Abstract
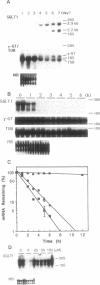
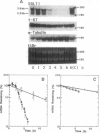
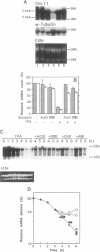
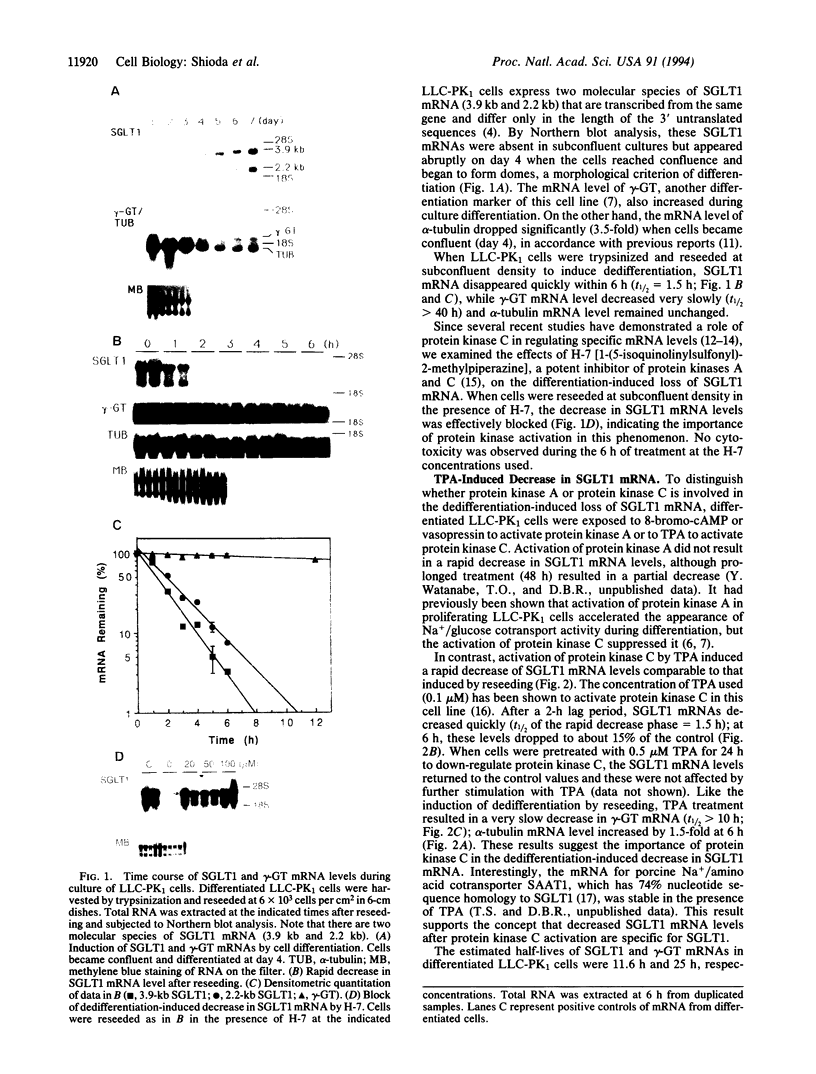
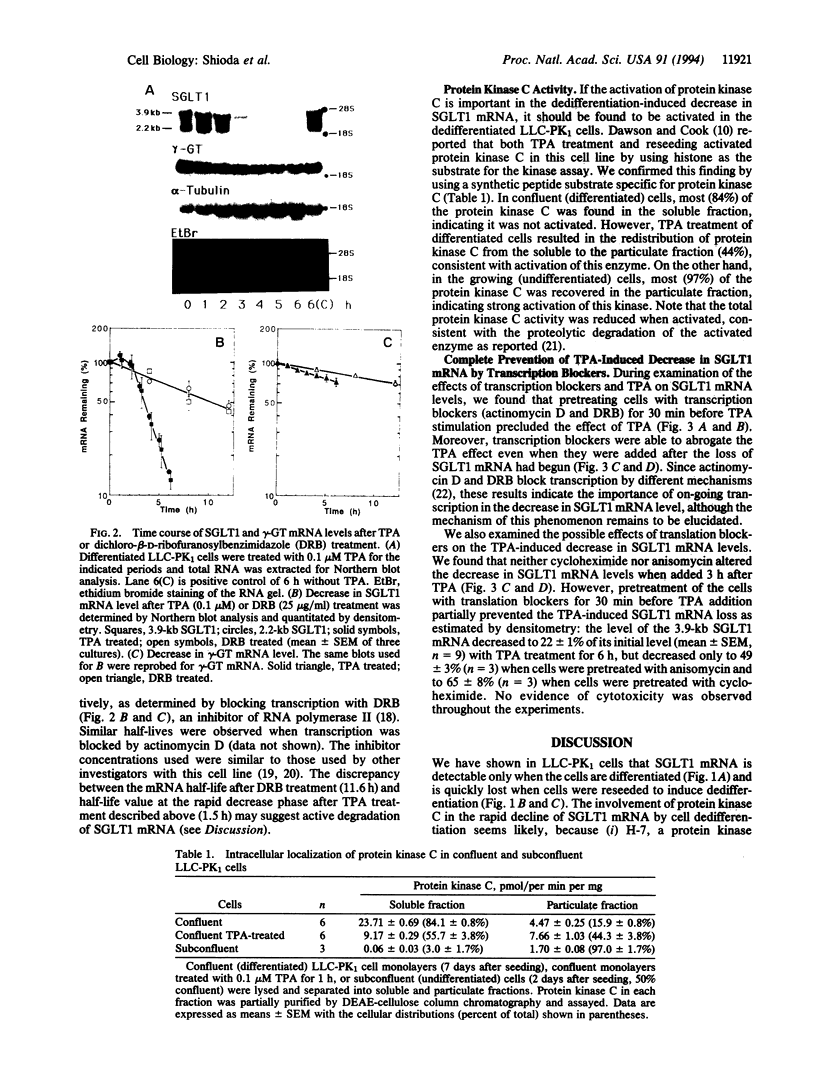
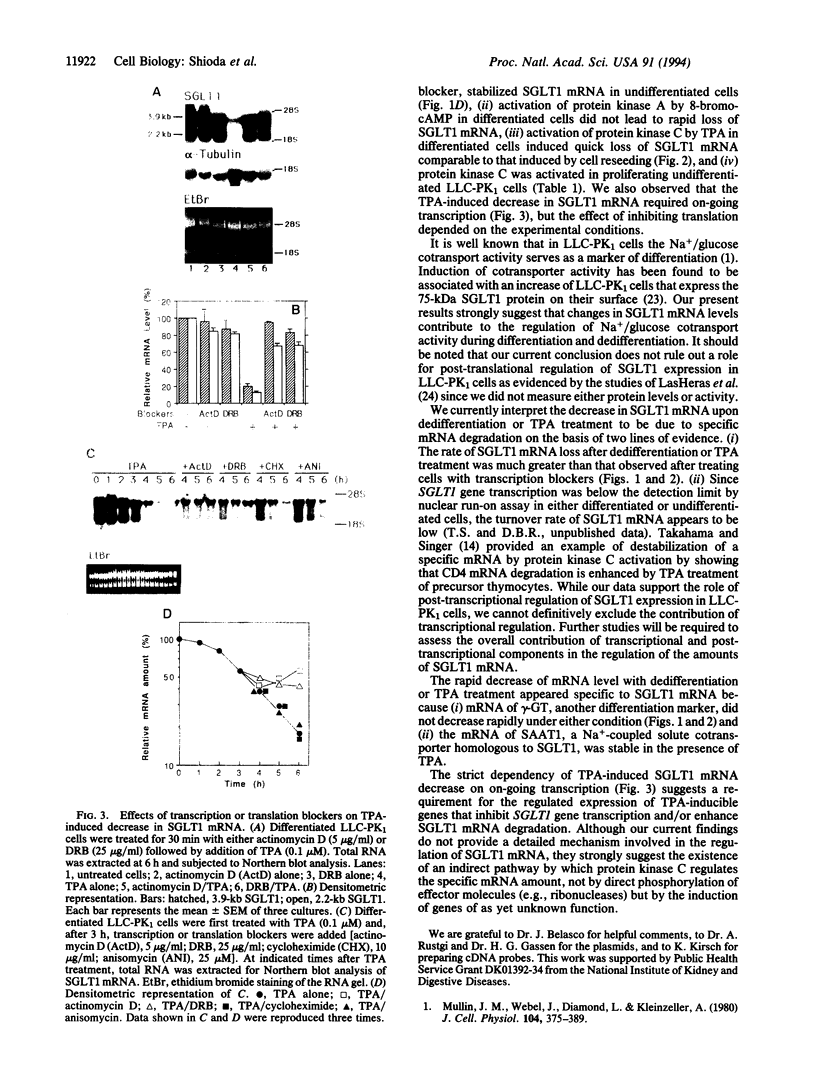
We examined changes in the mRNA level of SGLT1, a Na+/glucose cotransporter, by the differentiation status of LLC-PK1 renal epithelial cells. Proliferating (undifferentiated) cells revealed no detectable SGLT1 mRNA by Northern blot analysis. However, when cells became confluent and differentiated into polarized monolayers, there was an abrupt appearance of the SGLT1 mRNA. When confluent (differentiated) cells were dedifferentiated by reseeding at a subconfluent density, SGLT1 mRNA levels decreased quickly to nondetectable levels (t1/2 = 1.5 h), while the mRNA levels of gamma-glutamyltranspeptidase, another differentiation marker, decreased only slowly (t1/2 > 40 h). This decrease in SGLT1 mRNA was completely blocked by H-7, a protein kinase inhibitor. Since protein kinase C was highly activated in the undifferentiated cells and treatment of differentiated cells with a phorbol ester also induced quick and complete loss of SGLT1 mRNA (t1/2 = 1.5 h) but not of gamma-glutamyltranspeptidase mRNA, protein kinase C activation appears to be involved in the dedifferentiation-induced decrease in SGLT1 mRNA. Although the phorbol ester-induced decrease in the SGLT1 mRNA level was blocked completely by inhibition of transcription, inhibitors of translation blocked the decrease in mRNA levels only partially.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsler K., Cook J. S. Development of Na+-dependent hexose transport in a cultured line of porcine kidney cells. Am J Physiol. 1982 Jan;242(1):C94–101. doi: 10.1152/ajpcell.1982.242.1.C94. [DOI] [PubMed] [Google Scholar]
- Amsler K., Ghatani S., Hemmings B. A. cAMP-dependent protein kinase regulates renal epithelial cell properties. Am J Physiol. 1991 Jun;260(6 Pt 1):C1290–C1299. doi: 10.1152/ajpcell.1991.260.6.C1290. [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Breckon R., Dixon B. S. ATP receptor regulation of adenylate cyclase and protein kinase C activity in cultured renal LLC-PK1 cells. J Clin Invest. 1991 May;87(5):1732–1738. doi: 10.1172/JCI115191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ase K., Berry N., Kikkawa U., Kishimoto A., Nishizuka Y. Differential down-regulation of protein kinase C subspecies in KM3 cells. FEBS Lett. 1988 Aug 29;236(2):396–400. doi: 10.1016/0014-5793(88)80064-4. [DOI] [PubMed] [Google Scholar]
- Bennett E., Kimmich G. A. Na+ binding to the Na(+)-glucose cotransporter is potential dependent. Am J Physiol. 1992 Feb;262(2 Pt 1):C510–C516. doi: 10.1152/ajpcell.1992.262.2.C510. [DOI] [PubMed] [Google Scholar]
- Dawson W. D., Cook J. S. Parallel changes in amino acid transport and protein kinase C localization in LLC-PK1 cells treated with TPA or diradylglycerols. J Cell Physiol. 1987 Jul;132(1):104–110. doi: 10.1002/jcp.1041320114. [DOI] [PubMed] [Google Scholar]
- Gay D. A., Sisodia S. S., Cleveland D. W. Autoregulatory control of beta-tubulin mRNA stability is linked to translation elongation. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5763–5767. doi: 10.1073/pnas.86.15.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger M. A., Rhoads D. B. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994 Oct;74(4):993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Iwai Y., Bickel M., Pluznik D. H., Cohen R. B. Identification of sequences within the murine granulocyte-macrophage colony-stimulating factor mRNA 3'-untranslated region that mediate mRNA stabilization induced by mitogen treatment of EL-4 thymoma cells. J Biol Chem. 1991 Sep 25;266(27):17959–17965. [PubMed] [Google Scholar]
- Kong C. T., Yet S. F., Lever J. E. Cloning and expression of a mammalian Na+/amino acid cotransporter with sequence similarity to Na+/glucose cotransporters. J Biol Chem. 1993 Jan 25;268(3):1509–1512. [PubMed] [Google Scholar]
- Lasheras C., Scott J. A., Rabito C. A. Na+-sugar cotransport system as a polarization marker during organization of epithelial membrane. Am J Physiol. 1988 Dec;255(6 Pt 1):C745–C753. doi: 10.1152/ajpcell.1988.255.6.C745. [DOI] [PubMed] [Google Scholar]
- Mullin J. M., Weibel J., Diamond L., Kleinzeller A. Sugar transport in the LLC-PK1 renal epithelial cell line: similarity to mammalian kidney and the influence of cell density. J Cell Physiol. 1980 Sep;104(3):375–389. doi: 10.1002/jcp.1041040311. [DOI] [PubMed] [Google Scholar]
- Ohta T., Isselbacher K. J., Rhoads D. B. Regulation of glucose transporters in LLC-PK1 cells: effects of D-glucose and monosaccharides. Mol Cell Biol. 1990 Dec;10(12):6491–6499. doi: 10.1128/mcb.10.12.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandrikopoulou A., Frey A., Gassen H. G. Cloning and expression of gamma-glutamyl transpeptidase from isolated porcine brain capillaries. Eur J Biochem. 1989 Aug 15;183(3):693–698. doi: 10.1111/j.1432-1033.1989.tb21100.x. [DOI] [PubMed] [Google Scholar]
- Rabito C. A., Kreisberg J. I., Wight D. Alkaline phosphatase and gamma-glutamyl transpeptidase as polarization markers during the organization of LLC-PK1 cells into an epithelial membrane. J Biol Chem. 1984 Jan 10;259(1):574–582. [PubMed] [Google Scholar]
- Salvatori R., Bockman R. S., Guidon P. T., Jr A simple modification of the Peppel/Baglioni method for RNA isolation from cell culture. Biotechniques. 1992 Oct;13(4):510–512. [PubMed] [Google Scholar]
- Shyu A. B., Greenberg M. E., Belasco J. G. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989 Jan;3(1):60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Takahama Y., Singer A. Post-transcriptional regulation of early T cell development by T cell receptor signals. Science. 1992 Nov 27;258(5087):1456–1462. doi: 10.1126/science.1439838. [DOI] [PubMed] [Google Scholar]
- Van den Bosch L., De Smedt H., Borghgraef R. Influence of PMA and a low extracellular Ca2+ concentration on the development of the Na(+)-dependent hexose carrier in LLC-PK1 cells. Biochim Biophys Acta. 1991 Apr 17;1092(2):244–250. doi: 10.1016/0167-4889(91)90163-r. [DOI] [PubMed] [Google Scholar]
- Wager R. E., Assoian R. K. A phorbol ester-regulated ribonuclease system controlling transforming growth factor beta 1 gene expression in hematopoietic cells. Mol Cell Biol. 1990 Nov;10(11):5983–5990. doi: 10.1128/mcb.10.11.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. S., Lever J. E. Developmentally regulated 75-kilodalton protein expressed in LLC-PK1 cultures is a component of the renal Na+/glucose cotransport system. J Cell Biochem. 1989 May;40(1):83–89. doi: 10.1002/jcb.240400109. [DOI] [PubMed] [Google Scholar]
- Zandomeni R., Mittleman B., Bunick D., Ackerman S., Weinmann R. Mechanism of action of dichloro-beta-D-ribofuranosylbenzimidazole: effect on in vitro transcription. Proc Natl Acad Sci U S A. 1982 May;79(10):3167–3170. doi: 10.1073/pnas.79.10.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]