Abstract
Hypoxic injury is commonly associated with inflammatory-cell infiltration, and inflammation frequently leads to the activation of cellular hypoxia response pathways. The molecular mechanisms underlying this cross-talk during kidney injury are incompletely understood. Yamaguchi and colleagues identify CCAAT/enhancer-binding protein δ as a cytokine- and hypoxia-regulated transcription factor that fine-tunes hypoxia-inducible factor-1 signaling in renal epithelial cells and thus provide a novel molecular link between hypoxia and inflammation in kidney injury.
Hypoxia and inflammation frequently coexist in injured tissues and have been shown to modulate each other. Current investigations in this fascinating area of biology focus on understanding the effects of tissue hypoxia on inflammatory-cell recruitment and function and, conversely, aim at understanding the molecular mechanisms by which inflammatory cells modulate the activation of hypoxic signaling pathways at sites of tissue injury. Although important insights into these relationships have been gained from the study of various tumor and inflammatory disease models, not much is known about the mechanisms that underlie the cross-talk between hypoxia and inflammation in the context of kidney disease.
Yamaguchi and colleagues1 (this issue) now provide evidence that CCAAT/enhancer-binding protein δ (CEBPD) provides an important link between inflammation and hypoxic signaling in renal epithelial cells via the hypoxia-inducible factor (HIF) pathway (Figure 1). The investigators asked whether genes that had previously been identified as hypoxia-inducible in a renal artery stenosis model were involved in the regulation of HIF-1, a heterodimeric oxygen-sensitive transcription factor that functions as a key regulator of cellular hypoxia responses. Using a short hairpin RNA library screening approach, Yamaguchi and colleagues examined which of these hypoxia-inducible genes were required for the activation of a HIF-dependent oxygen-sensitive luciferase reporter in HeLa cells. Four genes were identified and validated: CEBPD, transforming growth factor-β-induced factor (TGIF), nuclear receptor super-family 4A member 1 (NR4A1), and P300/CBP-associated factor (PCAF), of which CEBPD had the most pronounced effect on HIF-1 activity under hypoxia. Yamaguchi and colleagues then established that CEBPD expression was increased in renal epithelial cells not only under conditions of systemic hypoxia or renal artery stenosis, but also in renal injury models such as ischemia–reperfusion injury, cisplatin nephrotoxicity, and 5/6 nephrectomy.
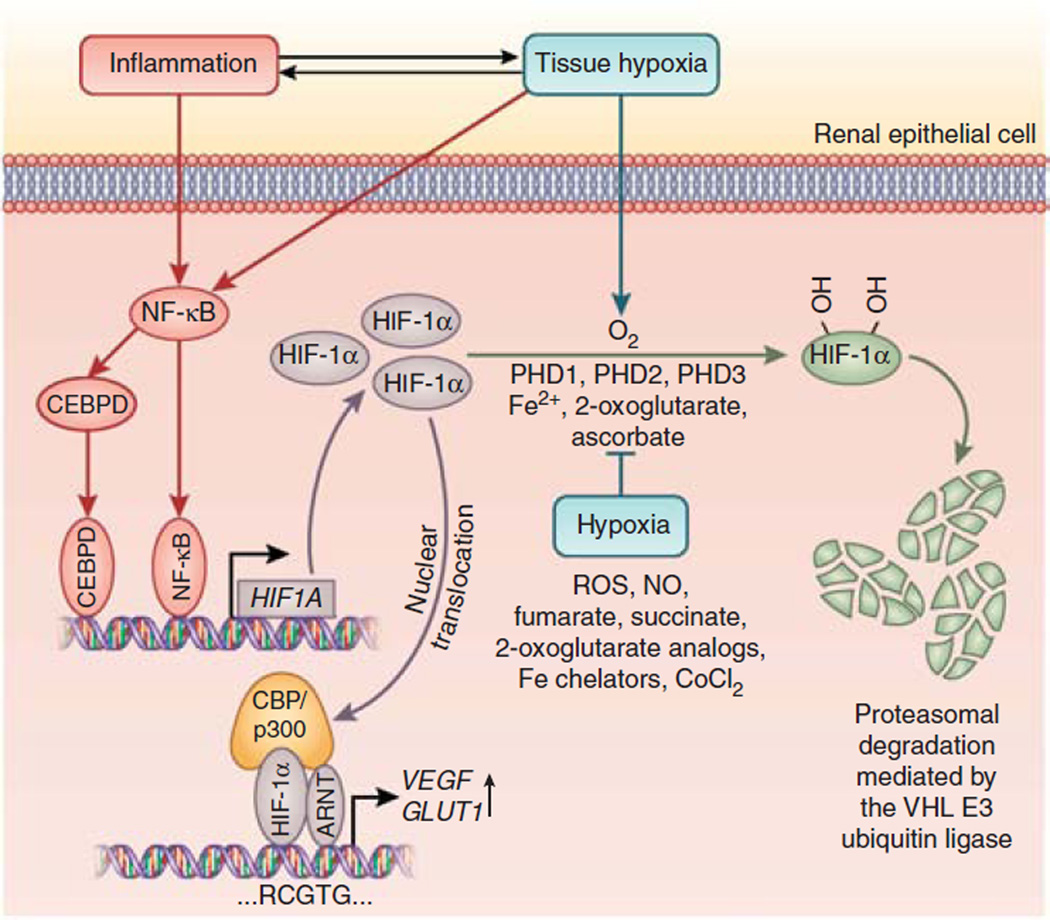
Figure 1. CEBPD links inflammation to HIF signaling in renal epithelial cells.
Inflammation and/or hypoxia result in increased nuclear factor-κB (NF-κB) and CEBPD activity, which in turn leads to increased HIF1A transcription. When oxygen is present, HIF-1α is hydroxylated by prolyl-4-hydroxylase domain proteins (PHDs) 1, 2, and 3 and targeted for proteasomal degradation by the pVHL–E3 ubiquitin ligase complex. Under hypoxic conditions prolyl-4-hydroxylation is inhibited, and HIF-1α is no longer degraded and translocates to the nucleus, where it heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT; also referred to as HIF-1β). HIF-1α/ARNT heterodimers bind to DNA at the HIF consensus site RCGTG. Nitric oxide (NO), reactive oxygen species (ROS), the Krebs cycle metabolites succinate and fumarate, cobalt chloride (CoCl2), and iron chelators such as desferrioxamine inhibit HIF prolyl-4-hydroxylases in the presence of oxygen. In normoxic cells, stimulation with interleukin-1β results in increased HIF1A transcription and HIF-1α protein levels, and increases expression of the HIF target genes VEGF (vascular endothelial growth factor) and GLUT1 (glucose transporter 1) irrespective of oxygen levels. Fe2+, ferrous iron.
CEBPD is a member of the CCAAT/enhancer-binding protein family of leucine zipper transcription factors, which interact with the CCAAT box motif and regulate genes involved in cellular proliferation and differentiation, metabolism, adipogenesis, immunity, and inflammation. It plays a critical role in the regulation of inflammation and immunity, as it functions as an inflammatory response protein that is induced by bacterial lipopolysaccharide, interferon-γ, interleukin-1β and -6, and tumor necrosis factor-α. Because of its hypoxia- and cytokine-inducibility and its role in the regulation of renal epithelial HIF-1 activity, Yamaguchi and colleagues hypothesized that CEBPD could provide a molecular link between hypoxia and inflammation during kidney injury. The authors used HK-2 cells to dissect this relationship on a molecular level and determined that activation of nuclear factor-κB (NF-κB) was required for the hypoxia- and cytokine-dependent induction of CEBPD, which binds to a specific regulatory element in the HIF1A promoter and enhances HIF1A transcription under conditions of hypoxia or interleukin-1β stimulation; interleukin-1β had been previously shown to activate HIF-1 in the presence of oxygen. The investigators then demonstrated that inhibition of CEBPD in HK-2 cells diminishes HIF--1α levels and profoundly reduces HIF target gene expression under these conditions.
While CEBPD has previously been shown to promote HIF-1α expression in cancer cell lines and macrophages by enhancing mammalian target of rapamycin (mTOR) signaling,2 Yamaguchi and colleagues1 investigate the CEBPD/HIF axis in the context of kidney injury and show that CEBPD regulates HIF-1α primarily at the transcriptional level, which is consistent with studies of HIF1A regulation in the setting of CEBPD overexpression.3 Transcriptional activation of HIF1A is likely to occur in synergy with NF-κB, which, aside from inducing CEBPD transcription, binds to the HIF1A promoter and induces HIF1A transcription (Figure 1).
Although cells synthesize HIF-1α continuously, it is normally rapidly degraded in the presence of molecular oxygen, unless the HIF degradation machinery is inhibited or overwhelmed as the result of increased HIF-1α translation. The latter has been proposed as the mechanism by which growth factors and cytokines lead to HIF activation. Key components of the HIF degradation machinery are Fe(II)- and 2-oxoglutarate-dependent prolyl-4-hydroxylase domain proteins (PHDs), which function as O2 sensors and control HIF-α degradation by catalyzing the hydroxylation of specific proline residues located within its oxygen-dependent degradation domain. Hydroxylated HIF-α is then targeted for proteasomal degradation by the VHL–E3 ubiquitin ligase complex (Figure 1). Structural analogs of 2-oxoglutarate, the Krebs cycle intermediates succinate and fumarate, reactive oxygen species, and nitric oxide are examples of molecules that are known to inhibit HIF-PHDs, thus leading to HIF stabilization in the presence of molecular oxygen. Some of these molecules are produced by inflammatory cells and have been shown to lead to activation of HIF signaling in nonimmune cells. A recent example is macrophage-derived nitric oxide, which stimulates renal erythropoietin production.4
The findings by Yamaguchi and colleagues predict that the renal HIF-1 response is blunted when CEBPD is inhibited. This could be easily tested in CEBPD knockout mice and is predicted to have negative effects on kidney disease outcome. In the non-cancerous kidney, HIF-1α has been shown to mediate epithelial hypoxia responses, while its homolog HIF-2α appears to be the predominant mediator of hypoxia responses in endothelial and interstitial fibroblast-like cells, the latter being the cellular source of erythropoietin. Renal HIF-2α does not appear to be regulated by CEBPD.1 HIF-1 and HIF-2 have both been shown to produce cytoprotective responses during acute and chronic kidney injury. While their role in the progression of chronic kidney disease remains controversial, several studies have demonstrated that diminished HIF responses are associated with adverse disease outcome.5,6 For example, HIF activation in response to hypoxia is submaximal or suppressed in diabetic kidneys and can be enhanced by the administration of CoCl2, which inhibits the development of diabetic nephropathy in streptozotocin-treated rats.5 Furthermore, preischemic HIF activation by pharmacologic means ameliorates ischemic injuries and holds great promise as a therapeutic strategy for the prevention of acute kidney injury.7
Support for the notion that inflammatory cells elicit tissue protection through the activation of HIF signaling in nonimmune cells comes from experimental models of inflammatory bowel disease. In this setting elegant in vitro and in vivo studies have shown that transmigrating neutrophils generate microenvironmental hypoxia sufficient enough to stabilize intestinal epithelial-cell HIF. This was dependent on respiratory burst activity and had positive effects on disease outcome.8 In contrast, mice with impaired respiratory burst activity developed more severe colitis, which was associated with exaggerated neutrophil infiltration and diminished hypoxia.8 These findings promote the concept that microenvironmental hypoxia, in this case generated by inflammatory cells, is critical for the resolution of inflammation. Whether and to what degree similar mechanisms operate in the injured kidney remain to be investigated.
While the studies by Yamaguchi and colleagues1 investigate a straightforward relationship between CEBPD signaling and HIF activation, the interplay between hypoxia, HIF signaling, and inflammation is far more complex and operates at multiple cellular and molecular levels in a bidirectional, cell type- and context-dependent manner. HIF-1, for example, shifts cellular metabolism toward anaerobic glycolysis, which permits inflammatory cells to operate in hypoxic microenvironments, and is important for bacterial killing and other immune-cell functions. While constitutive HIF activation in myeloid cells can lead to the exacerbation of certain inflammatory conditions, activation of HIF signaling in macrophages has also been shown to reduce inflammatory-cell infiltration in obstructive nephropathy.9 Although Yamaguchi and colleagues show that NF-κB activates HIF signaling via CEBPD in renal epithelial cells, NF-κB activation may inhibit certain HIF responses in other cell types; for example, the loss of HIF-2-dependent erythropoietin production in renal peritubular fibroblast-like cells has been associated with increased NF-κB activity.10 In endothelial cells, HIF-2 regulates cell adhesion and inflammatory-cell infiltration during ischemic kidney injury; its activation is protective and diminishes the development of fibrosis that is frequently associated with acute kidney injury.7
In summary, Yamaguchi and colleagues1 identify CEBPD as an important regulator of HIF signaling in renal epithelial cells and provide a molecular link between hypoxia and inflammation in the kidney. The authors’ work has implications for acute and chronic kidney injuries, including renal transplantation, and will stimulate future investigations into the molecular mechanisms that regulate the interplay between microenvironmental hypoxia, inflammation, HIF signaling, and renal repair.
ACKNOWLEDGMENTS
The author is supported by the Krick-Brooks Chair in Nephrology, by National Institutes of Health grants R01-DK081646, R01-DK080821, and R01-DK101791, and by a Department of Veterans Affairs Merit Award (1I01BX002348).
Footnotes
DISCLOSURE
The author declared no competing interests.
REFERENCES
- 1.Yamaguchi J, Tanaka T, Eto N, Nangaku M. Inflammation and hypoxia linked to renal injury by CCAAT/enhancer-binding protein δ. Kidney Int. 2015;88:262–275. doi: 10.1038/ki.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balamurugan K, Wang JM, Tsai HH, et al. The tumour suppressor C/EBPdelta inhibits FBXW7 expression and promotes mammary tumour metastasis. EMBO J. 2010;29:4106–4117. doi: 10.1038/emboj.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min Y, Ghose S, Boelte K, et al. C/EBP-delta regulates VEGF-C autocrine signaling in lymphangiogenesis and metastasis of lung cancer through HIF-1alpha. Oncogene. 2011;30:4901–4909. doi: 10.1038/onc.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakoda Y, Anand S, Zhao Y, et al. Herpesvirus entry mediator regulates hypoxia-inducible factor-1alpha and erythropoiesis in mice. J Clin Invest. 2011;121:4810–4819. doi: 10.1172/JCI57332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordquist L, Friederich-Persson M, Fasching A, et al. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol. 2015;26:328–338. doi: 10.1681/ASN.2013090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill P, Shukla D, Tran MG, et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008;19:39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapitsinou PP, Sano H, Michael M, et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014;124:2396–2409. doi: 10.1172/JCI69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell EL, Bruyninckx WJ, Kelly CJ, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi H, Gilbert V, Liu Q, et al. Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J Immunol. 2012;188:5106–5115. doi: 10.4049/jimmunol.1103377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souma T, Yamazaki S, Moriguchi T, et al. Plasticity of renal erythropoietin-producing cells governs fibrosis. J Am Soc Nephrol. 2013;24:1599–1616. doi: 10.1681/ASN.2013010030. [DOI] [PMC free article] [PubMed] [Google Scholar]