Graphical abstract
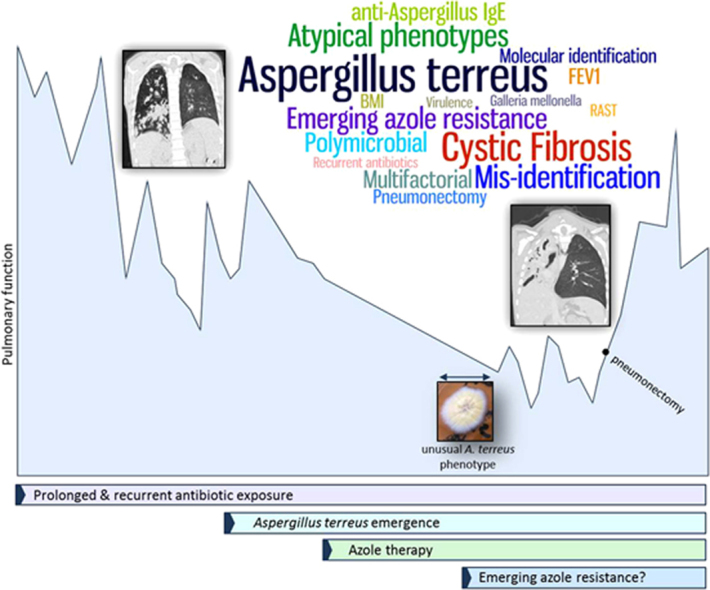
Keywords: Aspergillus terreus, Cystic Fibrosis, Paecilomyces variotii, Mis-identification, Atypical phenotype
1. Introduction
Cystic Fibrosis (CF) is the most common inherited life shortening condition affecting Caucasians. CF is characterised by mutations in the CF transmembrane conductance regulator (CFTR) gene, which codes for an ATP-driven pump that transports sodium and chloride ions across epithelial surfaces [1]. CF is a multiple organ disease; however up to 95% of morbidity and mortality is due to pulmonary infection. The CF lung has impaired mucociliary clearance and a build-up of thick mucus which creates an ideal environment to facilitate microbial colonisation. Excessive neutrophil recruitment and enhanced inflammation ensue which causes airway epithelial cell damage, decline in lung function and eventual respiratory failure.
Isolation of filamentous fungi, in particular Aspergillus spp. is common in respiratory secretions from CF patients [2]. Aspergillus terreus is the third most common filamentous fungus isolated from CF adult airway samples, being detected in 1.9 to 6.2% of CF patients [3,4]. In our clinic, 3 of 159 paediatric CF patients tested were A. terreus positive which is in line with the published literature on adults with CF (unpublished data). A. terreus has been reported to cause ABPA [5,6], infective endocarditis [6], pulmonary mycetoma [6] and invasive aspergillosis (IA) [7]. Until recently Aspergillus species identification was not thought to be therapeutically important; however different species within the genus can exhibit varying levels of antifungal drug resistance [8] and virulence in in vivo infection models [9]. Invasive disease caused by A. terreus can be as severe as IA caused by Apergillus fumigatus however A. terreus is inherently resistant to Amphotericin B [10]. Additionally IA caused by A. terreus is associated with long-term persistence of conidia and liver degeneration [11]. For these reasons A. terreus has the potential to cause complications post-transplant for people with CF. Here we present a case of a child with CF with a polymicrobial community in the airways among which A. terreus emerged and persisted as a dominant species.
2. Case
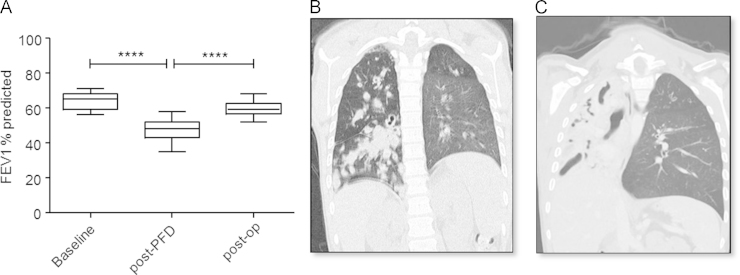
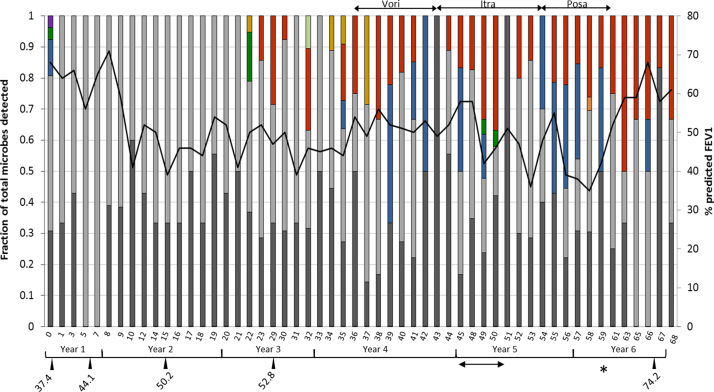
A 10-year-old boy with advanced CF lung disease diagnosed at 10 weeks old presented with a decline in pulmonary function. He had significant clinical manifestations of his disease, including chronic colonisation/infection with Pseudomonas aeruginosa and Staphylococcus aureus for more than 6 years necessitating multiple courses of antibiotic therapy, as well as gastrointestinal manifestation resulting in a body mass index (BMI) below the 0.4th centile and the requirement for gastrostomy feeding. His forced expiratory volume in 1 s (FEV1) was found to have significantly declined between months 8 and 10 from a score of 56–71% predicted to a score of 35–58% predicted (1 way ANOVA; p<0.0001) (Fig. 1A). This coincided with an anti-A. fumigatus IgG level (ImmunoCap) of 44.1 mg/L (above the 40 mg/L threshold [12]) (Fig. 2), high total IgG, high total IgA and high anti-A. fumigatus IgE levels (ImmunoCap) (Table S1). Ten months prior to pulmonary function decline (PFD), A. fumigatus was detected once by culture of patient sputum on malt extract agar (Fannin) and then once again thirteen months after PFD. Fourteen months after PFD A. terreus was detected by culture from sputum samples. The patient subsequently remained persistently colonised with A. terreus (Fig. 2) and only cultured A. fumigatus on 2 more occasions over the 68 months (Fig. 2). The patient also tested positive for A. flavus on one occasion in month 32. Following six consecutive isolations of A. terreus from the patient's sputa, treatment with voriconazole was commenced. Due to significant side effects, this was changed to itraconazole 7 months later, and after a further 11 months this was switched to posaconazole. Susceptibility of six of the A. terreus isolates to the azoles was measured using the TREK Sensititre YeastOne method. Isolates were classed as resistant (R), intermediate (I) or sensitive (S) to the azoles based on published epidemiological cutoff values (ECVs) (Table S2) [13]. Despite good in vitro susceptibility to the azoles, A. terreus was not eradicated during therapy. Of note sera levels of voriconazole, itraconazole and posaconazole ranged from 0.2–1.4 mg/L, 0.34–1.44 mg/L and 0.41–0.4 mg/L, respectively (reference ranges: >2 mg/L, >0.5 mg/L and 5–15 mg/L for voriconazole, itraconazole and posaconazole, respectively). The patient's lung function continued to worsen and radiological appearances declined (Fig. 1B and C).
Fig. 1.
Evidence of pulmonary function decline (A) Histogram depicting ranges of FEV1 scores before initial decline in lung function (baseline), after pulmonary function decline (post-PFD) and post-pneumonectomy (post-op). Chest CT scan in month 19 (B) and in month 45 (C) revealed progressive obstruction of the right lung. ****p<0.0001; 1-way ANOVA.
Fig. 2.
Microbiological culture results and associated FEV1 scores. A 100% stacked column histrogram presenting the microrganisms cultured from the patients airways as a fraction of the total microbial community detected by culture. P. aeruginosa (dark grey), S. aureus (light grey), Stenotrophomonas (blue), A. fumigatus (dark green), Streptococcus pneumoniae (purple), Candida (gold), A. terreus (red), A. flavus (light green) and Escherichia coli (orange) all colonised the airways of this patient. The secondary y-axis depicts the FEV1 scores (black line) at the time of each sample collection over a six year period (x-axis represents months). The star (*) represents the time of right lung pneumonectomy. The doubled-ended arrow below the timeline shows the period of time that A. terreus was mis-identified as P. variotii. Periods of antifungal drug treatment are represented by doubled-ended arrows above the stacked columns; vori=voriconazole, itra=itraconazole, posa=posaconazole. Anti-A. fumigatus IgG levels (mg/L) are represented by drop-down arrows from the timeline. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
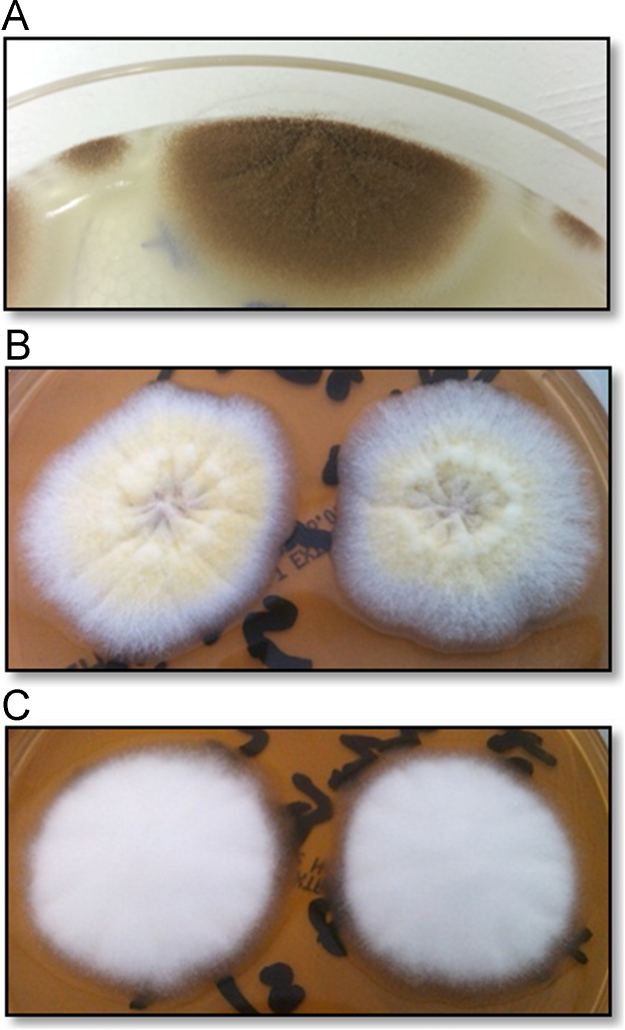
Twenty-two months after A. terreus was first identified, a fungus with a different morphological appearance was cultured on malt extract agar. In contrast to the typical cinnamon brown colonies of A. terreus cultured in month 40 (Fig. 3A), this isolate had yellow conidial masses (Fig. 3B) and some clonal variants grew only white hyphal masses with no spores (Fig. 3C). Microscopically these isolates had collapsed vesicles with reduced metulae and few phialides giving rise to sparse, short chains of conidia. Microscopically these isolates were similar to Penicillium however the yellow colour of the conidia led to their classification as Paecilomyces variotii. These isolates were sent for confirmatory macro- and microscopic identification and for sensitivity testing to the Health Protection Agency Mycology Reference Laboratory in Bristol, UK. These isolates were confirmed to be A. terreus complex, not P. variotii. Additionally the internal transcribed spacer (ITS), calmodulin and β-tubulin regions [14] from these isolates and five additional isolates collected from the patient were sequenced (Sourcebioscience), confirming all isolates as A. terreus (Table 1). Of interest the emergence of these atypical A. terreus isolates coincided with commencement of itraconazole therapy (Fig. 2). The earlier typical isolate (month 40) was sensitive to the azoles, and resistant to amphotericin B (Table 1). However the later atypical isolates (months 49 and 50) both showed intermediate resistance to voriconazole. These isolates were cultured from the patient's sputum 7 months after cessation of voriconazole therapy.
Fig. 3.
Atypical growth of A. terreus. Typical growth of A. terreus collected at month 40 (A) showing cinnamon brown colonies. Two clonal variants were observed in samples plated in month 50; (B) atypical yellow sporulation (isolate 50a) and (C) white colonies with no sporulation (isolate 50b). Colonies from (A)–(C) above, along with five more isolates were sent for sequencing and all were confirmed as A. terreus.
Table 1.
Confirmation of eight isolates as A. terreus by sequencing of the ITS, calmodulin and β-tubulin regions.
| Isolate no | Date isolate collected | Identification |
ITS |
Calmodulin |
β-tubulin |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identity (%) | Accession no. | Query cover (%) | Identity (%) | Accession no. | Query cover (%) | Identity (%) | Accession no. | Query cover (%) | |||
| 1 | Month 40 | A. terreus | 99 | JQ717316.1 | 100 | 99 | JF927626.1 | 99 | 99 | JX501420.1 | 100 |
| 2 | Month 41 | A. terreus | 99 | JQ717316.1 | 99 | 99 | LN734852.1 | 100 | 99 | JX501420.1 | 100 |
| 3 | Month 44 | A. terreus | 98 | JQ717316.1 | 100 | 99 | JF927626.1 | 99 | 99 | JX501420.1 | 100 |
| 4 | Month 46 | A. terreus | 99 | JQ717316.1 | 99 | 99 | LN734852.1 | 99 | 99 | JX501420.1 | 100 |
| 5 | Month 49 | A. terreus | 99 | JQ717316.1 | 100 | 99 | JF927626.1 | 99 | 99 | JX501420.1 | 100 |
| 6 | Month 50 a | A. terreus | 99 | JQ717316.1 | 100 | 99 | JF927626.1 | 99 | 99 | JX501420.1 | 100 |
| 7 | Month 50b | A. terreus | 99 | JQ717316.1 | 100 | 99 | JF927626.1 | 99 | 99 | JX501420.1 | 100 |
| 8 | Month 52 | A. terreus | 99 | JQ717316.1 | 100 | 99 | JF927625.1 | 100 | 99 | JX501420.1 | 100 |
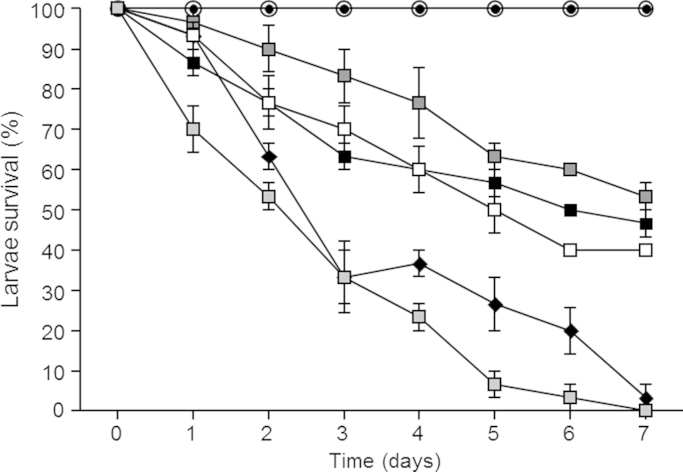
In order to establish whether the A. terreus isolates collected from this patient have the potential to be pathogenic, we inoculated Galleria mellonella larvae [15] with 1×106 conidia/20 μl and monitored larvae mortality over 7 days. An A. terreus reference strain (ATCC201901) and three A. terreus clinical isolates from the patient all caused mortality when tested in the G. mellonella infection model (Fig. 4). Interestingly one of the clinical A. terreus isolates with atypical growth (taken at month 46) caused 100% mortality by day 7 and was similar in virulence to A. fumigatus (ATCC26933).
Fig. 4.
Pathogenicity of A. terreus in the G. mellonella infection model. Ten Galleria were inoculated per treatment; uninjected (open circle), phosphate buffered saline (PBS) (closed circle), A. fumigatus reference strain (ATCC26933) (black diamond), A. terreus reference strain (ATCC201901) (black square) and three A. terreus isolates collected from the patient in month 40 (white squares), month 46 atypical isolate (light grey square) and month 50 (dark grey square). Percentage survival (y-axis) was monitored over 7 days (x-axis). These experiments were carried out on three independent occasions and error bars represent standard error.
Following complete collapse of the right lung the decision was taken to perform a right lung pneumonectomy. Post-pneumonectomy lung function returned to baseline levels however the patient continued to culture A. terreus and anti-A. fumigatus IgG levels continued to rise, with a most recent level of 74.2 mg/L. The patient was taken off posaconazole post-pneumonectomy in Newcastle in month 60 and has not been on antifungals since.
3. Discussion
There are few reports of A. terreus colonisation of the CF airways, all of which are from adult patients [3,4]. A. terreus emerged as a dominant and persistent member of the polymicrobial community present in this child's airway following long-term co-colonisation with P. aeruginosa and S. aureus and a prolonged period of exposure to high-dose antibiotics (Supplementary material). Exposure to high-dose antibiotics is a predisposing factor for development of fungal infections and in particular has been linked to the development of aspergillosis in CF [16]. People with CF have a high exposure to antibiotics over their life time and as such are predisposed to developing fungal infections.
A significant decline in lung function coincided with high anti-A. fumigatus IgG levels of 44.1 mg/L (above the 40 mg/L threshold [12]), high total IgG, total IgA and high anti-A. fumigatus IgE levels but not with A. fumigatus or A. terreus culture positivity. Thirteen months after detection of anti-A. fumigatus IgG, A. fumigatus was detected in the patients sputum however this was sporadic and no A. fumigatus was cultured from the following 19 sputum samples. Fourteen months after detection of anti-A. fumigatus IgG, A. terreus was detected from the patients sputum and was consistently present in the patients airways thereon. It has been previously reported that serum antibody levels can be elevated in advance of sputum culture positivity for Aspergillus species [17]. Monitoring anti-Aspergillus IgG and IgE levels in vulnerable patients such as CF patients could pick up early colonisation before culture positivity. In the case presented here culture methods were used to detect microorganisms however more sensitive molecular methods may have detected A. terreus colonisation earlier or may have detected the presence of A. fumigatus in more samples. A. fumigatus was only cultured on 4 occasions from this patient's sputa whereas the patient was chronically colonised with A. terreus following initial isolation. Despite the sporadic and limited isolation of A. fumigatus from this patient's sputa, high A. fumigatus-specific IgG and IgE antibodies were detected in the patient's serum. There are no reports in the literature of cross-reactivity between A. fumigatus and A. terreus allergens using the Phadia ImmunoCap M3 assay. Phadia's online information specifies that “A. terreus, A. flavus and A. nidulans hydrolyse collagen and were found to secrete an alkaline protease related to that of A. fumigatus”. Although the allergenic potential of this alkaline protease has not been determined it could be similar to other allergenic alkaline proteases from other fungal species and cause potential cross-reactivity with A. terreus using the ImmunoCap M3 assay. A. terreus has also been shown to produce allergens homologous to Asp f2 (UniProt: Q0CZU2_ASPTN), Asp f4 (UniProt: Q0CB03_ASPTN, Q0CTT7_ASPTN, Q0C9×7_ASPTN), Asp f7 (UniProt: Q0CL73_ASPTN) and Asp f15 (UniProt: Q0CYJ2_ASPTN) which could cause potential cross-reactivity as these are all identified A. fumigatus allergens. The potential for cross-reactivity within the Aspergilli genus using the ImmunoCap M3 assay warrants further exploration.
Lung function did not improve and radiological appearances worsened over a 4 year period despite treatment with numerous antibiotics and three azoles. Following complete collapse of the right lung the decision was taken to perform a right lung pneumonectomy. Post-pneumonectomy lung function returned to baseline levels however the patient continued to culture A. terreus and anti-Aspergillus IgG levels continued to rise, with a most recent level of 74.2 mg/L recorded in month 66. Baxter et al. [12] demonstrated that an Aspergillus IgG level of more than 75 mg/L can identify patients with ABPA serology or bronchitis with 90% sensitivity and 96% specificity. Baxter and colleagues suggest that “the use of IgG, using a cutoff of 75 mg/L, could become more clinically useful in practice for investigating and monitoring patients clinically suspected to have Aspergillus disease”. A study by Barton et al. [17] suggested a cutoff of 90 mg/L for separating patients with ABPA from those with Aspergillus sensitisation or controls. The patient represented in this case report had anti-Aspergillus IgG levels close to the 75 mg/L threshold post-pneumonectomy and with the trend of increasing anti-Aspergillus IgG levels observed over the past 4 years and continued culture of A. terreus from airway samples, the patients anti-Aspergillus IgG levels should be monitored closely.
Despite in vitro sensitivity of A. terreus isolates to the azoles, therapy failed to eradicate A. terreus from the patient's airways. It is also worth noting that there were difficulties in maintaining therapeutic levels of the azole drugs in the patient's sera. In this report later A. terreus isolates, post-voriconazole treatment, tested intermediate for resistance to voriconazole having been sensitive prior to this (Table S2). Here we used ECVs as there are no standardised breakpoints for antifungal drug resistance published. ECVs represent the MIC value identifying the upper limit of the wild type population and have been suggested to help characterise the susceptibility of isolates to antifungals [13]. Of note, these isolates were also independently determined as intermediately resistant to voriconazole by the Health Protection Agency Mycology Reference Laboratory in Bristol, UK (data not shown). A. terreus clinical isolates resistant to the triazoles have previously been reported [8,10]. These findings highlight the need for routine antifungal drug resistance testing and in particular, monitoring of patients that receive regular high-dose azole therapy such as CF patients.
During this study, atypical isolates of A. terreus were mis-identified as P. variotii. Previously, A. terreus isolates from this patient had grown with typical cinnamon brown conidial masses and the isolates mis-identified as P. variotii had yellow conidial masses and some clonal variants grew only white hyphal masses. Microscopically these isolates were also atypical of A. terreus having collapsed vesicles with reduced numbers of metulae (eliminating the circular ring of medulla typical of the species) and few phialides giving rise to sparse, short chains of conidia. Microscopically these isolates appeared similar to Penicillium however their yellow conidia led to their speciation as P. variotii. Due to this sudden appearance of P. variotii, a fungal species rarely isolated from CF airway samples, we sought confirmatory macro- and microscopic identification from the fungal reference laboratory in Bristol, UK and we also sequenced the ITS, β-tubulin and calmodulin regions from the atypical isolates. Also antifungal sensitivity testing was performed as P. variotii is susceptible to Amphotericin B [18] while A. terreus is intrinsically resistant to Amphotericin B. The isolates were subsequently confirmed as A. terreus. It is interesting that the atypical growth of A. terreus emerged when the patient switched from voriconazole to itraconazole therapy. Atypical growth of Aspergillus exposed to azoles has been previously reported to interfere with fungal identification [19]. A. terreus and P. variotii are usually easily distinguishable by macro- and microscopic methods however the atypical growth of the A. terreus isolates, perhaps due to influences of azole therapy, resulted in mis-identification. A patient's exposure to antifungals should be considered when using morphological characteristics to identify colonising fungal species.
The A. terreus isolates collected in this study were pathogenic in the G. mellonella infection model. Galleria are invertebrates with significant similarities between their immune system and the innate immune system of mammals [20]. There are also good correlations between results obtained from mice infection studies and those obtained in the galleria [21]. Interestingly, the atypical A. terreus isolate collected in month 46 (post-azole treatment) displayed increased virulence in the G. mellonella infection model. There are no reports in the literature of azoles increasing the virulence of A. terreus and this warrants further investigation.
Previous studies have linked declines in pulmonary function to Aspergillus sensitisation [22] and Aspergillus colonisation in the absence of ABPA [23]. Although A. terreus could not be definitively linked to this patients pulmonary function decline due to the polymicrobial nature of the patient's airway, A. terreus could be contributing to reduced lung function and disease progression. The ultimate aim of CF care is to prevent damage to the airways caused by microbial infection and inflammation and in turn prevent lung function decline. Considering this, it is vital to establish whether rare, novel and emerging CF microorganisms are capable of impairing lung function and contributing to CF airway disease.
Conflict of interest
Dr Julie Renwick receives salary from the NCH, Tallaght hospital to carry out research. For all other authors there are no conflicts of interest.
Acknowledgements
Firstly we thank the CF patient and his family for consent to publish this case. We would like to acknowledge the hard work and dedication of the CF nurses and physicians in the National Children's Hospital, Tallaght hospital, Dublin 24, Ireland. We would like to thank the medical microbiology laboratory team, specifically Eddie McCullagh, for his support of this work.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.mmcr.2015.07.002.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Boucher R.C. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 2004;23(1):146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 2.Pihet M. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis—a review. Med. Mycol. 2009;47(4):387–397. doi: 10.1080/13693780802609604. [DOI] [PubMed] [Google Scholar]
- 3.Cimon B. Aspergillus terreus in a cystic fibrosis clinic: environmental distribution and patient colonization pattern. J. Hosp. Infect. 2003;53(1):81–82. doi: 10.1053/jhin.2002.1343. [DOI] [PubMed] [Google Scholar]
- 4.Sabino R. Molecular epidemiology of Aspergillus collected from cystic fibrosis patients. J. Cyst. Fibros. 2014 doi: 10.1016/j.jcf.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Vincken W., Schandevul W., Roels P. Allergic bronchopulmonary aspergillosis caused by Aspergillus terreus. Am. Rev. Respir. Dis. 1983;127(3):388–389. doi: 10.1164/arrd.1983.127.3.388b. [DOI] [PubMed] [Google Scholar]
- 6.Laham M.N., Carpenter J.L. Aspergillus terreus, a pathogen capable of causing infective endocarditis, Pulmonary mycetoma, and allergic bronchopulmonary aspergillosis. Am. Rev. Respir. Dis. 1982;125(6):769–772. doi: 10.1164/arrd.1982.125.6.769. [DOI] [PubMed] [Google Scholar]
- 7.Hachem R. Invasive aspergillosis caused by Aspergillus terreus: an emerging opportunistic infection with poor outcome independent of azole therapy. J. Antimicrob. Chemother. 2014;69(11):3148–3155. doi: 10.1093/jac/dku241. [DOI] [PubMed] [Google Scholar]
- 8.Mortensen K.L. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 2010;54(11):4545–4549. doi: 10.1128/AAC.00692-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhtar N. In vivo pathogenicity studies of Aspergilli in Lepidopteran Model host Galleria mellonella. APCBEE Procedia. 2014;8(0):293–298. [Google Scholar]
- 10.Arendrup M.C. In vivo emergence of Aspergillus terreus with reduced azole susceptibility and a Cyp51a M217I alteration. J. Infect. Dis. 2012;206(6):981–985. doi: 10.1093/infdis/jis442. [DOI] [PubMed] [Google Scholar]
- 11.Slesiona S. Murine infection models for Aspergillus terreus pulmonary aspergillosis reveal long-term persistence of conidia and liver degeneration. J. Infect. Dis. 2012;205(8):1268–1277. doi: 10.1093/infdis/jis193. [DOI] [PubMed] [Google Scholar]
- 12.Baxter C.G. Novel immunologic classification of aspergillosis in adult cystic fibrosis. J. Allergy Clin. Immunol. 2013;132(3):560–566. doi: 10.1016/j.jaci.2013.04.007. (e10) [DOI] [PubMed] [Google Scholar]
- 13.Espinel-Ingroff A. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document) J. Clin. Microbiol. 2010;48(9):3251–3257. doi: 10.1128/JCM.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arabatzis M., Velegraki A. Sexual reproduction in the opportunistic human pathogen Aspergillus terreus. Mycologia. 2013;105(1):71–79. doi: 10.3852/11-426. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh K., Reeves E.P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004;28(1):101–112. doi: 10.1016/j.femsre.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Bargon J. Prophylactic antibiotic therapy is associated with an increased prevalence of Aspergillus colonization in adult cystic fibrosis patients. Respir. Med. 1999;93(11):835–838. doi: 10.1016/s0954-6111(99)90270-6. [DOI] [PubMed] [Google Scholar]
- 17.Barton R.C. Serologic diagnosis of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis through the detection of immunoglobulin G to Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 2008;62(3):287–291. doi: 10.1016/j.diagmicrobio.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Houbraken J. Identification of Paecilomyces variotii in clinical samples and settings. J. Clin. Microbiol. 2010;48(8):2754–2761. doi: 10.1128/JCM.00764-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt M.E. Atypical Aspergillus flavus isolates associated with chronic azole therapy. J. Clin. Microbiol. 2009;47(10):3372–3375. doi: 10.1128/JCM.00671-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mylonakis E. Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathologia. 2008;165(1):1–3. doi: 10.1007/s11046-007-9082-z. [DOI] [PubMed] [Google Scholar]
- 21.Brennan M. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002;34(2):153–157. doi: 10.1111/j.1574-695X.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 22.Kanthan S.K. Factors effecting impact of Aspergillus fumigatus sensitization in cystic fibrosis. Pediatr. Pulmonol. 2007;42(9):785–793. doi: 10.1002/ppul.20656. [DOI] [PubMed] [Google Scholar]
- 23.Amin R. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest. 2010;137(1):171–176. doi: 10.1378/chest.09-1103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material