–1896. doi: 10.1200/JCO.1999.17.6.1891. [DOI] [PubMed] [Google Scholar]
- Finck S.J., Giuliano A.E., Morton D.L. LDH and melanoma. Cancer. 1983;51(5):840–843. doi: 10.1002/1097-0142(19830301)51:5<840::aid-cncr2820510516>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Flaherty K., Daud A., Weber J.S. 32(9010) 2014. Updated overall survival (OS) for BRF113220, a phase 1–2 study of dabrafenib (D) alone versus combined dabrafenib and trametinib (D + T) in pts with BRAF V600 mutation-positive (+) metastatic melanoma (MM) (ASCO Meeting Abstracts). [Google Scholar]
- Fleming N.H., Zhong J., da Silva I.P. Serum-based miRNAs in the prediction and detection of recurrence in melanoma patients. Cancer. 2015;121(1):51–59. doi: 10.1002/cncr.28981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E.B., Shang S., de Miera E.V. Serum microRNAs as biomarkers for recurrence in melanoma. J. Transl. Med. 2012;10:155. doi: 10.1186/1479-5876-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- Greenberg E., Besser M.J., Ben-Ami E. 18(6) 2013. A comparative analysis of total serum miRNA profiles identifies novel signature that is highly indicative of metastatic melanoma: a pilot study; pp. 502–508. (Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals). [DOI] [PubMed] [Google Scholar]
- Guo H.B., Stoffel-Wagner B., Bierwirth T., Mezger J., Klingmuller D. Clinical significance of serum S100 in metastatic malignant melanoma. Eur. J. Cancer. 1995;31A(6):924–928. doi: 10.1016/0959-8049(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Hodi F.S., O'Day S.J., McDermott D.F. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T., Hornik K., Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J. Comput. Graph. Stat. 2006;15(3):651–674. [Google Scholar]
- Joseph R.W., Elassaiss-Schaap J., Wolchok J.D. 32(3015) 2014. Baseline tumor size as an independent prognostic factor for overall survival in patients with metastatic melanoma treated with the anti-PD-1 monoclonal antibody MK-3475. (ASCO Meeting Abstracts). [Google Scholar]
- Karakousis C.P., Balch C.M., Urist M.M., Ross M.M., Smith T.J., Bartolucci A.A. Local recurrence in malignant melanoma: long-term results of the multiinstitutional randomized surgical trial. Ann. Surg. Oncol. 1996;3(5):446–452. doi: 10.1007/BF02305762. [DOI] [PubMed] [Google Scholar]
- Kruijff S., Bastiaannet E., Kobold A.C., van Ginkel R.J., Suurmeijer A.J., Hoekstra H.J. S-100B concentrations predict disease-free survival in stage III melanoma patients. Ann. Surg. Oncol. 2009;16(12):3455–3462. doi: 10.1245/s10434-009-0629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidinger P., Keller A., Borries A. High-throughput miRNA profiling of human melanoma blood samples. BMC Cancer. 2010;10:262. doi: 10.1186/1471-2407-10-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter U., Eigentler T., Garbe C. Follow-up in patients with low-risk cutaneous melanoma: is it worth it? Melanoma Manag. 2014;1(2):115–125. doi: 10.2217/mmt.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margue C., Reinsbach S., Philippidou D. Comparison of a healthy miRNome with melanoma patient miRNomes: are microRNAs suitable serum biomarkers for cancer? Oncotarget. 2015 doi: 10.18632/oncotarget.3661. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P.S., Parkin R.K., Kroh E.M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. U. S. A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D.W., Rehli M., Bosserhoff A.K. MiRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J. Invest. Dermatol. 2009;129(7):1740–1751. doi: 10.1038/jid.2008.452. [DOI] [PubMed] [Google Scholar]
- Noren Hooten N., Fitzpatrick M., Wood W.H., 3rd Age-related changes in microRNA levels in serum. Aging. 2013;5(10):725–740. doi: 10.18632/aging.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S., Oyama T., Lam S., Chong K., Foshag L.J., Hoon D.S. A direct plasma assay of circulating microRNA-210 of hypoxia can identify early systemic metastasis recurrence in melanoma patients. Oncotarget. 2015;6(9):7053–7064. doi: 10.18632/oncotarget.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristau J., Staffa J., Schrotz-King P. Suitability of circulating miRNAs as potential prognostic markers in colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2014;23(12):2632–2637. doi: 10.1158/1055-9965.EPI-14-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y., Ishiguro M., Kitagawa G. D. Reidel Publishing Company; 1986. Akaike Information Criterion Statistics. [Google Scholar]
- SEER . National Cancer Institute; Bethesda, MD: 2014. SEER Cancer Statistics Review, 1975–2011. [Google Scholar]
- Sirott M.N., Bajorin D.F., Wong G.Y. Prognostic factors in patients with metastatic malignant melanoma. A multivariate analysis. Cancer. 1993;72(10):3091–3098. doi: 10.1002/1097-0142(19931115)72:10<3091::aid-cncr2820721034>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Smit L.H., Korse C.M., Hart A.A. Normal values of serum S-100B predict prolonged survival for stage IV melanoma patients. Eur. J. Cancer. 2005;41(3):386–392. doi: 10.1016/j.ejca.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Sosman J.A., Kim K.B., Schuchter L. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 2012;366(8):707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M.S., Bonazzi V.F., Boyle G.M. MiR-514a regulates the tumour suppressor NF1 and modulates BRAFi sensitivity in melanoma. Oncotarget. 2015 doi: 10.18632/oncotarget.3924. (Advance Online Publications: Page 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher K.L., Zhu W., Lehmann K.P. A novel oncogenic role for the miRNA-506–514 cluster in initiating melanocyte transformation and promoting melanoma growth. Oncogene. 2012;31(12):1558–1570. doi: 10.1038/onc.2011.345. [DOI] [PubMed] [Google Scholar]
- Tembe V., Schramm S.J., Stark M.S. MicroRNA and mRNA expression profiling in metastatic melanoma reveal associations with BRAF mutation and patient prognosis. Pigment Cell Melanoma Res. 2015;28(3):254–266. doi: 10.1111/pcmr.12343. [DOI] [PubMed] [Google Scholar]
- Vermont J., Bosson J.L., Francois P., Robert C., Rueff A., Demongeot J. Strategies for graphical threshold determination. Comput. Methods Prog. Biomed. 1991;35(2):141–150. doi: 10.1016/0169-2607(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Weide B., Elsasser M., Buttner P. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br. J. Cancer. 2012;107(3):422–428. doi: 10.1038/bjc.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide B., Richter S., Buttner P. Serum S100B, lactate dehydrogenase and brain metastasis are prognostic factors in patients with distant melanoma metastasis and systemic therapy. PLoS One. 2013;8(11):e81624. doi: 10.1371/journal.pone.0081624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple C.A., Brinckerhoff C.E. BRAF (V600E) melanoma cells secrete factors that activate stromal fibroblasts and enhance tumourigenicity. Br. J. Cancer. 2014;111(8):1625–1633. doi: 10.1038/bjc.2014.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wians F.H.J. Clinical laboratory tests: which, why, and what do the results mean? Lab. Med. 2009;40(2):105–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1 and 2. Supplementary Figures 1–10.