Abstract
Objective
Perinatal hypoxic-ischemic encephalopathy (HIE) and prolonged febrile seizures (pFS) are common neurologic problems that occur during childhood. However, there is insufficient evidence from experimental studies to conclude that pFS directly induces hippocampal injury. We studied cognitive function and histological changes in a rat model and investigated which among pFS, HIE, or a dual pathologic effect is most detrimental to the health of children.
Methods
A rat model of HIE at postnatal day (PD) 7 and a pFS model at PD10 were used. Behavioral and cognitive functions were investigated by means of weekly open field tests from postnatal week (PW) 3 to PW7, and by daily testing with the Morris water maze test at PW8. Pathological changes in the hippocampus were observed in the control, pFS, HIE, and HIE+pFS groups at PW9.
Results
The HIE priming group showed a seizure-prone state. The Morris water maze test revealed a decline in cognitive function in the HIE and HIE+pFS groups compared with the pFS and control groups. Additionally, the HIE and HIE+pFS groups showed significant hippocampal neuronal damage, astrogliosis, and volume loss, after maturation. The pFS alone induced minimal hippocampal neuronal damage without astrogliosis or volume loss.
Conclusion
Our findings suggest that pFS alone causes no considerable memory or behavioral impairment, or cellular change. In contrast, HIE results in lasting memory impairment and neuronal damage, gliosis, and tissue loss. These findings may contribute to the understanding of the developing brain concerning conditions caused by HIE or pFS.
Keywords: Hypoxic-ischemic encephalopathy, Febrile seizure, Epilepsy, Hippocampal injury
INTRODUCTION
Perinatal hypoxic-ischemic encephalopathy (HIE) is one of the most common causes of neonatal brain injury. This disease has a high incidence of occurrence of approximately 1-6 per 1000 live births, and results in significant neurodevelopmental disabilities1,18). Approximately 20-40% of HIE survivors develop neurological impairments such as epilepsy, cerebral palsy (CP), mental retardation, or psychiatric problems. These patients often have a lifelong disability, bear financial and social burdens, and have a higher risk for epilepsy, which occurs in 15-60% of children with CP11). The occurrence of seizures during fever episodes may be the first manifestation of epilepsy or an underlying neurologic condition, and patients with CP may be more prone to seizures during fevers.
Febrile seizures (FS) are the most common type of seizures, occurring in 2-5% of children during early childhood. Although simple FS is clinically benign and produces few neurologic sequelae, prolonged FS (pFS) is known to be associated with hippocampal sclerosis, which is a commonly observed structural abnormality in patients with temporal lobe epilepsy (TLE)15,20,23,24). In a previous animal study, prolonged seizures induced by fever in immature rats resulted in hippocampal damage and spontaneous TLE7). However, a previous population study did not detect any relation between hippocampal sclerosis and TLE with pFS. The immature brain seems to be less vulnerable to seizure-induced brain injuries12), but more vulnerable to HIE13), than the mature brain. Furthermore, the causative roles of childhood pFS and hippocampal sclerosis in adult TLE remain uncertain.
There are some possible hypotheses that could explain this relation : 1) Abnormalities of the hippocampus may already be present due to a predisposing neurologic condition before a febrile seizure occurs. This condition can make a patient more susceptible to febrile seizures, although pFS will not increase hippocampal damage. 2) Damage to the hippocampus may result in a seizure-susceptible condition, and subsequent seizures can cause additional damage. 3) The pFS alone could produce hippocampal damage.
This study was done to gain insight into whether predisposing perinatal HIE and pFS, and how the combination of these injuries influences cognitive-behavioral function and hippocampal damage. Four experimental animal groups were used in this study. We first determined whether pFS could cause damage in immature rat brains. Second, we studied whether rats with a history of HIE would be more vulnerable to pFS. Third, we studied which insults caused by pFS and HIE would cause more severe damage in immature rat brains.
MATERIALS AND METHODS
Animal models
Animal model of perinatal hypoxic-ischemic brain injury
In this study, we employed a well-established unilateral cerebral hypoxic-ischemic model with postnatal day (PD) 7 Sprague-Dawley pups14,22,28). This age was chosen because the brains of pups at this age are similar to those of a 32 to 34-week-old human fetus. The PD7 rat pups were anesthetized with inhaled isoflurane, and the right common carotid artery was completely severed through electrocauterization (UM880; Umeco Co., Seoul, Korea). After recovery in an incubator at 36.5℃ for 1 hour, the pups were exposed to 8% oxygen (balanced gas of N2 and H2O, 1 ppm; Jung Ang Sanso Co. Ltd., Seoul, Korea) in a warm incubator at 36.5℃ for 2 hours to induce hypoxic-ischemic brain damage. After a 30-minutes (min) recovery period, the surviving rats were transferred to the dam in the animal facility. In this hypoxic-ischemic injury rodent model, damage is induced with brain ischemia, hypoxia, and reperfusion.
Animal model of pFS
PD10 pups were used because the hippocampal developmental age of these pups is generally similar to that of a human infant9). Lipopolysaccharide (LPS, Escherichia coli 055 : B5) was administered to a pFS experimental model4,7) to induce an innate inflammatory response. LPS (200 µg/kg) was injected intraperitoneally 2 hours before seizure induction. The PD10 rat pups were kept in a 3-L glass, and were exposed to a heated air stream from a hair dryer placed 50 cm above the pups to induce seizures5). The pups were exposed to heat for 36 min, and the heat was controlled to maintain the seizure for ≥20 min. The types and duration of seizures were determined through serial measurement of rectal temperature every 2 min. To avoid brain injury caused by hyperthermia, rectal temperature was maintained at 40-42.5℃ and was not allowed to exceed 43℃ during heating. The pups were allowed to rest on cool surfaces if their temperature exceeded 42.5℃.
Experimental groups
This study was approved by the Animal Use Committee of the Korea University College of Medicine. Animal suffering was minimized, and all procedures involving animals were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Only male pups were used in this study. The overall strategy was to compare the differences in cognitive-behavioral function and neuronal injury between the four experimental groups. 1) Controls (n=32) were maintained as normothermic according to age (rectal temperature, 33-34℃), had not undergone any prior procedure, and underwent the open field test and Morris water maze test throughout the experiments. 2) The pFS-only group (n=40) was subjected to induced hyperthermia and developed seizures, but did not undergo the HIE procedure. 3) The HIE-only group (n=33) underwent a procedure in which the right common carotid artery was completely severed. These pups were not heated. 4) The HIE+pFS group (n=27) underwent the HIE procedure and were subjected to hyperthermic conditions leading to the development of seizures.
Cognitive and behavioral tests
Open field test
The open field activity levels of the rats were tested weekly from postnatal week (PW) 3 to PW7 by using a black Plexiglas box (60×60×25 cm). Rats were exposed to the open black box for 10 min. Locomotive behaviors were monitored and analyzed by using an auto-tracking system (SmarTrack), in which locomotion was calculated in pixel units by dividing the center area (25×25 cm) by the entire area.
Morris water maze test
The Morris water maze test was performed at PW8 to measure cognitive function. The facility for the Morris water maze experiment was a circular stainless-steel tank (155 cm in diameter, 60 cm in depth) filled with water to a depth of 40 cm (27.0±1.0℃) that was made opaque by the addition of skim milk. For this task, rats learned to find a hidden circular platform (10 cm in diameter, 1.5 cm below the surface of the water) in a fixed area in one quadrant of the tank.
Spatial acquisition test (invisible test)
In each trial, the rat was placed in the water, facing the wall at one of five designated start points. Animals that failed to find the platform within 90 seconds (s) were guided by an investigator, placed onto the platform, and kept there for 30 s. Escape latency (time to find the platform) as a measure of learning ability was recorded in each trial by using an auto-tracking system (SmarTrack, Smartech, Madison, WI, USA). The times were averaged during five trials per day, for 5 days. Various extra-maze cues (posters, doors, and computers) were maintained throughout the course of experimental data collection.
Reference memory test (probe trial)
Two hours after the last invisible test, a probe test was performed to determine the retention of spatial memory. In this test, the platform was removed from its fixed location (the target quadrant), and the time and number of visits made to the target quadrant during 90 s was recorded. The number of visits to the target quadrant was scored as an index of memory recall of the platform location in the spatial acquisition trials.
Visible test
The visible test was performed the day after the reference memory test. The submerged platform was reinstalled in a different quadrant from the one used in the spatial acquisition test with the addition of an orange flag on the platform (8×8 cm2). The same testing method was used.
Neurohistopathologic analyses
For immunohistochemical analyses, the rats were sacrificed with perfusion of 4% paraformaldehyde at PW9, and their brains were postfixed in the same fixative for 24 hours. The brains were then placed in 30% sucrose for cryo-protection and coronally sectioned (20 µm). In all groups, the hippocampi were cut serially on both the contralateral/ipsilateral sides, and every sixth sections were used. Immunostaining was performed with free-floating brain sections. The following primary antibodies were applied overnight : anti-neuronal specific nuclear protein (NeuN), 1 : 10000 (Calbiochem, La Jolla, CA, USA); anti-glial fibrillary acidic protein (anti-GFAP), 1 : 25000 (Sigma, St. Louis, MO, USA). After several washes with phosphate buffer saline, appropriate secondary antibodies were applied for 30 min. Next, the sections were washed, mounted, and observed with a fluorescence or confocal microscope. Three rats were used for each group, ipsilateral hippocampal areas were analyzed, and three randomly selected regions in CA1 (150×150×20 µm3) were analyzed for cell counting.
Statistical analysis
Statistical analyses were performed by using the Statistical Package for the Social Sciences software, version 11.5 (SPSS Inc., Chicago, IL, USA). The results are expressed as mean±standard error of the mean. Escape latencies and swimming speeds in the maze task were analyzed with Kruskal-Wallis test with the unpaired t-test as a multiple comparison post hoc test when appropriate. A p-value of <0.05 was considered to be statistically significant.
RESULTS
The pFS latency and seizure duration
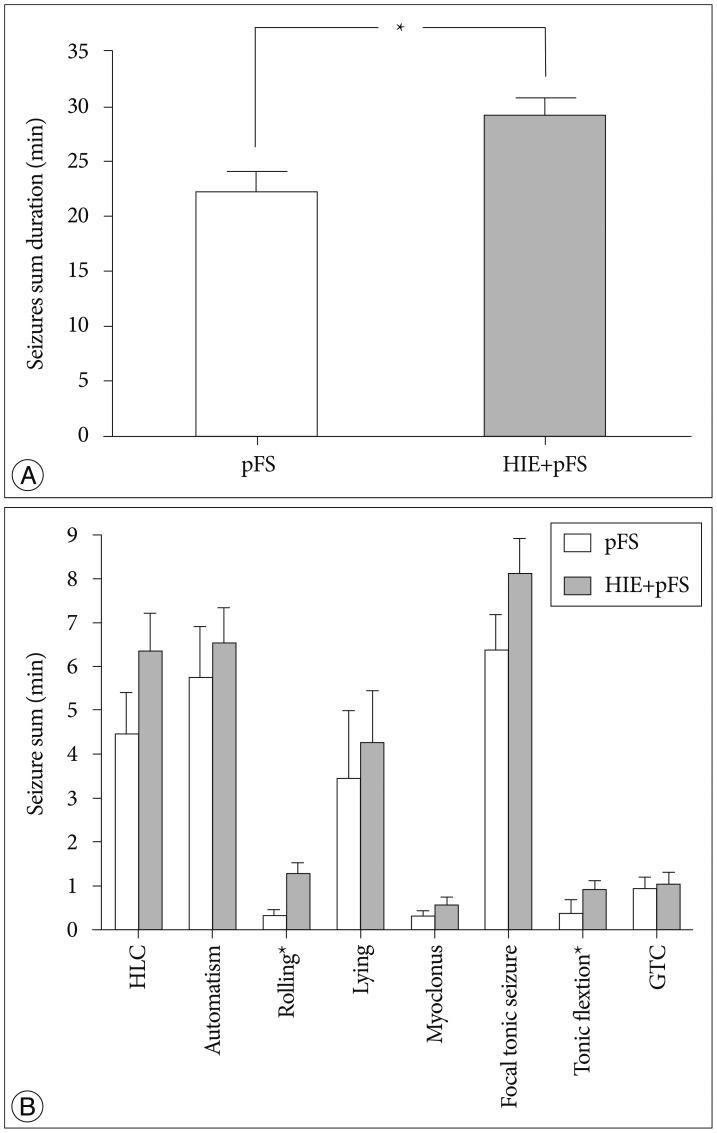
Rats in which hyperthermic seizures had been induced displayed motion arrest and subsequent convulsive seizures that were typically characterized by automatic forelimb movement and smacking, followed by lying on one side or on their backs, rolling, hind-limb clonus, focal tonic limb, generalized tonic flexion, and clonic contractions. The HIE+pFS group developed seizures with a mean latency time of 3.64±0.53 min, and the pFS group developed seizures with a mean latency time of 4.73±0.80 min; there was no statistically significant difference between these two groups. Rectal temperature was not different between the pFS and HIE+pFS groups during the first seizure episode. The HIE+pFS group showed a longer duration of total convulsive seizures (t-test, 29.03±11.46 min in HIE+pFS, 21.94±8.80 min in pFS; p=0.032) (Fig. 1A) and more frequent seizure of all types than the pFS group; however, there was no statistical significance in rolling (Mann-Whitney test, p=0.009) and tonic flexion (Mann-Whitney test, p=0.009) (Fig. 1B). The duration and intensity of hyperthermia applied to the groups and the mean rectal temperature did not differ between the pFS and HIE+pFS groups.
Fig. 1. Duration of seizures. The total seizure duration was significantly longer in the HIE+pFS group (*p<0.05; t-test, p=0.032) (A). In the HIE+ pFS group, all types of seizures occurred more frequently than in the pFS-only group; however, only rolling (Mann-Whitney test, p=0.009) and tonic flexion (Mann-Whitney test, p=0.009) had a statistically significant difference (B). Data are expressed as the mean±standard error of the mean. pFS : prolonged febrile seizures, HIE : hypoxic-ischemic encephalopathy, HLC : hind-limb clonus, GTC : generalized tonic clonic seizures.
Behavioral tests
Open field test
The total traveled distance was not different between the four groups (Fig. 2A). The percentage of time in the central zone out of the total time traveled between the groups, which is an indicator of anxiety-like behavior, was analyzed16). There was no significant difference between the four groups (Fig. 2B).
Fig. 2. Whole traveled distance and central entries in the open field activities test. The whole traveled distance in the open field tests were not different between groups (A) (Kruskal-Wallis test, p>0.005). The entries into the central zone were identical between groups (B) (Kruskal-Wallis test, p>0.005). Cont : control, pFS : prolonged febrile seizures, HIE : hypoxic-ischemic encephalopathy.
Morris water maze test
Spatial acquisition test (invisible test)
All four groups showed the ability to learn to escape with a decreasing latency period in days. No difference in speed and total swimming distance (Kruskal-Wallis test, p=0.68; 690.0 cm in HIE+pFS, 653.9 cm in HIE, 493.4 cm in pFS, and 550.8 cm in the control) was observed on the first day between the four groups during the Morris water maze test. There was no difference in escape latency to the hidden platform between the different groups on the first day of testing; however, a difference was seen at the second day (Fig. 3). The HIE and HIE+pFS groups showed poorer performance than the pFS and control groups on the third (Kruskal-Wallis test, p=0.002), fourth (Kruskal-Wallis test, p<0.001), and fifth (Kruskal-Wallis test, p=0.005) day.
Fig. 3. Morris water maze test (invisible test). On the first 2 days of testing, there was no difference in the escape latency periods between the groups; however, after day 2, there were distinct differences in the escape latency periods to the hidden platform between the different groups. The HIE and HIE+pFS groups showed poorer performances than the pFS and control groups (*p<0.05; Kruskal-Wallis test, third day p=0.002, fourth day p<0.001, fifth day p=0.005). Cont : control, pFS : prolonged febrile seizures, HIE : hypoxic-ischemic encephalopathy.
Reference memory test (probe trial)
In the probe test, there was no difference in swimming time or time remaining in the platform region (Kruskal-Wallis test, p=0.27; 29.42% in HIE+pFS, 38.42% in HIE, 45.91% in pFS, and 47.18% in the control).
Visible test
There was no difference in the escape latency period between the four groups during the visible platform test (Kruskal-Wallis test, p=0.28; 89.1 s in HIE+pFS, 69.2 s in HIE, 55.8 s in pFS, and 58.2 s in the control)
Immunohistochemistry
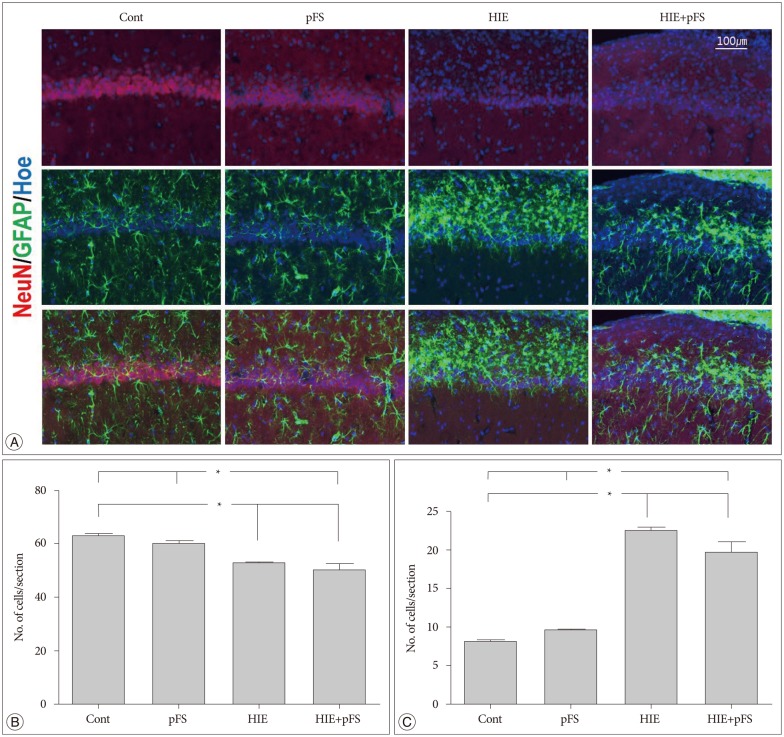
The size of the ipsilateral hippocampus was smaller in the HIE and HIE+pFS groups than in the control group (HIE+pFS vs. Cont, p=0.009; HIE vs. Cont, p=0.044) (Fig. 4). In the HIE and HIE+pFS groups, NeuN-positive cells were sparse in the hippocampus CA1 (150×150×20 µm3) (HIE+pFS vs. Cont, p=0.000; HIE+pFS vs. pFS, p=0.001; HIE vs. Cont, p=0.001; HIE vs. pFS, p=0.018) (Fig. 5A, B). At high magnification (200×), a marked increase in GFAP-positive cells was observed in the CA1 (150×150×20 µm3) of the ipsilateral hippocampus in the HIE and HIE+pFS groups as compared with the control and pFS groups (HIE+pFS vs. Cont, p=0.000; HIE+pFS vs. pFS, p=0.000; HIE vs. Cont, p=0.000; HIE vs. pFS, p=0.000) (Fig. 5A, C).
Fig. 4. Morphology of the ipsilateral hippocampus. Immunofluorescence of the ipsilateral rat hippocampus (20 µm thickness) at PW9. The nucleus is labeled with Hoechst (blue), astrocytes with GFAP (green), and neurons with NeuN (red). The ipsilateral hippocampus had shrunk and the NeuN signals decreased in the HIE and HIE+pFS groups (A). The size of the ipsilateral hippocampus was smaller in the HIE and HIE+pFS groups than in the control group (B) (*p<0.05; Kruskal-Wallis test : Cont vs. HIE, p=0.044; Cont vs. HIE+pFS, p=0.009). Cont : control, pFS : prolonged febrile seizures, HIE : hypoxic-ischemic encephalopathy, PW9 : postnatal week 9, GFAP : glial fibrillary acidic protein, NeuN : neuronal specific nuclear protein.
Fig. 5. Image of the ipsilateral hippocampus CA1. Images were immunostained with the following neuronal markers : NeuN/glial marker, GFAP/nucleus marker, Hoechst (A). The number of NeuN-positive cells decreased in the HIE and HIE+pFS groups compared with the pFS and control groups (B) (Kruskal-Wallis test : Cont vs. HIE, p=0.001; Cont vs. HIE+pFS, p=0.000; pFS vs. HIE, p=0.018; pFS vs. HIE+pFS, p=0.001). The number of GFAP-positive cells was markedly increased in the CA1 of the HIE and HIE+pFS groups (C) (*p<0.05; Kruskal-Wallis test : Cont vs. HIE+pFS, p=0.000; pFS vs. HIE+pFS, p=0.000; Cont vs. HIE, p=0.000; pFS vs. HIE, p=0.000). Cont : control, pFS : prolonged febrile seizures, HIE : hypoxic-ischemic encephalopathy, NeuN : neuronal specific nuclear protein, GFAP : glial fibrillary acidic protein.
DISCUSSION
The main finding of this study is that pFS alone did not increase anxiety or cause major cellular abnormalities, whereas HIE priming produced more frequent febrile seizures and considerable learning impairment. In addition, the HIE and HIE+pFS groups were identified to have neuronal cell loss and gliosis in the hippocampus. In a rodent, hippocampus was shown to play a critical role in learning ability, especially concerning spatial memory26). Also, in humans, the hippocampus is used for spatial memory2). In rodents, the hippocampus can play several additional roles, not only in spatial memory but also in fear conditioning21) and trace conditioning (stimulus selection)3). Entries into the central zone in the open field test are matched to fearless hippocampal dysfunction. Another study showed that FS insults increased anxiety in the early period after seizures in the open field tests10). However, it was transient; there were no lasting effects like in the present study. This is a consistent intellectual prognosis that is mostly normal in patients with pFS without a preceding neurologic abnormality29).
The Morris water maze test is a well-established standard technique for evaluating spatial learning and memory dependent on hippocampal functioning19,30). In the present study, learning and spatial memory impairment was observed after HIE priming at PW8. This shows that HIE in immature brains can produce late spatial memory deficit.
The HIE priming groups showed a reduction in the NeuN signal in the hippocampus. It is well known that NeuN expression is reduced in damaged neurons27). Additionally, astrocyte reactivity, as determined by GFAP staining, was significantly higher in the ipsilateral hippocampus of the HIE priming groups. Unlike in control and pFS groups, the cognitive dysfunction during the Morris water maze tests was correlated with neuronal damage and astrogliosis proved by neurohistopathology in two HIE priming groups. These findings suggest that predisposing damage other than pFS can produce hippocampal damage and be a pathology model of hippocampal sclerosis.
The general understanding is that status epilepticus can cause structural damage to the brain6). Hippocampal volume loss after febrile status epilepticus has been noted in patients with TLE and other forms of epilepsy. However, there are studies presenting opposing results25), and another stating that combined injuries can produce more damage17). Even now, it is unclear whether febrile status epilepticus results in hippocampal volume loss. In a previous study, seizures were shown to directly aggravate the hypoxic state in neonatal rats with HIE8). This study demonstrated a decrease in NeuN immunoreactivity that was insufficient to induce definite volume loss, astrogliosis, and/or neuronal inactivity.
Although the HIE priming groups had a grossly atrophic hippocampal change, there was no hemiparesis or exercise intolerance. The swimming distance and speed were the same between the groups. This means that the HIE insult was relatively mild in clinically common CP experiences. Furthermore, there were no discriminable changes in the pFS group, although the pFS procedure was fatal with a slightly longer time and higher temperature.
In summary, the present study demonstrated that perinatal hypoxic-ischemia can lead to a seizure-prone condition, induce long-term cognitive deficits, result in a decline in hippocampal volume loss, and can cause NeuN hypo-immunoreactivity and astrogliosis, which is consistent with neuronal damage to the hippocampus. The pFS alone has no deficit on behavior and spatial memory function and causes no change in NeuN immunoreactivity, astrogliosis, or volume loss. In conclusion, persons with a predisposing neurologic condition and with hippocampal damage such as HIE are prone to febrile seizures or pFS, and impaired neurocognitive function. The pFS alone has no discriminable effect on the hippocampus. The HIE insult is more of a burden in regard to cognitive deficits and hippocampal damage than pFS insult.
CONCLUSION
Perinatal HIE is one of the most common causes of neonatal brain injury. The first seizure during childhood often occurs with fever. Only 2% of children with febrile convulsion have epilepsy by age. Patients with intractable TLE frequently have a history of febrile seizure in childhood. This study was performed to investigate whether predisposing perinatal HIE, pFS, and the combination of these injuries influence cognitive-behavioral function and hippocampal damage. An HIE rat model at PD7 and a pFS model at PD10 were used to evaluate behavioral and cognitive functions. We observed the results of an open field test, Morris water maze test, and changes in the hippocampus in four groups : control, pFS, HIE, and HIE+pFS. The main finding of this study is that pFS alone did not increase anxiety or cause major cellular abnormalities, whereas HIE priming produced more frequent febrile seizures and significant learning impairment in the Morris water maze test. In addition, the HIE priming groups were identified to have neuronal cell loss and gliosis in the hippocampus. These findings suggest that predisposing damage such as HIE other than pFS produces cognitive dysfunction and hippocampal damage, and can be a pathology model of hippocampal sclerosis.
Acknowledgements
This work was supported by a grant from Korea University College of Medicine.
References
- 1.Ambalavanan N, Carlo WA, Shankaran S, Bann CM, Emrich SL, Higgins RD, et al. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084–2093. doi: 10.1542/peds.2006-1591. [DOI] [PubMed] [Google Scholar]
- 2.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 3.Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus : the importance of contiguity. J Neurosci. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baram TZ, Gerth A, Schultz L. Febrile seizures : an appropriate-aged model suitable for long-term studies. Brain Res Dev Brain Res. 1997;98:265–270. doi: 10.1016/s0165-3806(96)00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender RA, Dubé C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:399–412. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronen RA. The status of status : seizures are bad for your brain's health. AJNR Am J Neuroradiol. 2000;21:1782–1783. [PMC free article] [PubMed] [Google Scholar]
- 7.Dubé C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures : prospective analysis. Brain. 2006;129(Pt 4):911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzhala V, Ben-Ari Y, Khazipov R. Seizures accelerate anoxia-induced neuronal death in the neonatal rat hippocampus. Ann Neurol. 2000;48:632–640. [PubMed] [Google Scholar]
- 9.Gottlieb A, Keydar I, Epstein HT. Rodent brain growth stages : an analytical review. Biol Neonate. 1977;32:166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- 10.Kwon SK, Kovesdi E, Gyorgy AB, Wingo D, Kamnaksh A, Walker J, et al. Stress and traumatic brain injury : a behavioral, proteomics, and histological study. Front Neurol. 2011;2:12. doi: 10.3389/fneur.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwong KL, Wong SN, So KT. Epilepsy in children with cerebral palsy. Pediatr Neurol. 1998;19:31–36. doi: 10.1016/s0887-8994(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 12.Lado FA, Sankar R, Lowenstein D, Moshé SL. Age-dependent consequences of seizures : relationship to seizure frequency, brain damage, and circuitry reorganization. Ment Retard Dev Disabil Res Rev. 2000;6:242–252. doi: 10.1002/1098-2779(2000)6:4<242::AID-MRDD3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Lafemina MJ, Sheldon RA, Ferriero DM. Acute hypoxia-ischemia results in hydrogen peroxide accumulation in neonatal but not adult mouse brain. Pediatr Res. 2006;59:680–683. doi: 10.1203/01.pdr.0000214891.35363.6a. [DOI] [PubMed] [Google Scholar]
- 14.Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis DV. Losing neurons : selective vulnerability and mesial temporal sclerosis. Epilepsia. 2005;46(Suppl 7):39–44. doi: 10.1111/j.1528-1167.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 16.Lipkind D, Sakov A, Kafkafi N, Elmer GI, Benjamini Y, Golani I. New replicable anxiety-related measures of wall vs center behavior of mice in the open field. J Appl Physiol (1985) 2004;97:347–359. doi: 10.1152/japplphysiol.00148.2004. [DOI] [PubMed] [Google Scholar]
- 17.Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995;118(Pt 1):105–118. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- 18.McLean C, Ferriero D. Mechanisms of hypoxic-ischemic injury in the term infant. Semin Perinatol. 2004;28:425–432. doi: 10.1053/j.semperi.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Provenzale JM, Barboriak DP, VanLandingham K, MacFall J, Delong D, Lewis DV. Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis. AJR Am J Roentgenol. 2008;190:976–983. doi: 10.2214/AJR.07.2407. [DOI] [PubMed] [Google Scholar]
- 21.Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci. 2010;21:1–17. doi: 10.1515/revneuro.2010.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 23.Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Hippocampal abnormalities after prolonged febrile convulsion : a longitudinal MRI study. Brain. 2003;126(Pt 11):2551–2557. doi: 10.1093/brain/awg262. [DOI] [PubMed] [Google Scholar]
- 24.Sloviter RS, Kudrimoti HS, Laxer KD, Barbaro NM, Chan S, Hirsch LJ, et al. "Tectonic" hippocampal malformations in patients with temporal lobe epilepsy. Epilepsy Res. 2004;59:123–153. doi: 10.1016/j.eplepsyres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Thom M, Zhou J, Martinian L, Sisodiya S. Quantitative post-mortem study of the hippocampus in chronic epilepsy : seizures do not inevitably cause neuronal loss. Brain. 2005;128(Pt 6):1344–1357. doi: 10.1093/brain/awh475. [DOI] [PubMed] [Google Scholar]
- 26.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 27.Unal-Cevik I, Kilinç M, Gürsoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss : a cautionary note. Brain Res. 2004;1015:169–174. doi: 10.1016/j.brainres.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 28.Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, et al. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55:158–163. doi: 10.1002/(SICI)1097-4547(19990115)55:2<158::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Verity CM, Ross EM, Golding J. Outcome of childhood status epilepticus and lengthy febrile convulsions : findings of national cohort study. BMJ. 1993;307:225–228. doi: 10.1136/bmj.307.6898.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vorhees CV, Williams MT. Morris water maze : procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]