Abstract
Introduction
Awareness and knowledge of treatment as prevention (TasP) was assessed among HIV-positive and HIV-negative gay, bisexual and other men who have sex with men (GBMSM) in Vancouver, Canada.
Methods
Baseline cross-sectional survey data were analyzed for GBMSM enrolled, via respondent-driven sampling (RDS), in the Momentum Health Study. TasP awareness was defined as ever versus never heard of the term “TasP.” Multivariable logistic regression identified covariates of TasP awareness. Among those aware of TasP, men's level of knowledge of TasP was explored through an examination of self-perceived knowledge levels, risk perceptions and short-answer definitions of TasP which were coded as “complete” if three TasP-related components were identified (i.e. HIV treatment, viral suppression and prevention of transmission). Information source was also assessed. Analyses were stratified by HIV status and RDS adjusted.
Results
Of 719 participants, 23% were HIV-positive, 68% Caucasian and median age was 33 (Interquartile range (IQR) 26,47). Overall, 46% heard of TasP with differences by HIV status [69% HIV-positive vs. 41% HIV-negative GBMSM (p<0.0001)]. In adjusted models: HIV-positive GBMSM were more likely to have heard of TasP if they were Canadian born, unemployed, not using party drugs and had higher CD4 counts; HIV-negative GBMSM were more likely to have heard of TasP if they were Caucasian (vs. Aboriginal), students, had higher education, a regular partner and multiple sexual partners. Among those aware of TasP 91% of HIV-positive and 69% of HIV-negative GBMSM (p<0.0001) felt they knew “a lot” or “a bit in general” about TasP; 64 and 41% (p=0.002) felt HIV treatment made the risk of transmission “a lot lower”; and 21 and 13% (p<0.0001) demonstrated “complete” TasP definitions. The leading information source was doctors (44%) for HIV-positive GBMSM and community agencies (38%) for HIV-negative GBMSM, followed by gay media for both populations (34%).
Conclusions
Nearly half of GBMSM in this study reported having heard of TasP, yet only 14% demonstrated complete understanding of the concept. Variations in TasP awareness and knowledge by HIV status, and key socio-demographic, behavioural and clinical factors, highlight a need for health communication strategies relevant to diverse communities of GBMSM in order to advance overall TasP health literacy.
Keywords: treatment as prevention, men who have sex with men, HIV, health literacy, TasP knowledge, TasP awareness
Introduction
Globally and in Canada, gay, bisexual, and other men who have sex with men (GBMSM) are at high-risk for HIV infection [1]. In British Columbia (BC), Canada, GBMSM comprise 45% of the estimated 9300–13,500 individuals living with HIV and 63% of all new HIV diagnoses in 2012 (150 cases) [2]. The ManCount Survey of GBMSM in Vancouver, the epicentre of BC's epidemic, reported an HIV prevalence of 18% overall, although that figure was approaching one in three for men aged ≥45 years [3].
Treatment as Prevention (TasP) has been actively promoted in Vancouver since 2010, and more recently province-wide, as a critical strategy to reduce HIV morbidity and mortality among individuals living with HIV [4] and at the same time to reduce the transmission of HIV at the population level [5], by lowering viral loads in people with HIV through HIV treatment. This policy, called STOP HIV/AIDS (or “Seek and Treat for Optimal Prevention of HIV/AIDS”), involves the expansion of antiretroviral therapy (ART) to all people living with HIV in BC free-of-charge (for further details: [6]). In 2014, TasP was formally adopted by the United Nations as the global authority's new 90-90-90 strategy (90% diagnosed, 90% on treatment, 90% virally suppressed) to reduce the burden of HIV/AIDS worldwide [7]. As several countries throughout the world incorporate TasP into policy and practice, efforts are needed to understand TasP health literacy among key affected populations.
A 2011–2013 study of TasP among GBMSM in Australia found that, despite generally positive attitudes towards the early initiation of ART, the overwhelming majority (97%) remained sceptical that ART prevented transmission [8]. In qualitative work with people living with HIV in the same setting, despite recognizing the preventive benefits of TasP, participants remained reluctant to take up this approach due to concerns regarding rapidly changing treatment guidelines, the effects of initiating life-long medications, the perception that TasP prioritizes public good over individual agency, and the impact of changing beliefs about infectiousness on people's personal approaches to managing risk and prevention [9]. Similar barriers to TasP acceptability were found in the United Kingdom with inequalities in TasP awareness and literacy levels observed by serostatus; for example, HIV-negative men were less likely to understand key concepts such as the meaning of undetectable viral load and its link to HIV transmission [10]. These findings raise questions about the possible limits of TasP under real world conditions if levels of community awareness and knowledge of TasP are relatively low.
The primary objective of this study was to examine the prevalence of awareness of TasP and analyze associations with key socio-demographic, clinical, and behavioural variables among HIV-positive and HIV-negative GBMSM in Vancouver. Among those aware of TasP, we also examined men's current level of knowledge of TasP, exploring how GBMSM access, understand and perceive this information. To our knowledge, this is the first study in Canada to provide an estimate of TasP awareness and knowledge among GBMSM living with and at-risk for HIV in a setting where a natural experiment for TasP has taken place.
Methods
Study population
Baseline cross-sectional data were analyzed for participants enrolled in the Momentum Health Study, a longitudinal bio-behavioural prospective cohort study of HIV-positive and HIV-negative GBMSM (≥16 years) in Vancouver, Canada. Data were collected at participants’ first study visit between February 2012 and February 2014.
Recruitment and study procedures
Respondent-driven sampling (RDS) was used to recruit GBMSM in the Greater Vancouver area [11]. A computer-assisted, self-administrated (CASI) questionnaire was used to collect socio-demographic and behavioural variables. The CASI was completed at a private computer booth in a study office located in Vancouver's West End traditional gay neighbourhood. Data regarding family doctor and any disclosure to this provider regarding sexual identity and same-sex behaviour were collected through a nurse-administered questionnaire. Data on HIV viral load and CD4 cell count were provided through linkage with administrative data at the British Columbia Centre for Excellence in HIV/AIDS [12]. Participants received honouraria of $50 for completing the study visit (paid in cash and/or prize draw entries for travel or electronics gift cards) and $10 for each person they successfully recruited into the study.
Outcomes: TasP awareness and knowledge
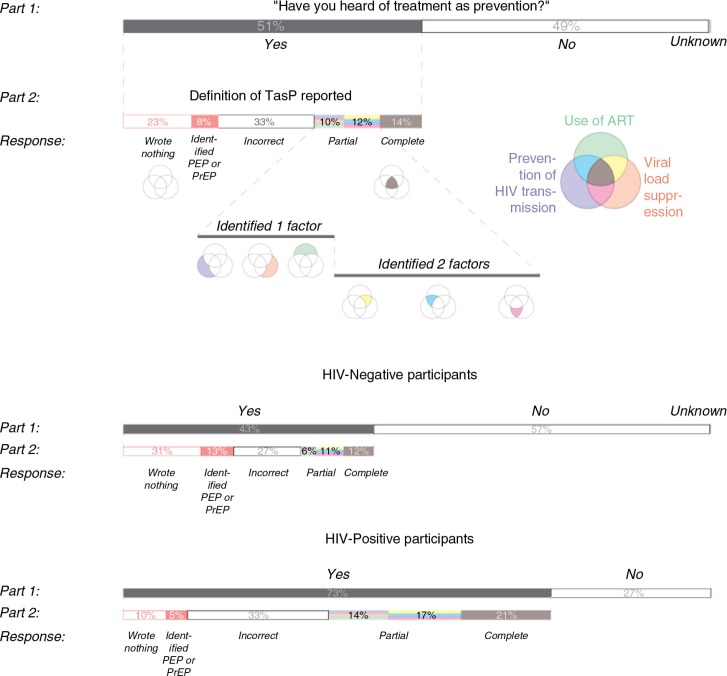
The primary outcome in this study was TasP awareness (ever vs. never heard of the term), assessed through the following question: Have you ever heard of the term “TasP”? Among those aware of TasP, participants were then asked how much they thought they knew about TasP, from whom or where they learnt about it, and to provide a definition in their own words (TasP knowledge) (Figure 1). Definitions were prompted using the following question stem: Please could you give a brief description of what you understand “TasP” to be. Responses were qualitatively assessed for completeness using a pre-determined three-part definition, developed using TasP literature [13] and refined through a sample of responses to determine appropriate language and scope [14]. The three components of a “complete” definition of TasP included: ART use, viral suppression, and prevention of HIV transmission [among HIV-positive people, as compared with PrEP/PEP (pre-exposure prophylaxis/post-exposure prophylaxis) used in those at-risk]. As shown in Figure 1, these data were then re-coded for quantitative analysis of participant's extent of knowledge, with responses re-coded as complete TasP knowledge (three factors identified), partial TasP knowledge (one or two factors identified), or incorrect TasP knowledge (no factors identified). We also coded those who wrote nothing or described PrEP/PEP.
Figure 1.
Classification of participants’ self-reported definitions of TasP.
Independent variables of interest
Independent variables included socio-demographics (age, race/ethnicity, sexual orientation, highest formal education attained, current student status, country of birth, first language, current neighbourhood, employment status, income, and regular partnership status), behavioural factors [any drug use, party drug use (Cocaine, Crystal, Mushrooms, Nitrous Oxide, LSD, Other Hallucinogens, GHB, Ketamine, Ecstasy, Poppers), injection drug use, number of male anal sex partners in the past six months, any condom-less anal intercourse with a male partner of unknown HIV status, any work as an escort or in the sex industry], and clinical variables (most recent CD4 cell count and viral load).
Statistical analyses
All analyses were conducted using SAS® version 9.3 (SAS, North Carolina, United States) and adjusted by weights generated using RDSAT version 7.1.46 to better reflect population estimates. Descriptive statistics include crude frequencies and RDS-adjusted proportions. Bivariate and multivariable logistic regression was used to identify covariates of TasP awareness, stratified by HIV status. Model selections were conducted using a backward stepwise elimination technique based on two criteria [Akaike Information Criterion (AIC) and Type III p-values] until the final model reached the optimum (minimum) AIC [15]. All statistical tests were two-sided and considered significant at α<0.05.
Ethical statement
All participants provided voluntary informed consent at study enrolment. The Research Ethics Boards of Simon Fraser University, University of British Columbia/Providence Health, and the University of Victoria provided ethical approval for all study procedures.
Results
A total of 719 participants were included in this study, of whom 119 (17%) were recruited as seeds. After RDS-adjustment, 23% of this total were HIV-positive, 68% were Caucasian, 81% identified as gay and median age was 33 [IQR 26,47]. Overall, 86% of the sample reported high school education or greater, 52% were currently employed, 53% had annual incomes <$18,500, 34% reported a current regular partner, 75% were born in Canada and 52% lived in Vancouver's downtown/West End area, which is the historic neighbourhood with a substantial gay men's population. Other demographics are shown in Table 1. Among HIV-positive participants, 89% were receiving ART, of whom 67% were ≥95% adherent to treatment in the past six months (based on pharmacy refill data), 83% had a CD4 ≥350 cells/mm3 and 72% had an undetectable VL (<50 copies/mL). From this total, two men refused to answer TasP questions and were excluded from subsequent analyses.
Table 1.
Sample demographics
| n | RDS % | RDS (95% CI) | |
|---|---|---|---|
| HIV positive | |||
| No | 520 | 76.6 | (68.7, 83.9) |
| Yes | 199 | 23.4 | (16.1, 31.3) |
| Age | |||
| 16–24 | 139 | 21.4 | (15.0, 28.7) |
| 25–39 | 305 | 41.9 | (34.7, 48.7) |
| 40+ | 275 | 36.7 | (28.2, 45.4) |
| Ethnicity | |||
| Caucasian | 539 | 68.0 | (61.0, 74.2) |
| Asian | 72 | 9.8 | (6.3, 14.7) |
| Aboriginal ancestry | 50 | 10.3 | (5.5, 15.9) |
| Other | 58 | 11.9 | (7.3, 17.0) |
| Sexual orientation | |||
| Gay | 612 | 80.7 | (76.2, 85.3) |
| Bisexual | 66 | 15.3 | (10.6, 19.5) |
| Other | 41 | 4.0 | (2.4, 6.2) |
| Education | |||
| Some high school or less | 61 | 14.5 | (10.1, 20.8) |
| Completed high school (only) | 107 | 20.2 | (14.5, 25.0) |
| Any post-secondary education | 537 | 65.3 | (58.0, 72.3) |
| Current student | |||
| No | 568 | 81.0 | (75.9, 86.0) |
| Yes | 150 | 19.0 | (14.0, 24.1) |
| Born in Canada | |||
| No | 162 | 25.3 | (19.5, 32.1) |
| Yes | 557 | 74.7 | (67.9, 80.5) |
| First language | |||
| English | 597 | 79.1 | (72.9, 84.7) |
| Other | 122 | 20.9 | (15.3, 27.1) |
| Neighbourhood | |||
| Downtown/West End | 356 | 51.9 | (44.1, 59.2) |
| Elsewhere in Vancouver | 223 | 30.4 | (24.1, 36.3) |
| Outside Vancouver | 140 | 17.7 | (13.1, 23.7) |
| Currently employed | |||
| No | 264 | 48.0 | (41.3, 55.2) |
| Yes | 455 | 52.0 | (44.8, 58.7) |
| Income | |||
| <$18,500 | 328 | 52.5 | (46.2, 59.2) |
| $18,500–44,999 | 247 | 32.8 | (26.9, 38.3) |
| $50,000–74,999 | 101 | 9.4 | (6.2, 12.8) |
| $75,000+ | 43 | 5.3 | (2.8, 8.1) |
| Relationship with regular partner | |||
| No | 446 | 65.6 | (58.4, 71.5) |
| Yes | 232 | 34.4 | (28.5, 41.6) |
| Any reported drug use in the past 6 months | |||
| No | 258 | 34.7 | (28.9, 41.2) |
| Yes | 461 | 65.3 | (58.8, 71.1) |
| Any reported party drug use in the past 6 months | |||
| No | 288 | 40.7 | (34.6, 46.7) |
| Yes | 431 | 59.3 | (53.3, 65.4) |
| Any reported injection drug use in the past 6 months | |||
| No | 662 | 90.5 | (86.3, 94.5) |
| Yes | 57 | 9.5 | (5.5, 13.7) |
| Number of male anal sex partners in the past 6 months | |||
| 0–1 | 229 | 35.0 | (29.1, 41.6) |
| 2–5 | 208 | 25.7 | (21.2, 31.3) |
| 6+ | 195 | 25.6 | (19.0, 30.7) |
| No anal sex in the past 6 months | 87 | 13.8 | (9.9, 18.4) |
| Unprotected anal sex with opposite or unknown status partner | |||
| No | 441 | 64.1 | (58.0, 70.7) |
| Yes | 262 | 35.9 | (29.3, 42.0) |
| Worked as an escort or in the sex industry | |||
| No | 588 | 79.4 | (73.6, 84.6) |
| Yes, in the past 6 months | 43 | 8.5 | (4.5, 13.3) |
| Yes, but not in the past 6 months | 88 | 12.1 | (8.3, 16.3) |
| Current CD4 cell count | |||
| <200 | 13 | 6.6 | (2.3, 11.8) |
| 200–349 | 23 | 11.6 | (4.5, 23.0) |
| 350+ | 159 | 81.7 | (69.2, 90.8) |
| Current viral load <50 | |||
| No | 60 | 28.1 | (19.6, 45.2) |
| Yes | 139 | 71.9 | (54.8, 80.4) |
| Currently has a family doctor | |||
| No | 232 | 34.2 | (27.4, 41.3) |
| Yes | 486 | 65.8 | (58.7, 72.6) |
| Out to family doctor | |||
| No | 80 | 18.8 | (12.5, 27.9) |
| Yes | 400 | 81.2 | (72.1, 87.5) |
| Told family doctor about male partners | |||
| No family doctor | 232 | 34.9 | (28.5, 42.3) |
| Did not tell | 80 | 14.6 | (10.5, 19.5) |
| Told doctor | 400 | 50.5 | (43.1, 57.5) |
RDS=respondent-driven sampling; 95% CI=95% confidence interval.
TasP awareness
Overall, 46% of GBMSM had heard of TasP. HIV-positive men were more likely to have heard of TasP (69%) compared with HIV-negative men (41%, p<0.0001). Tables 2 and 3 show the RDS-adjusted demographic and risk factors, prevalence of TasP awareness and univariate associations for HIV-positive GBMSM (n=199) and HIV-negative GBMSM (n=520), respectively. The adjusted multivariable logistic regression models stratified by HIV status are shown in Table 4. In the adjusted models, among HIV-positive GBMSM, TasP awareness was significantly higher among men born in Canada (vs. not) [AOR (95% CI)=4.05 (1.52–10.80)] and men with a current CD4 cell count of ≥350 (vs. <200) [6.30 (1.30–30.64)]; and significantly lower among men who identified as bisexual (vs. gay) [0.15 (0.05–0.47)], currently employed (vs. not) [0.28 (0.13–0.62)] and had used any party drugs in the past six months (vs. none) [0.35 (0.13–0.95)]. Among HIV-negative GBMSM, TasP awareness was significantly higher among men who completed high school [3.33 (1.40–7.95)] or any post-secondary education [3.49 (1.60–7.61)] (vs. some or no high school), were a current student (vs. not) [1.67 (1.09–2.59)], had a regular partner (vs. not) [1.91 (1.27–2.87)] and had ≥6 [1.94 (1.07–3.52)] or 2–5 [1.77 (1.06–2.95)] male anal sex partners in the past six months (vs. 0–1 partners); and significantly lower among men who identified as bisexual (vs. gay) [0.45 (0.24–0.85)] and Aboriginal (vs. Caucasian) [0.38 (0.15–0.97)].
Table 2.
Demographic and risk factors, prevalence of TasP awareness and univariate associations for HIV-positive GBMSM (n=199)
| Total (HIV positive) | Aware of TasP | Univariate associations | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| n | RDS % | RDS (95% CI) | n | RDS % | RDS (95% CI) | p | OR (95% CI) | |
| Age | ||||||||
| 16–24 | 0 | 0 | ||||||
| 25–39 | 48 | 24.4 | (15.9, 32.9) | 34 | 77.4 | (62.9, 92.0) | 0.1379 | Reference |
| 40+ | 151 | 75.6 | (67.1, 84.1) | 110 | 64.8 | (52.1, 77.5) | 0.54 (0.24–1.22) | |
| Ethnicity | ||||||||
| Caucasian | 150 | 67.5 | (56.9, 78.1) | 115 | 71.5 | (59.8, 83.3) | 0.5860 | Reference |
| Asian | 13 | 6.7 | (2.5, 10.9) | 8 | 62.3 | (27.4, 97.1) | 0.66 (0.18–2.34) | |
| Aboriginal ancestry | 23 | 17.1 | (8.0, 26.2) | 12 | 61.0 | (30.2, 91.8) | 0.62 (0.27–1.46) | |
| Other | 13 | 8.7 | (1.5, 16.0) | 9 | 59.5 | (7.8, 100.0) | 0.58 (0.19–1.78) | |
| Sexual orientation | ||||||||
| Gay | 171 | 83.0 | (74.6, 91.4) | 128 | 72.1 | (61.1, 83.1) | 0.0139 | Reference |
| Bisexual | 21 | 12.5 | (4.8, 20.2) | 11 | 39.1 | (3.5, 74.7) | 0.25 (0.10–0.64) | |
| Other | 7 | 4.5 | (0.5, 8.6) | 5 | 76.0 | (32.7, 100.0) | 1.23 (0.22–6.76) | |
| Education | ||||||||
| Some high school or less | 24 | 13.4 | (6.5, 20.4) | 14 | 54.9 | (29.0, 80.7) | 0.4093 | Reference |
| Completed high school (only) | 39 | 20.8 | (11.5, 30.0) | 27 | 64.6 | (39.2, 90.0) | 1.50 (0.47–4.80) | |
| Any post-secondary education | 132 | 65.8 | (55.5, 76.1) | 99 | 70.3 | (57.3, 83.3) | 1.95 (0.71–5.37) | |
| Current student | ||||||||
| No | 180 | 90.5 | (85.4, 95.6) | 129 | 67.6 | (56.4, 78.7) | 0.7547 | Reference |
| Yes | 19 | 9.5 | (4.4, 14.6) | 15 | 71.4 | (44.5, 98.3) | 1.20 (0.38–3.75) | |
| Born in Canada | ||||||||
| No | 35 | 17.3 | (9.2, 25.4) | 22 | 41.8 | (17.0, 66.6) | 0.0012 | Reference |
| Yes | 164 | 82.7 | (74.6, 90.8) | 122 | 73.7 | (63.4, 84.0) | 3.91 (1.71–8.90) | |
| First language | ||||||||
| English | 165 | 83.0 | (74.9, 91.1) | 121 | 72.5 | (62.2, 82.8) | 0.0087 | Reference |
| Other | 34 | 17.0 | (8.9, 25.1) | 23 | 46.8 | (19.9, 73.6) | 0.33 (0.15–0.76) | |
| Neighbourhood | ||||||||
| Downtown/West End | 136 | 68.5 | (59.0, 78.0) | 95 | 65.7 | (52.7, 78.7) | 0.4996 | Reference |
| Elsewhere in Vancouver | 37 | 18.8 | (10.5, 27.2) | 29 | 68.5 | (42.6, 94.4) | 1.14 (0.49–2.64) | |
| Outside Vancouver | 26 | 12.7 | (6.8, 18.6) | 20 | 78.9 | (60.4, 97.3) | 1.95 (0.64–5.92) | |
| Currently employed | ||||||||
| No | 116 | 60.9 | (50.6, 71.2) | 86 | 79.1 | (69.9, 88.2) | 0.0003 | Reference |
| Yes | 83 | 39.1 | (28.8, 49.4) | 58 | 51.5 | (34.1, 69.0) | 0.28 (0.14–0.56) | |
| Income | ||||||||
| <$18,500 | 114 | 61.1 | (50.7, 71.4) | 78 | 67.5 | (55.3, 79.7) | 0.9353 | Reference |
| $18,500–44,999 | 55 | 25.5 | (16.0, 34.9) | 42 | 66.6 | (42.9, 90.2) | 0.96 (0.45–2.05) | |
| $50,000–74,999 | 25 | 12.7 | (6.1, 19.4) | 20 | 71.3 | (36.1, 100.0) | 1.20 (0.43–3.36) | |
| $75,000+ | 5 | 0.7 | (0.0, 1.6) | 4 | 92.7 | (68.2, 100.0) | n/a | |
| Relationship with regular partner | ||||||||
| No | 125 | 71.2 | (61.4, 81.1) | 86 | 66.8 | (53.6, 80.0) | 0.7135 | Reference |
| Yes | 55 | 28.8 | (18.9, 38.6) | 42 | 70.0 | (49.4, 90.5) | 1.16 (0.53–2.53) | |
| Any reported drug use in the past 6 months | ||||||||
| No | 44 | 16.5 | (10.2, 22.7) | 36 | 82.6 | (69.4, 95.8) | 0.0786 | Reference |
| Yes | 155 | 83.5 | (77.3, 89.8) | 108 | 65.0 | (53.0, 77.0) | 0.39 (0.14–1.11) | |
| Any reported party drug use in the past 6 months | ||||||||
| No | 56 | 24.8 | (16.4, 33.1) | 44 | 82.1 | (70.2, 94.0) | 0.0280 | Reference |
| Yes | 143 | 75.2 | (66.9, 83.6) | 100 | 63.2 | (50.4, 76.0) | 0.37 (0.16–0.90) | |
| Any reported injection drug use in the past 6 months | ||||||||
| No | 168 | 87.8 | (81.4, 94.1) | 128 | 68.5 | (57.0, 80.0) | 0.6849 | Reference |
| Yes | 31 | 12.2 | (5.9, 18.6) | 16 | 64.1 | (40.2, 87.9) | 0.82 (0.31–2.15) | |
| Number of male anal sex partners in the past 6 months | ||||||||
| 0–1 | 55 | 30.6 | (20.9, 40.3) | 41 | 72.7 | (54.9, 90.5) | 0.5790 | Reference |
| 2–5 | 52 | 22.3 | (14.5, 30.1) | 40 | 71.9 | (55.7, 88.0) | 0.96 (0.36–2.45) | |
| 6+ | 69 | 32.4 | (22.0, 42.8) | 49 | 64.6 | (43.6, 85.7) | 0.69 (0.30–1.59) | |
| No anal sex in the past 6 months | 23 | 14.7 | (6.7, 22.7) | 14 | 58.9 | (26.3, 91.5) | 0.54 (0.20–1.47) | |
| Unprotected anal sex with opposite or unknown status partner | ||||||||
| No | 107 | 54.9 | (44.2, 65.6) | 77 | 70.4 | (57.9, 83.0) | 0.8183 | Reference |
| Yes | 88 | 45.1 | (34.4, 55.8) | 66 | 68.7 | (52.2, 85.3) | 0.92 (0.47–1.83) | |
| Worked as an escort or in the sex industry | ||||||||
| No | 136 | 67.0 | (56.7, 77.3) | 101 | 67.8 | (55.1, 80.5) | 0.4891 | Reference |
| Yes, in the past 6 months | 15 | 6.4 | (0.8, 12.0) | 12 | 83.8 | (58.5, 100.0) | 2.45 (0.46–12.99) | |
| Yes, but not in the past 6 months | 48 | 26.6 | (16.8, 36.3) | 31 | 64.1 | (42.4, 85.9) | 0.85 (0.40–1.80) | |
| Current CD4 cell count | ||||||||
| <200 | 13 | 5.4 | (1.5, 9.3) | 5 | 31.9 | (0.0, 74.3) | 0.0729 | Reference |
| 200–349 | 23 | 12.5 | (4.7, 20.2) | 17 | 66.0 | (29.2, 100.0) | 4.16 (0.80–21.66) | |
| 350+ | 159 | 82.1 | (73.7, 90.5) | 119 | 71.1 | (59.8, 82.4) | 5.27 (1.26–22.06) | |
| Current viral load <50 | ||||||||
| No | 60 | 29.0 | (19.0, 39.0) | 37 | 65.6 | (46.2, 85.0) | 0.6724 | Reference |
| Yes | 139 | 71.0 | (61.0, 81.0) | 107 | 69.0 | (56.5, 81.4) | 1.17 (0.58–2.37) | |
| Currently has a family doctor | ||||||||
| No | 6 | 4.7 | (0.0, 10.0) | 2 | 65.5 | (0.7, 100.0) | 0.8802 | Reference |
| Yes | 193 | 95.3 | (90.0, 100.0) | 142 | 68.1 | (57.4, 78.7) | 1.12 (0.25–5.08) | |
| Out to family doctor | ||||||||
| No | 9 | 5.2 | (1.3, 9.2) | 4 | 46.7 | (0.0, 94.6) | 0.1903 | Reference |
| Yes | 182 | 94.8 | (90.8, 98.7) | 136 | 69.1 | (57.9, 80.2) | 2.54 (0.63–10.29) | |
| Told family doctor about male partners | ||||||||
| No family doctor | 6 | 4.7 | (0.0, 10.1) | 2 | 65.5 | (0.7, 100.0) | 0.4203 | Reference |
| Did not tell | 9 | 5 | (1.2, 8.8) | 4 | 46.7 | (0.0, 94.6) | 0.46 (0.06–3.41) | |
| Told doctor | 182 | 90.3 | (83.9, 96.7) | 136 | 69.1 | (57.9, 80.2) | 1.18 (0.26–5.34) | |
GBMSM=gay, bisexual, and other men who have sex with men; RDS= respondent-driven sampling; 95% CI=95% confidence interval.
Table 3.
Demographic and risk factors, prevalence of TasP awareness and univariate associations for HIV-negative GBMSM (n=520)
| Total (HIV positive) | Aware of TasP | Univariate associations | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| n | RDS % | RDS (95% CI) | n | RDS % | RDS (95% CI) | p | OR (95% CI) | |
| Age | ||||||||
| 16–24 | 139 | 27.5 | (22.1, 32.9) | 52 | 31.6 | (21.1, 42.2) | 0.1905 | Reference |
| 25–39 | 257 | 48.5 | (42.4, 54.6) | 113 | 39.9 | (31.3, 48.5) | 1.44 (0.95–2.18) | |
| 40+ | 124 | 24.0 | (18.8, 29.2) | 57 | 40.2 | (28.3, 52.1) | 1.45 (0.89–2.37) | |
| Ethnicity | ||||||||
| Caucasian | 389 | 70.1 | (64.1, 76.0) | 173 | 37.9 | (31.3, 44.4) | 0.0343 | Reference |
| Asian | 59 | 11.0 | (7.5, 14.4) | 19 | 41.3 | (24.1, 58.6) | 1.16 (0.67–2.00) | |
| Aboriginal ancestry | 27 | 6.8 | (3.1, 10.4) | 8 | 16.4 | (2.0, 30.9) | 0.32 (0.13–0.77) | |
| Other | 45 | 12.2 | (7.5, 16.9) | 22 | 45.3 | (23.5, 67.1) | 1.36 (0.81–2.29) | |
| Sexual orientation | ||||||||
| Gay | 441 | 82.0 | (77.1, 86.9) | 186 | 39.4 | (33.0, 45.9) | 0.0042 | Reference |
| Bisexual | 45 | 13.3 | (8.6, 18.0) | 16 | 21.2 | (8.1, 34.3) | 0.41 (0.23–0.75) | |
| Other | 34 | 4.7 | (2.9, 6.5) | 20 | 52.8 | (32.3, 73.3) | 1.72 (0.77–3.83) | |
| Education | ||||||||
| Some high school or less | 37 | 11.7 | (7.0, 16.4) | 12 | 14.2 | (3.1, 25.3) | 0.0004 | Reference |
| Completed high school (only) | 68 | 17.2 | (12.1, 22.3) | 23 | 35.4 | (19.2, 51.7) | 3.31 (1.45–7.58) | |
| Any post-secondary education | 405 | 71.1 | (65.0, 77.2) | 183 | 42.0 | (35.4, 48.5) | 4.37 (2.09–9.15) | |
| Current student | ||||||||
| No | 388 | 74.5 | (69.3, 79.7) | 160 | 34.7 | (28.2, 41.3) | 0.0153 | Reference |
| Yes | 131 | 25.5 | (20.3, 30.7) | 62 | 46.3 | (34.5, 58.1) | 1.62 (1.10–2.39) | |
| Born in Canada | ||||||||
| No | 127 | 28.4 | (22.7, 34.2) | 50 | 40.7 | (28.5, 52.9) | 0.3529 | Reference |
| Yes | 393 | 71.6 | (65.8, 77.3) | 172 | 36.4 | (30.0, 42.9) | 0.84 (0.57–1.22) | |
| First language | ||||||||
| English | 432 | 78.9 | (73.5, 84.3) | 193 | 36.7 | (30.6, 42.9) | 0.3962 | Reference |
| Other | 88 | 21.1 | (15.7, 26.5) | 29 | 41.0 | (26.0, 56.1) | 1.20 (0.80–1.82) | |
| Neighbourhood | ||||||||
| Downtown/West End | 220 | 45.0 | (38.9, 51.2) | 97 | 40.7 | (31.2, 50.2) | 0.2786 | Reference |
| Elsewhere in Vancouver | 186 | 31.4 | (26.1, 36.8) | 86 | 37.2 | (28.1, 46.4) | 0.86 (0.58–1.29) | |
| Outside Vancouver | 114 | 23.6 | (18.5, 28.6) | 39 | 32.4 | (20.6, 44.2) | 0.70 (0.45–1.09) | |
| Currently employed | ||||||||
| No | 148 | 36.8 | (30.6, 43.0) | 58 | 29.4 | (19.8, 39.0) | 0.0024 | Reference |
| Yes | 372 | 63.2 | (57.0, 69.4) | 164 | 42.5 | (35.5, 49.5) | 1.77 (1.22–2.56) | |
| Income | ||||||||
| <$18,500 | 214 | 47.0 | (40.9, 53.2) | 91 | 37.1 | (28, 46.2) | 0.9759 | Reference |
| $18,500–44,999 | 192 | 35.2 | (29.5, 40.9) | 81 | 37.8 | (28.4, 47.2) | 1.03 (0.70–1.51) | |
| $50,000–74,999 | 76 | 9.8 | (6.7, 12.8) | 34 | 40.3 | (25, 55.6) | 1.15 (0.63–2.09) | |
| $75,000+ | 38 | 8.0 | (4.8, 11.2) | 16 | 37.1 | (17.1, 57) | 0.10 (0.52–1.93) | |
| Relationship with regular partner | ||||||||
| No | 321 | 65.9 | (60.2, 71.7) | 129 | 34.4 | (27.2, 41.7) | 0.0109 | Reference |
| Yes | 177 | 34.1 | (28.3, 39.8) | 85 | 45.9 | (35.7, 56.1) | 1.61 (1.12–2.33) | |
| Any reported drug use in the past 6 months | ||||||||
| No | 214 | 43.5 | (37.5, 49.6) | 94 | 43.6 | (34.5, 52.7) | 0.0114 | Reference |
| Yes | 306 | 56.5 | (50.4, 62.5) | 128 | 33.1 | (25.8, 40.3) | 0.64 (0.45–0.90) | |
| Any reported party drug use in the past 6 months | ||||||||
| No | 232 | 47.4 | (41.3, 53.5) | 99 | 41.5 | (32.7, 50.2) | 0.0826 | Reference |
| Yes | 288 | 52.6 | (46.5, 58.7) | 123 | 34.3 | (26.7, 41.9) | 0.74 (0.52–1.04) | |
| Any reported injection drug use in the past 6 months | ||||||||
| No | 494 | 94.3 | (90.8, 97.9) | 212 | 38.7 | (32.8, 44.7) | 0.0388 | Reference |
| Yes | 26 | 5.7 | (2.1, 9.2) | 10 | 20.0 | (1.7, 38.2) | 0.40 (0.16–0.95) | |
| Number of male anal sex partners in the past 6 months | ||||||||
| 0–1 | 174 | 35.2 | (29.3, 41.1) | 71 | 34.5 | (24.8, 44.1) | 0.0365 | Reference |
| 2–5 | 156 | 29.2 | (24.0, 34.4) | 60 | 31.9 | (22.5, 41.3) | 0.90 (0.57–1.39) | |
| 6+ | 126 | 22.9 | (17.4, 28.3) | 58 | 44.2 | (30.3, 58.0) | 1.50 (0.95–2.38) | |
| No anal sex in the past 6 months | 64 | 12.7 | (8.8, 16.6) | 33 | 48.1 | (31.2, 65.0) | 1.76 (1.01–3.07) | |
| Unprotected anal sex with opposite or unknown status partner | ||||||||
| No | 334 | 68.9 | (63.4, 74.5) | 146 | 35.6 | (28.6, 42.6) | 0.0927 | Reference |
| Yes | 174 | 31.1 | (25.5, 36.6) | 74 | 43.2 | (32.7, 53.7) | 1.38 (0.95–2.00) | |
| Worked as an escort or in the sex industry | ||||||||
| No | 452 | 85.6 | (81.1, 90.2) | 195 | 40.0 | (33.6, 46.3) | 0.0070 | Reference |
| Yes, in the past 6 months | 28 | 7.9 | (3.8, 12.0) | 10 | 14.8 | (2.2, 27.4) | 0.26 (0.11–0.60) | |
| Yes, but not in the past 6 months | 40 | 6.5 | (4.1, 8.9) | 17 | 35.7 | (17.9, 53.5) | 0.83 (0.41–1.69) | |
| Currently has a family doctor | ||||||||
| No | 226 | 44.9 | (38.8, 51) | 84 | 37.5 | (28.1, 46.8) | 0.9496 | Reference |
| Yes | 293 | 55.1 | (49, 61.2) | 137 | 37.7 | (30.4, 45) | 1.01 (0.72–1.43) | |
| Out to family doctor | ||||||||
| No | 71 | 32.3 | (24.4, 40.2) | 30 | 33.7 | (19.7, 47.7) | 0.2996 | Reference |
| Yes | 218 | 67.7 | (59.8, 75.6) | 105 | 39.9 | (31.3, 48.6) | 1.31 (0.79–2.18) | |
| Told family doctor about male partners | ||||||||
| No family doctor | 226 | 45.2 | (39.1, 51.4) | 84 | 37.5 | (28.1, 46.8) | 0.5793 | Reference |
| Did not tell | 71 | 17.7 | (12.7, 22.6) | 30 | 33.7 | (19.7, 47.7) | 0.85 (0.52–1.39) | |
| Told doctor | 218 | 37.1 | (31.4, 42.8) | 105 | 39.9 | (31.3, 48.6) | 1.11 (0.76–1.62) | |
GBMSM=gay, bisexual, and other men who have sex with men; RDS= respondent-driven sampling; 95% CI=95% confidence interval.
Table 4.
Multivariable models of TasP awareness stratified by HIV status
| HIV-negative GBMSM AOR (95% CI) |
HIV-positive GBMSM AOR (95% CI) |
|
|---|---|---|
| Ethnicity | ||
| Caucasian | Reference | |
| Asian | 0.91 (0.51–1.63) | |
| Aboriginal ancestry | 0.38 (0.15–0.97) | |
| Other | 1.42 (0.81–2.49) | |
| Sexual orientation | ||
| Gay | Reference | Reference |
| Bisexual | 0.45 (0.24–0.85) | 0.15 (0.05–0.47) |
| Other | 1.75 (0.75–4.11) | 0.71 (0.10–5.21) |
| Education | ||
| Some high school or less | Reference | |
| Completed high school (only) | 3.33 (1.40–7.95) | |
| Any post-secondary education | 3.49 (1.60–7.61) | |
| Current student | ||
| No | Reference | |
| Yes | 1.67 (1.09–2.58) | |
| Born in Canada | ||
| No | Reference | |
| Yes | 4.05 (1.52–10.80) | |
| Currently employed | ||
| No | Reference | |
| Yes | 0.28 (0.13–0.62) | |
| Relationship with regular partner | ||
| No | Reference | |
| Yes | 1.91 (1.27–2.87) | |
| Any reported party drug use in the past 6 months | ||
| No | Reference | |
| Yes | 0.35 (0.13–0.95) | |
| Number of male anal sex partners in the past 6 months | ||
| 0–1 | Reference | |
| 2–5 | 0.75 (0.46–1.21) | |
| 6+ | 1.77 (1.06–2.95) | |
| No anal sex in the past 6 months | 1.94 (1.07–3.52) | |
| Current CD4 cell count | ||
| <200 | Reference | |
| 200–349 | 4.12 (0.69–24.64) | |
| 350+ | 6.30 (1.30–30.64) |
GBMSM=gay, bisexual, and other men who have sex with men; RDS=respondent-driven sampling; 95% CI=95% confidence interval.
TasP knowledge
Among HIV-positive (n=144) and HIV-negative (n=222) GBMSM aware of TasP, Table 5 presents their self-perceived knowledge level, risk perceptions and information source. After RDS-adjustment, 91% of HIV-positive men who had heard of TasP felt they knew “a lot” or “a bit in general” about TasP compared with 69% of HIV-negative men who had heard of TasP (p<0.0001). In addition, 64 and 41%, respectively, felt HIV treatment made the risk of transmitting or acquiring HIV “a lot lower” (p=0.0020). The leading information sources for HIV-positive GBMSM were doctors (44%) (vs. 10% for HIV-negative men, p<0.0001) and community agencies (38%) (vs. 25% for HIV-negative men, p=0.0338). Gay media was also an important information source for men regardless of HIV status (34% for both HIV-positive and HIV-negative men, p=0.9517). Other sources included friends (20% HIV-positive vs. 32% HIV-negative men, p=0.0392) and sex partners (10% vs. 17%, p=0.1211).
Table 5.
Self-perceived knowledge, source of awareness and impact on HIV transmission of Treatment as Prevention (TasP)
| Total sample | HIV-negative GBMSM | HIV-positive GBMSM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| n | RDS % | RDS (95% CI) | n | RDS % | RDS (95% CI) | n | RDS % | RDS (95% CI) | p | |
| How much do you think you know about what TasP means? (n=366) | <0.0001 | |||||||||
| Not much, or nothing at all | 77 | 20.0 | (10.0, 28.5) | 66 | 30.6 | (22.4, 38.8) | 11 | 9.5 | (1.0, 17.9) | |
| A bit in general | 201 | 57.1 | (47.0, 68.6) | 126 | 57.1 | (48.1, 66.0) | 75 | 52.4 | (40.0, 64.8) | |
| A lot | 88 | 22.9 | (14.3, 33.8) | 30 | 12.3 | (7.2, 17.4) | 58 | 38.1 | (26.2, 50.1) | |
| Who or where did you learn about TasP from? (n=289) [all that apply] | ||||||||||
| Friends | 84 | 27.6 | (13.7, 38.6) | 56 | 31.5 | (20.8, 42.2) | 28 | 19.8 | (9.2, 30.4) | 0.0392 |
| Sex partners | 40 | 15.8 | (6.0, 25.9) | 21 | 17.1 | (7.4, 26.9) | 19 | 10.2 | (1.6, 18.9) | 0.1211 |
| Community agency | 106 | 31.7 | (29.1, 55.4) | 47 | 25.3 | (15.8, 34.8) | 59 | 37.7 | (25.6, 49.8) | 0.0338 |
| Doctor | 76 | 27.8 | (16.5, 41.4) | 17 | 9.6 | (4.2, 14.9) | 59 | 44.0 | (31.4, 56.7) | <0.0001 |
| Gay media | 102 | 31.1 | (25.1, 53.2) | 57 | 33.9 | (23.1, 44.7) | 45 | 34.2 | (21.8, 46.7) | 0.9517 |
| How do you think that TasP changes your current risk of getting or transmitting HIV? (n=289) | 0.0020 | |||||||||
| A lot lower | 143 | 57.5 | (42.6, 69.2) | 54 | 40.5 | (28.7, 52.4) | 89 | 63.6 | (51.4, 75.8) | |
| A little lower | 86 | 28.6 | (19.4, 45.7) | 65 | 41.0 | (29.7, 52.4) | 21 | 19.1 | (8.2, 30.1) | |
| No difference | 50 | 10.9 | (4.9, 15.4) | 31 | 16.2 | (9.5, 22.9) | 19 | 13.7 | (6.5, 20.8) | |
| A little higher | 5 | 1.4 | (0.0, 2.1) | 3 | 0.9 | (0, 1.9) | 2 | 2.3 | (0, 6.5) | |
| A lot higher | 5 | 1.6 | (0.0, 4.0) | 3 | 1.3 | (0, 3.1) | 2 | 1.3 | (0, 3.2) | |
GBMSM=gay, bisexual, and other men who have sex with men; RDS=respondent-driven sampling; 95% CI=95% confidence interval.
Qualitative analysis of participants’ short-answer definitions of TasP revealed that only 14% of participants who had heard of TasP demonstrated complete TasP knowledge with all three factors identified (ART use, viral suppression and prevention of HIV transmission), while 12% identified two out of three TasP factors, and 43% identified one or none. The remaining men provided no definition (23%) or described PrEP/PEP (7%). By HIV status, 21% of HIV-positive men and 13% of HIV-negative men (p<0.0001) identified all three TasP factors. The factor identified most was “ART use” (48% HIV-positive vs. 29% HIV-negative men, p<0.0001). The factor omitted most was “viral suppression” (30% HIV-positive vs. 14% HIV-negative men, p<0.0001). Figure 1 illustrates the division of TasP definitions.
An illustrative example of a complete definition was reported by a participant who said: By getting treatment, viral load goes to “non-detectable” (ideally) therefore lessening chances of transmission (HIV-positive, Caucasian, 52 years). However, the vast majority of men were unable to clearly express a complete understanding of TasP. For example, one participant explained: The more regular testing you get, the more you are exposed to STI/HIV information/education and the more likely you are to practice safer sex and prevent infections (HIV-negative, Latin American, 29 years). This incorrect definition does, however, highlight testing, which is one element to the implementation of BC's TasP policy overall. In other cases, men were unable to articulate essential differences between PrEP, PEP and TasP, for example: Taking the new drug for neg people to use if they have a poz partner or are seeing many poz guys or high risk behaviours (HIV-negative, Caucasian, 58 years). A sample of participants’ definitions is shown in Table 6.
Table 6.
A sample of participants’ definitions of TasP
| Complete (3 factors identified) |
|
|
| Two factors identified |
|
|
| One factor identified |
|
|
| Incorrect (No factors identified) |
|
|
| PrEP/PEP only |
|
|
Discussion
This cross-sectional survey of GBMSM in Vancouver, Canada, indicates that while TasP awareness was high among HIV-positive men (69%), it was relatively low among HIV-negative men (41%) and varied by key socio-demographic, clinical and behavioural factors among both populations. Further, men's articulation of their knowledge of TasP was poor, albeit better among HIV-positive men. To our knowledge, this is first study to provide an estimate of TasP awareness and knowledge among GBMSM living with and at-risk for HIV in a setting where a natural experiment for TasP has taken place, and the results have important implications for HIV care, prevention and education in BC and globally as jurisdictions scale-up the implementation of TasP into practice.
We suspect that some of the differences in TasP awareness and knowledge observed by HIV status are because TasP messaging and practices are largely targeted and taken by people with HIV as they are the recipient of ART in this strategy. The personal health benefits of ART for HIV-positive people, in terms of reduced morbidity and mortality [4], may explain some of the difference in incentive for HIV-positive GBMSM to learn about TasP. Indeed, in previous research [8–10], HIV-negative GBMSM have been shown to demonstrate lower TasP literacy with a lack of understanding of undetectable viral loads and scepticism that highly active antiretroviral therapy (HAART) prevents transmission. The benefits of TasP for HIV-negative people, in terms of prevention of transmission [16], require that information on TasP be made available and accessible to diverse communities of GBMSM irrespective of HIV status. As bioethicists have highlighted, “a treatment-as-prevention strategy that places all the emphasis upon the positive person's adherence … carries a disproportionate burden of responsibility” [17, pp. 63]. TasP is an important strategy in the arsenal of HIV prevention tools for all men, along with access to a combination of other evidence-based biomedical (e.g. PrEP/PEP), behavioural (e.g. consistent and correct use of condoms and lubricant), and structural (e.g. reducing stigma) HIV prevention interventions [18,19]. Meaningful engagement of HIV uninfected men in TasP initiatives are critical so that they can incorporate this information into their sexual decision-making and support their own health and the health of their partners and communities. Within this context, it is important to understand how HIV-negative versus HIV-positive GBMSM differentially access, perceive, and use TasP information, with special consideration given to men's own personal risk reduction strategies as well as the wider barriers to TasP such as the criminalization of HIV transmission, widespread stigma, and other social constraints [8–10].
A patient's health literacy can play an important role in overall health and clinical outcomes across many health issues [20]. In our study, HIV-positive GBMSM with higher CD4 cell counts were more likely to be aware of TasP; however, no association was found between TasP awareness and ART adherence or viral suppression. In addition, factors associated with increased HIV transmission were investigated in this study, with different patterns found by HIV status. For HIV-negative men, reporting two or more recent male anal sex partners was positively associated with TasP awareness. However, among HIV-positive men, any party drug use was negatively associated with TasP awareness, suggesting a greater lack of awareness of TasP among those with a potential greater risk of HIV transmission. There is concern that the public health benefits of TasP could be overwhelmed by increased risk behaviours, commonly referred to as risk compensation [21]. However, recent intervention research with GBMSM has demonstrated that exposure to multiple messages regarding HIV prevention strategies (PrEP/PEP, rectal microbicides) did not affect men's intentions to use condoms nor their attitudes regarding unprotected sex [22]. This is consistent with other studies of ART [23] and PrEP/PEP [24,25], which have reported no evidence of risk compensation that would offset the benefits of using HIV treatment as an effective prevention strategy. Future research will be conducted using longitudinal Momentum Health Study data to explore the relationship between TasP awareness and knowledge, treatment optimism, and risk compensation in this population.
Consistent with previous research [26,27], study findings also indicate important cultural and structural barriers to access to information regarding TasP. For example, HIV-negative Aboriginal men were less likely than their Caucasian counterparts to be aware of TasP as were HIV-positive men not born in Canada and HIV-negative men without high school education, highlighting a need for TasP messaging that is culturally relevant, responsive to literacy levels, and aware of other barriers in health care. Further, for HIV-positive men, TasP awareness was associated with unemployment. While this may seem counterintuitive, we suspect that this may be linked to men who have been living with HIV for longer (and thus likely more aware of issues such as TasP) and who have removed themselves from the job market to deal with their illness. Finally, regardless of HIV status, gay men were more likely to have heard of TasP than bisexual men. Previous research regarding biomedical approaches to HIV prevention has demonstrated the need for increased levels of community education to raise awareness and capacity within communities of GBMSM [28]. The differential access to and uptake of health promotion messaging among bisexual and other non-gay identified GBMSM in this study must be considered in future education campaigns and interventions.
Among those aware of TasP, despite a majority reporting that they felt they knew “a lot” or “a bit in general” about TasP (91% HIV-positive vs. 69% HIV-negative men), men's articulation of their knowledge of TasP was poor – only 21% of HIV-positive and 13% of HIV-negative GBMSM demonstrated complete TasP knowledge in their short-answer definitions. The factor omitted most was “viral suppression,” perhaps suggesting a lack of understanding of the mechanism through which ART prevents illness among HIV-positive people as well as transmission. Although, with the open-ended nature of this question, participants may have assumed viral suppression was implied. Further, 13% of HIV-negative men and 5% of HIV-positive men described PrEP/PEP only, reflecting a general understanding of ART-based prevention approaches but highlighting a gap in knowledge on the essential differences between PrEP, PEP and TasP and underscoring a need to improve men's literacy of the various approaches [29]. Further, among those aware of TasP, only 64 and 41%, respectively, felt HIV treatment made the risk of transmitting or acquiring HIV “a lot lower,” despite a growing evidence base that suggests the high efficacy of this approach [30,31]. This echoes previous research showing that HIV-positive men and those engaging in practices that put them at an increased risk for infection are more likely to believe in the preventive benefits of ART [8]. Continued promotion of the individual health and preventative benefits of ART remains critical, particularly among HIV-negative and other communities of GBMSM who may be missed in current TasP promotional efforts.
The results of this study also shed light on how GBMSM access information related to TasP, with information sources varying considerably by HIV status. While doctors were the leading information source for HIV-positive GBMSM (44%), they were the least likely source for HIV-negative GBMSM (10%), highlighting how physicians can be important gatekeepers of information that they feel is relevant to their patients’ health. This is despite 95% of HIV-positive men and 68% of HIV-negative men being “out” to their family doctors. Notably, GBMSM were also unlikely to report learning about TasP from sex partners (10% HIV-positive men versus 17% HIV-negative men). These results may indicate challenges GBMSM have around participating in conversations with doctors and sex partners about HIV, sexuality, and ART-based prevention strategies such as TasP [32], particularly within a background of persistent stigma towards HIV-positive people and the risks of criminal charges related to HIV non-disclosure. This emphasizes the importance of continued work to de-stigmatize HIV, within which the negative impact of criminalization of HIV non-disclosure must be considered [33]. Community agencies (38% HIV-positive men vs. 25% HIV-negative men) and gay media (34% for both HIV-positive and HIV-negative men) were also key sources of information. Although not explored in this survey, HIV and sexually transmitted infections (STI) testing services as well as various online modes (e.g. mobile phone applications, and social media campaigns) may also be important population-based vehicles through which this kind of education could occur.
Overall, these findings indicate that generating and disseminating TasP messages cannot take a one-size-fits-all approach. Rather, it requires a consideration of the diversity of the target audience as well as gay men's health and media literacy (or the ways in which they use, interpret, and respond to information). This is consistent with previous research highlighting how effectively targeting HIV prevention messaging to diverse communities of GBMSM requires the development of a variety of health promotion messages at both an individual- and population-level, and that are also grounded in and culturally relevant to both venue/mode (e.g. Internet, bars, clinics) and person characteristics (e.g. age, culture, education levels) [29].
A study limitation is that we used baseline data collected from participants over a two-year period. Any potential shifts in TasP awareness and knowledge over this time will be investigated in future work. Further, participants’ definitions of TasP may not be a complete proxy for and likely under estimates their entire understanding of the concept, as the open-ended nature of the survey question may have precluded some individuals from demonstrating their full knowledge. More direct closed-ended questions specifically addressing each of the three identified components of TasP knowledge may actually have produced a more accurate assessment of men's knowledge of this concept. The study was strengthened by its use of RDS to develop weighted population estimates.
Conclusions
To our knowledge, this is the first study to specifically report on TasP awareness and knowledge among GBMSM using a more representative sampling approach (i.e. RDS). For GBMSM to make use of TasP as a tool for their own health and the health of their communities, they must understand it. Health communication strategies relevant to diverse communities of GBMSM are critical to advancing TasP health literacy.
Acknowledgements
This work was supported by the Canadian Institutes for Health Research [107544]; National Institutes for Health, National Institute for Drug Abuse [R01DA031055] and Health Canada. We thank our community colleagues at the Health Initiative for Men, YouthCO HIV & HepC Society of BC and Positive Living BC for their support. We also thank the research participants for sharing their important data with the Momentum Health Study, and James Nakagawa for the design and creation of Figure 1 in this manuscript. DMM is supported by a Scholar Award from the Michael Smith Foundation for Health Research.
Competing interests
The authors have no competing interest to declare.
Authors' contributions
RSH has full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis and the final decision to submit for publication. AC, JF and RSH led the conceptualization and design of the study, with contributions from all authors. PS prepared the data set and ZC ran the analysis of data. AC interpreted the results and wrote the first draft of the paper, with contributions from all authors. The initial draft of the manuscript was critically reviewed and edited by all authors for important intellectual content. All authors approved the final version to submit for publication.
Source and role of funding
The Canadian Institutes of Health Research (Grant No. 107544) and the National Institutes of Health (Grant No. 1R01DA031055-01A1). The sponsors had no role in the design and conduct of the study; in the collection, management, analysis and interpretation of the data; or in the preparation, review or approval of the manuscript.
Ethics committee approval
Ethical approval was provided by the Research Ethics Boards of the University of British Columbia, Simon Fraser University, and the University of Victoria.
References
- 1.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–77. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BC Centre for Disease Control. Vancouver: Division of STI/HIV Prevention and Control, BC Centre for Disease Control; 2012. HIV Annual Report 2012. [Google Scholar]
- 3.Moore DM, Kanters S, Michelow W, Gustafson R, Hogg RS, Kwag M, et al. Implications for HIV prevention programs from a serobehavioural survey of men who have sex with men in Vancouver, British Columbia: The ManCount study. Can J Public Health. 2011;103(2):142–6. doi: 10.1007/BF03404220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anema A, Lima VD, Johnston K, Levy A, Montaner JS. Expanded highly active antiretroviral therapy coverage – a powerful strategy to curb progression to AIDS, death and new infections. Eur Infect Dis. 2009;3(1):41. [PMC free article] [PubMed] [Google Scholar]
- 5.Lourenço L, Lima VD, Heath K, Nosyk B, Gilbert M, Colley G, et al. Process monitoring of an HIV treatment as prevention program in British Columbia, Canada. J Acquir Immune Defic Syndr. 2014;67(3):e94–109. doi: 10.1097/QAI.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BC Centre for Excellence in HIV/AIDS. STOP HIV/AIDS: seek and treat for optimal prevention of HIV/AIDS Vancouver 2013 [cited 2015 May 7] Available from: http://stophivaids.ca.
- 7.UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic; Geneva: UNAIDS; 2014. [Google Scholar]
- 8.Holt M, Lea T, Murphy DA, Ellard J, Rosengarten M, Kippax SC, et al. Australian gay and bisexual men's attitudes to HIV treatment as prevention in repeated, national surveys, 2011–2013. PLoS One. 2014;9(11):e112349. doi: 10.1371/journal.pone.0112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman C, de Wit J, Persson A, Holt M, Slavin S, Kidd M, et al. Understanding concerns about treatment-as-prevention among people with HIV who are not using antiretroviral therapy. AIDS Behav. 2015;19(15):821–31. doi: 10.1007/s10461-014-0959-9. [DOI] [PubMed] [Google Scholar]
- 10.Young I, Flowers P, McDaid L. Key factors in the acceptability of treatment as prevention (TasP) in Scotland: a qualitative study with communities affected by HIV. Sex Transm Infect. 2015;91(4):269–74. doi: 10.1136/sextrans-2014-051711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrest JI, Stevenson B, Rich A, Michelow W, Pai J, Jollimore J, et al. Community mapping and respondent-driven sampling of gay and bisexual men's communities in Vancouver, Canada. Cult Health Sex. 2014;16(3):288–301. doi: 10.1080/13691058.2014.881551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogg RS, Yip B, Chan KJ, Wood E, Craib KJP, O'Shaughnessy MV, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286(20):2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 13.Montaner JSG, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368(9534):531–6. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 14.Racey CS, Zhang W, Brandson EK, Fernandes KA, Tzemis D, Harrigan PR, et al. HIV antiviral drug resistance: patient comprehension. AIDS Care. 2010;22(7):816–26. doi: 10.1080/09540120903431355. [DOI] [PubMed] [Google Scholar]
- 15.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21(9):1175–83. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 16.Montaner JS, Lima VD, Harrigan PR, Lourenço L, Yip B, Nosyk B, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the “HIV Treatment as Prevention” Experience in a Canadian Setting. PLoS One. 2014;9(2):e87872. doi: 10.1371/journal.pone.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haire B, Kaldor JM. Ethics of ARV based prevention: treatment-as-prevention and PrEP. Deve World Bioeth. 2013;13(2):63–9. doi: 10.1111/dewb.12026. [DOI] [PubMed] [Google Scholar]
- 18.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Current HIV/AIDS Rep. 2011;8(1):62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan PS, Carballo-Diéguez A, Coates T, Goodreau SM, McGowan I, Sanders EJ, et al. Successes and challenges of HIV prevention in men who have sex with men. Lancet. 2012;380(9839):388–99. doi: 10.1016/S0140-6736(12)60955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeWalt DA, Berkman ND, Sheridan S, Lohr KN, Pignone MP. Literacy and health outcomes. J Gen Intern Med. 2004;19(12):1228–39. doi: 10.1111/j.1525-1497.2004.40153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson DP. HIV treatment as prevention: natural experiments highlight limits of antiretroviral treatment as HIV prevention. PLoS Med. 2012;9(7):e1001231. doi: 10.1371/journal.pmed.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustanski B, Ryan DT, Sanchez T, Sineath C, Macapagal K, Sullivan PS. Effects of messaging about multiple biomedical and behavioral HIV prevention methods on intentions to use among US MSM: results of an experimental messaging study. AIDS Behav. 2014;18:1651–60. doi: 10.1007/s10461-014-0811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuyper L, Milloy M-J, Marshall BD, Zhang R, Kerr T, Montaner JS, et al. Does initiation of HIV antiretroviral therapy influence patterns of syringe lending among injection drug users? Addict Behav. 2011;36(5):560–3. doi: 10.1016/j.addbeh.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus JL, Glidden DV, Mayer KH, Liu AY, Buchbinder SP, Amico KR, et al. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS One. 2013;8(12):e81997. doi: 10.1371/journal.pone.0081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin JN, Roland ME, Neilands TB, Krone MR, Bamberger JD, Kohn RP, et al. Use of postexposure prophylaxis against HIV infection following sexual exposure does not lead to increases in high-risk behavior. AIDS. 2004;18(5):787–92. doi: 10.1097/00002030-200403260-00010. [DOI] [PubMed] [Google Scholar]
- 26.Koelmeyer R, English DR, Smith A, Grierson J. Association of social determinants of health with self-rated health among Australian gay and bisexual men living with HIV. AIDS Care. 2014;26(1):65–74. doi: 10.1080/09540121.2013.793273. [DOI] [PubMed] [Google Scholar]
- 27.Knight R, Shoveller J, Carson A, Contreras-Whitney J. Examining clinicians’ experiences providing sexual health services for LGBTQ youth: considering social and structural determinants of health in clinical practice. Health Educ Res. 2014;29(4):662–70. doi: 10.1093/her/cyt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poynten I, Jin F, Prestage G, Kaldor J, Imrie J, Grulich A. Attitudes towards new HIV biomedical prevention technologies among a cohort of HIV-negative gay men in Sydney, Australia. HIV Med. 2010;11(4):282–8. doi: 10.1111/j.1468-1293.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- 29.Kingdon MJ, Storholm ED, Halkitis PN, Jones DC, Moeller RW, Siconolfi D, et al. Targeting HIV prevention messaging to a new generation of gay, bisexual, and other young men who have sex with men. J Health Commun. 2013;18(3):325–42. doi: 10.1080/10810730.2012.727953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodger ABT, Cambiano V, Vernazza P, Estrada V, Van Lunzen J, Collins S, et al., editors. HIV transmission risk through condomless sex if HIV+ partner on suppressive ART: PARTNER study. 21st Conference on Retroviruses and Opportunistic Infections (CROI 2014); Boston, MA: 2014. Mar 3–6, [Google Scholar]
- 32.Prestage G, Mao L, McGuigan D, Crawford J, Kippax S, Kaldor J, et al. HIV risk and communication between regular partners in a cohort of HIV-negative gay men. AIDS Care. 2006;18(2):166–72. doi: 10.1080/09540120500358951. [DOI] [PubMed] [Google Scholar]
- 33.Adam BD, Elliott R, Corriveau P, English K. Impacts of criminalization on the everyday lives of people living with HIV in Canada. Sex Res Social Policy. 2014;11(1):39–49. [Google Scholar]