Abstract
Air pollution has been classified as Group 1 carcinogenic to humans, but the underlying tumorigenesis remains unclear. In Xuanwei City of Yunnan Province, the lung cancer incidence is among the highest in China attributed to severe air pollution generated by combustion of smoky coal, providing a unique opportunity to dissect lung carcinogenesis of air pollution. Here we analyzed the somatic mutations of 164 non-small cell lung cancers (NSCLCs) from Xuanwei and control regions (CR) where smoky coal was not used. Whole genome sequencing revealed a mean of 289 somatic exonic mutations per tumor and the frequent C:G → A:T nucleotide substitutions in Xuanwei NSCLCs. Exome sequencing of 2010 genes showed that Xuanwei and CR NSCLCs had a mean of 68 and 22 mutated genes per tumor, respectively (p < 0.0001). We found 167 genes (including TP53, RYR2, KRAS, CACNA1E) which had significantly higher mutation frequencies in Xuanwei than CR patients, and mutations in most genes in Xuanwei NSCLCs differed from those in CR cases. The mutation rates of 70 genes (e.g., RYR2, MYH3, GPR144, CACNA1E) were associated with patients' lifetime benzo(a)pyrene exposure. This study uncovers the mutation spectrum of air pollution-related lung cancers, and provides evidence for pollution exposure–genomic mutation relationship at a large scale.
Keywords: Air pollution, Lung cancer, Whole genome sequencing, Exome sequencing, Exposure
Highlights
-
•
Somatic genomic mutations of air pollution-related lung cancer were characterized.
-
•
Mutations in air pollution-related lung cancers were 3 times as high as lung cancers from control regions.
-
•
167 of the 2010 genes had higher mutation rates in patients from highly polluted region than those from control regions.
-
•
The mutation rates of 70 genes were associated with patients' lifetime benzo(a)pyrene exposure.
1. Introduction
Air pollution is a significant environmental risk factor for lung cancer. For every increase of 5 μg/m3 of particulate matter (PM) smaller than 2.5 μm in diameter (PM2.5) in the environment, the risk of lung cancer rises by 18%; for every elevation of 10 μg/m3 in PM smaller than 10 μm (PM10), the risk increases by 22% (Raaschou-Nielsen et al., 2013). Anthropogenic PM2.5 is associated with 220,000 lung cancer mortalities annually (Anenberg et al., 2010). Based on sufficient evidence of carcinogenicity, the International Agency for Research on Cancer (IARC) Working Group recently classified outdoor air pollution and related PM as Group 1 carcinogenic to humans (Loomis et al., 2013). However, the carcinogenic mechanism of air pollution remains to be dissected using systematic approaches.
Xuanwei (XW) City in Yunnan Province of China (Fig. S1), provides an example of the epidemiological association between PM10, PM2.5 and lung cancer (Xiao et al., 2012, Cao and Gao, 2012, Mumford et al., 1987). This city and the neighboring Fuyuan (FY) county have a large deposit of smoky coal (Mumford et al., 1987). Until the 1970s, residents of these regions used smoky coal in unvented indoor fire pits for domestic cooking and heating, all processes that release high concentrations of PM10 and PM2.5. These airborne particles contain high concentrations of polycyclic aromatic hydrocarbons (PAHs) including benzo(a)pyrene (BaP) and polar compounds that are highly mutagenic (Mumford et al., 1987). Lung cancer incidence in XW is among the highest in China (Mumford et al., 1987, Xiao et al., 2012), and a reduction in lung cancer morbidity was noted in the 1990s after stove improvement in central XW, supporting the association between air pollution and lung cancer (Lan et al., 2002). The findings in XW had been cited in the IARC monograph classifying indoor emissions from household combustion of coal as “carcinogenic to humans (Group 1)” (World Health Organization International Agency for Research on Cancer, 2010). However, the overall lung cancer incidence in this region has been increasing (Xiao et al., 2012, Chen, 2008), possibly due to pollutants generated by coal-burning industrial plants that moved into the area (Cao and Gao, 2012). In 2011, a survey (Li et al., 2011) of 52,833 residents living in 382 rural villages in XW/FY reported 363 subjects diagnosed with lung cancer, with the world age-standardized rate (ASR) of 426/100,000 in some regions of XW. Population in these highly polluted regions (HPR) lends a unique opportunity to dissect the carcinogenesis that is specifically related to air pollution, and we took this opportunity by sequencing the whole genomes of lung cancers from these regions to provide a comprehensive landscape of genomic alterations in this study.
2. Materials and Methods
2.1. Study Design
We sequenced the whole genomes of 14 non-small-cell lung cancers (NSCLCs) from HPR, and performed targeted exome sequencing of 2010 genes in additional 150 primary NSCLCs from HPR and control regions (CR) in the rest of Yunnan and Guangdong Province where the level of air pollution and lung cancer incidence was comparable to most parts of China (van et al., 2010, Chen, 2008). The mutation patterns of HPR and CR NSCLCs were compared, and the exposure–response relationship was analyzed (Fig. S1).
2.2. Patients
The study was approved by the Local Research Ethics Committees of all participating sites. Tumor and adjacent normal lung tissues and peripheral blood samples were obtained from 164 patients with previously untreated NSCLCs (Tables 1, S1, Figs. 1 and S2). The diagnosis and TNM stage were established as previously described (Brambilla et al., 2001, Goldstraw et al., 2007). The patients resided in their communities and rarely (or never) stayed in other regions for a long time, and had regular life routines and regular daily time-spent indoors and outdoors. The exposure dosages of the patients to BaP were estimated by historical measurements in various regions of China that used different fuels and their smoking histories (Sinton et al., 1995, Sullvivan and Krieger, 2014). The most recent 10 years were excluded to allow for a hypothesized 10-year latency period between exposure and clinical recognition of lung cancer. Whole-genome sequencing (WGS) was performed in 14 HPR patients (Table S1 and Figures S), and exome sequencing was conducted in additional 65 HPR and 85 CR patients (Table S1).
Table 1.
The demographic characteristics of the 164 NSCLC patients from HPR or CR.
| Characteristics | Total (n = 164) | HPR (n = 79) | CR (n = 85) |
|---|---|---|---|
| Gender | |||
| Male | 101 | 42 | 59 |
| Female | 61 | 37 | 24 |
| n.d. | 2 | 0 | 2 |
| Age | |||
| < 65 | 122 | 61 | 61 |
| ≥ 65 | 40 | 18 | 22 |
| n.d. | 2 | 0 | 2 |
| Median, range | 56 [34, 78] | 57 [36, 76] | 59 [34, 78] |
| Residence | |||
| Xuanwei/Fuyuan | 79 | 79 | 0 |
| Rest of Yunnan | 24 | 0 | 24 |
| Guangdong | 61 | 0 | 61 |
| Smoking | |||
| Smoker | 81 | 38 | 43 |
| Non-smoker | 81 | 41 | 40 |
| n.d. | 2 | 0 | 2 |
| Histology | |||
| Adenocarcinoma | 112 | 64 | 48 |
| Squamous-cell carcinoma | 46 | 14 | 32 |
| Adenosquamous carcinoma | 0 | 0 | 0 |
| Large-cell carcinoma | 6 | 0 | 0 |
| TNM stage | |||
| IA | 18 | 12 | 6 |
| IB | 47 | 25 | 22 |
| IIA | 13 | 2 | 11 |
| IIB | 22 | 9 | 13 |
| IIIA | 28 | 13 | 15 |
| IIIB | 15 | 9 | 6 |
| IV | 16 | 9 | 7 |
| n.d. | 5 | 0 | 5 |
n.d.: not determined.
Fig. 1.
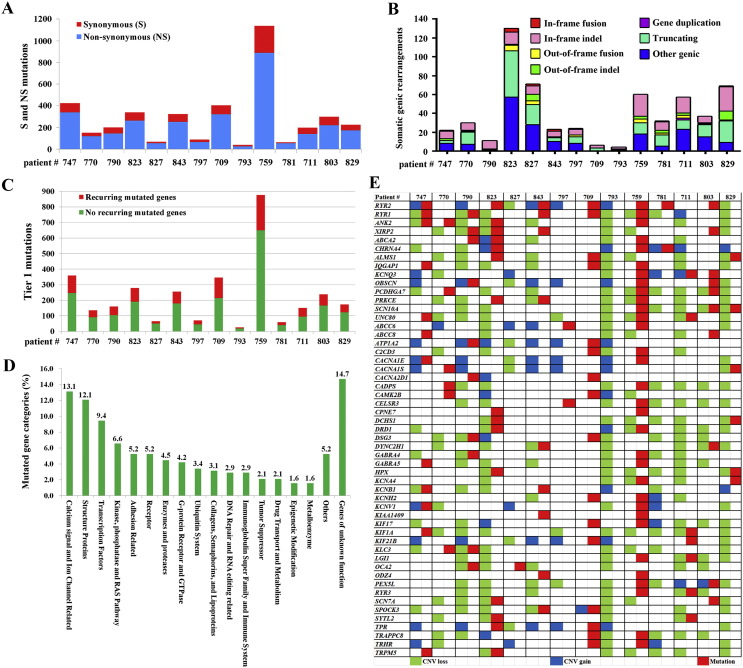
Mutation landscape of lung cancer from HPR. (A): A stacked bar graph representing the total number of non-synonymous versus synonymous mutations in each patient. (B): Summary of somatic genic rearrangements in each patient. “Other Genic” indicates rearrangements linking an intergenic region to the 3′ portions of a genic footprint. (C): Total numbers of recurring and non-recurring mutations in each patient. (D): The 381 recurring mutated genes (with dN/dS > 2), classified into 18 categories. (E): Mutations and copy number variations in calcium signal and ion channel genes.
2.3. Analytic Platforms
DNAs and RNAs were isolated from cancer or counterpart normal tissues, sequencing libraries were constructed, and sequenced using the Illumina Hiseq2000 platform. A SNP array using Illumina High Density Genome Wide Human 660WQuad_v1 was performed to detect somatic copy number alterations (SCNAs) throughout the genomes. Mutations of 2010 genes identified by WGS were screened in 150 additional NSCLCs by exome captured and sequencing. The protocols are detailed in eMethods.
3. Results
3.1. Somatic Mutation Profile in HPR NSCLCs
For whole genome sequencing (WGS), cancer DNAs were sequenced to an average of 65.74 × (range, 61.02 ×–74.64 ×) and normal controls of 43.16 × (30.07 ×–78.70 ×) coverage. Point mutations, indels, somatic structural variations, and somatic copy number alterations (SCNAs) were found throughout the 14 NSCLC genomes (Fig. S3, Fig. 1 and Table S1, Table S2, Table S3). We reported a mean of 12.75 somatic genomic mutations/Mb, 8.16 exonic mutations/Mb, and 289 exonic mutations/tumor (Table S1). Only an average of 0.74% mutations was found in coding sequences (CDS) (Fig. S4A, B). Using capillary sequencing, 331/361 (91.7%) mutations in CDS were validated. Smoker, non-smokers, squamous cell carcinoma (SCC) and adenocarcinoma (AD) patients had approximately equal mutations in their genomes and CDS (Fig. S4C). The numbers of non-synonymous mutations, synonymous mutations (Fig. 1A), chromosomal rearrangements (Table S2) and somatic gene rearrangements (Fig. 1B) were not associated with smoking status or age. Within predicted promoter regions, there is a positive correlation between GC content and somatic mutation rate (Fig. S4D).
We analyzed the CDS mutations and reported a mean of 158 non-recurring and 31 recurring (defined as mutated in at least 2 samples) mutated genes per tumor (Fig. 1C and Table S4). Three genes, MUC16, RYR2 and TP53, were mutated in 7 (50%) of the 14 patients. CSMD3, RYR1, TTN and ZNF831 were mutated in 5 (35.7%) of the patients. There were 18 (including XIRP2 and ANK2), 65 (including EGFR and KRAS), 338 (including CACNA1E, CACNA1S, CACNA2D1 and RYR3) and 2209 genes mutated in 4 (28.57%), 3 (21.43%), 2 (14.29%), and 1 (7.14%) of the 14 patients, respectively (Table S4). Among the 428 recurrently mutated genes, 381 ones had a ratio of non-synonymous to synonymous mutations (dN/dS) > 2. These genes fall into 18 categories (Table S4 and Fig. 1D), with calcium signaling and ion channel-related genes (Fig. 1E), structural proteins, and transcription factors as the three most frequently mutated gene categories.
3.2. Somatic Copy Number Alterations (SCNAs)
A total of 479 SCNA segments and a mean of 34 copy number variations (CNVs) per tumor were detected (Table S3 and Fig. S5A). We identified 5 regions of significant CNVs: copy loss in 13q12.3-q34 (containing 13 genes including BRCA2, ERCC5, and RB1), 4p16.1-p13 (containing 6 genes), 4q22.1-q35.2 (containing 18 genes including CASP3, EGF, and FGF2), and 3p24.3-12.2 (containing 17 genes including TGFBR2 and SETD2); copy gain in 1q21.1-q44 (containing 35 genes including ABL2, IL6R, and MCL1) (Table S3).
Some genes including CYP1A1, CYP1B1, CAT, and ERCC1, affect PAH metabolism, detoxification, PAH-DNA adduct formation and repair (Irigaray and Belpomme, 2010). We assessed 23 genes involved in these processes, and reported that 20 ones had copy loss in 12/14 patients, and 1 gene (EPHX1) had copy gain in 4/14 patients at the DNA level (Fig. S5B). By quantitative real-time RT-PCR analysis of 17 of these genes, we found that 13 genes were down-regulated in at least 7 patients, with down-regulated CYP3A4 seen in 14 patients, decreased CAT, CYP1A1, and NAT in 13 patients, and down-regulated GSTM1 in 12 cases (Fig. S5C). CNV was also frequently seen in DNA repair genes in the patients, with a mean of 114 copy loss and 17 copy gain genes per tumor (Table S3).
3.3. Genomic Rearrangements
We used the CREST method (Wang et al., 2011) to detect and map the breakpoints of the somatic rearrangements among the 14 HPR patients. Totally, 992 chromosomal rearrangements including 573 (57.8%) gene rearrangements and 419 (42.2%) purely intergenic events were identified, with a mean of 71 genomic rearrangements per tumor (Table S2 and Fig. 1B). We identified six previously unreported interchromosomal in-frame fusion transcripts: ARHGEF10-IMMP2L, COL13A1-DLD, PCDH15-SOX5, CACNA1B-FAF1, MCF2L2-PHF3, and ABCC8-C3orf55 in 5 patients (Fig. S6, A-F), and five previously unreported intrachromosomal in-frame-fusion transcripts (PLCB1-CRLS1, DOCK2-TENM2, SOX5-ST8SIA1, TADA2B-TBC1D19 and NINJ2-NTF3) in four patients (Fig. S7, A-F). Sanger sequencing of PCR products using genomic DNA of the samples was conducted, and 99/108 (91.7%) tested structural variations were validated.
3.4. Genomic Signatures
In 12/14 (85.7%) HPR patients, the C:G → A:T transversions were the most frequent nucleotide substitutions (Fig. S8A). The percentage of transversions ranged from 17.7% to 58.8% (Table S5; p = 0.008). In males, the percentage of A:T → G:C mutations were much higher than in females (Table S5; p = 0.008); in 2 of the 6 males, the most prevalent mutation was A:T → T:A (Fig. S8A). The most frequently observed dinucleotide change was GG → TT/CC → AA (p < 0.001) (Fig. S8B). Among the trinucleotide alterations, XpCpG → XpApG was detected in 13/14 patients. CpCpX → CpApX and XpCpG → XpTpG were also detected (Fig. S8C).
3.5. Mutations in Calcium Signaling Genes
Gene Ontology Analysis showed that motor activity, calcium ion binding, extracellular matrix structural constituent, ion binding, cation binding, and metal ion binding activity were affected (Table S6). Pathway analysis using single-nucleotide variation and indel data revealed that the differentially altered genes were significantly enriched in 46 KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways, including pathways involved in focal adhesion, calcium signaling and pathogenesis of NSCLC (p < 0.0001) (Table S6). Indeed, some of the most frequently mutated genes (Table S4) were related to calcium signaling, e.g., RYR1-3, which encode RYRs calcium release channels located on the membrane of the endoplasmic reticulum (Lanner et al., 2010); ANK2, which is required for cardiac sinoatrial node Ca2 + homeostasis; XIRP2, which is a target of the calcium-dependent transcription factor MEF2A; CACNA1E (or Cav2.3) (Soong et al., 1993), CACNA1S and CACNA2D1, which encode plasma membrane calcium channel subunits (Pallone et al., 2012).
3.6. HPR NSCLCs Have Much More Mutations Than CR Patients
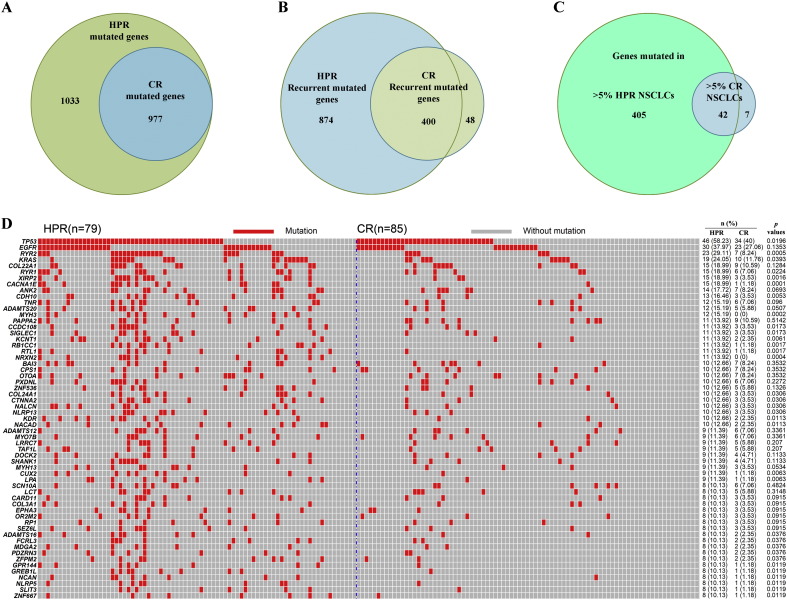
To unveil the difference in somatic mutations between HPR and CR NSCLCs (Table 1), exons of 2010 of the 2637 (76.2%) mutated genes found in WGS were captured and sequenced (Table S7), and the results were confirmed by sequencing of PCR products using according primers (Table S8). Cancer DNAs were sequenced to an average of 140 × (54.8 ×–261 ×) and normal controls of 145.2 × (46.6 ×–218.5 ×) coverage. We found that the 79 HPR and 85 CR NSCLCs had a mean of 68 and 22 mutated genes per tumor (Table 2), respectively (p < 0.0001). The HPR NSCLCs had much more mutations, e.g., the total number of mutated genes (Fig. 2A), recurrent mutated genes (Fig. 2B), and genes mutated in more than 3%–10% tumor samples (Fig. 2C, Table 2), than the CR patients. In HPR, the number of genes mutated in > 3% samples was 785, more than 3 times higher than that in CR (213 genes; Table 2). There were 59 genes which were mutated in > 10% tumor samples in HPR NSCLCs, but only 5 genes (TP53, EGFR, KRAS, COL22A1, PAPPA2) were mutated in > 10% tumor samples in CR (Fig. 2D). Among the 1529 recurrent mutated genes (Table S7), 167 genes (including TP53, RYR2, KRAS, CACNA1E, XIRP2) had statistically significantly higher mutation rates in HPR than CR patients, but no gene had significantly higher mutation rate in CR than HPR patients. One gene, TMEM132C, was mutated in 7.59% HPR and 14.12% CR samples, respectively (p = 0.182; Fig. 3C).
Table 2.
Comparison of mutations in the 2010 genes in HPR and CR NSCLCs.
| HPR (n = 79) |
CR (n = 85)⁎ |
p (HPR vs CR) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | S (n = 38) | NS (n = 41) | p | Total | S (n = 43) | NS (n = 40) | p | ||
| Mutation/Mb (mean) | 15.90 | 18.31 | 13.66 | 0.818 | 6.34 | 7.98 | 4.49 | 0.0297 | 3.611E-06 |
| G:C → T:A substitution | 54.79% | 54.03% | 55.73% | 0.114 | 41.92% | 45.26% | 38.08% | 3.421E-05 | 1.711E-39 |
| Mutated genes/tumor | 67.99 | 74.87 | 61.61 | 0.930 | 22.06 | 28.16 | 15.58 | 0.017 | 9.966E-07 |
| Non-silent mutations/tumor | 72.66 | 81.24 | 64.71 | 0.895 | 23.13 | 29.67 | 16.22 | 0.015 | 8.796E-07 |
| Genes mutated in > 3% samples | 785 | 703 | 617 | 0.062 | 213 | 273 | 97 | 6.339E-10 | 9.052E-21 |
S, smoker; NS, non-smoker.
The smoking status of two CR patients was unknown.
Fig. 2.
Comparison of mutations in HPR lung cancer with those in CR NSCLCs. (A): HPR NSCLCs bore more mutated genes than CR patients. (B): Comparison of recurrent mutated genes in HPR with those in CR NSCLCs. (C): Genes mutated in > 5% tumor samples from HPR and CR. (D): Mutations in 59 genes whose mutation frequencies are > 10% of HPR NSCLCs. Tumors are arranged from left to right in the top track.
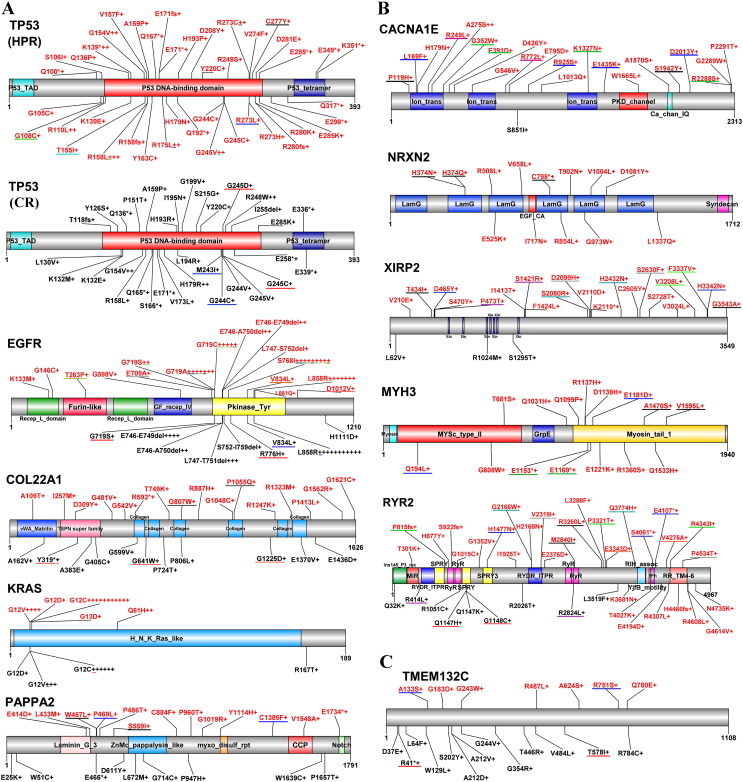
Fig. 3.
Mutations in some representative genes. Schematic representations of proteins encoded by the genes are shown. Numbers refer to amino acid residues. Mutations found in HPR and CR patients are shown in red and black, respectively. Each “+” corresponds to an independent, mutated tumor sample, and “*” indicates a nonsense (truncating) mutation. Mutations underlined with a same-colored line are found in the same patient. (A): The five genes which were mutated in > 10% tumor samples in both regions. (B): Representative five genes whose mutation rates in HPR lung cancer were significantly higher than in CR NSCLCs. (C): Mutations in TMEM132C which were mutated in 6/79 (7.59%) HPR NSCLCs and 12/85 (14.12%) CR lung cancers (p = 0.182).
3.7. Comparison of Somatic Mutations in HPR and CR NSCLCs
We compared the mutation patterns in HPR and CR NSCLCs. Among the total 80 mutations (46 mutations in HPR and 34 ones in CR NSCLCs) of TP53, 9 common mutations (G154V, R158L, A159P, E171*, Y220C, G244C, G245C, G245V, and E285K) were seen in both regions (Fig. 3A). However, most of the TP53 mutations in HPR were different from those in CR cases. HPR NSCLCs had equal EGFR mutation rate to CR cases (p = 0.135). However, CR non-smokers had higher EGFR mutation rate (40%) than smokers (16.3%; p = 0.0158), while in HPR non-smokers (43.9%) had equal mutation rate to smokers (31.6%; p = 0.26). In HPR, 14/43 (32.6%) mutations occurred in G719, while in CR only 1/25 (4%) alterations was observed in this amino acid; 9 (20.9%) mutations occurred in S768 in HPR cases, but no mutation was seen in this site in CR NSCLCs (Fig. 3A). Mutations in RYR2, COL22A1, PAPPA2 and TMEM132C in HPR NSCLCs were distinct to those found in CR patients (Fig. 3). Interestingly, 16/19 (84.2%) KRAS mutations in HPR NSCLCs and 10/11 (90.9%) KRAS mutations in CR patients were found in G12 (Fig. 3A), suggesting that NSCLCs of the two regions also had a few similar points in mutation patterns.
3.8. Association Between BaP Exposure and Gene Mutation
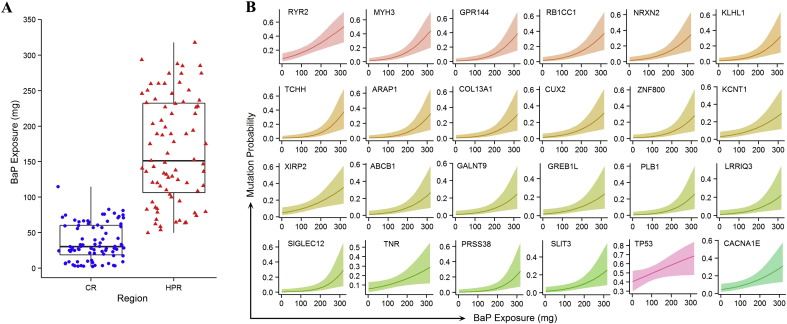
Lifetime exposure to BaP was calculated by applying air concentration (ng/m3) reported for various regions of China that used different fuels for cooking and heating (Sinton et al., 1995), an average inhalation rate of 20 m3/day, and the duration of exposure. Smoking a pack of filtered cigarettes per day was assigned a BaP exposure of 0.4 μg/day (Sullvivan and Krieger, 2014). The median value of BaP exposure in the HPR was 151.0 mg, five times as high as in the CR (30.1 mg) (Fig. 4A). By logistic regression, we found that the mutation frequencies of 70 genes (including RYR2, MYH3, GPR144, RBCC1, NRXN2, KLHL1, TCHH, ARAP1, COL13A1, CUX2, ZNF800, KCNT1, XIRP2, CACNA1E and TP53) were associated with BaP exposure (p < 0.05; Table S9, Fig. 4B and Fig. S9).
Fig. 4.
Association between mutations and exposure to BaP. (A): Estimated doses of the patients' exposure to BaP. Sources of combustion of smoky coal and cigarette smoke were included. (B): Exposure–response relationship between BaP exposure and the mutation probability of 24 representative genes. See also Figure S9.
4. Discussion
In this study, we examined the cancer genomes of HPR NSCLCs to identify the genomic mutation profile associated with prolonged exposure to smoky coal pollutants. Of the 14 WGS patients, there was a mean of 12.75 somatic genomic mutations/Mb, 8.16 exonic mutations/Mb, and 289 exonic mutations/tumor. Among the 2010 genes sequenced by targeted exome sequencing, the HPR patients had 68 mutated genes/tumor, 3 times higher than that in CR cases. Previous studies demonstrated that smoker NSCLCs bear more somatic mutations than never-smokers (Imielinski et al., 2012, Govindan et al., 2012). However, in HPR, the smokers and non-smokers harbored equal numbers of mutations and gene rearrangements in their genome. In CR, stages III–IV cancers had more mutations in 6 genes (KRAS, MYH13, TNR, ADAMTS20, PXDNL and SEZ6L) than stages I–II tumors, while patients ≥ 65 years harbored more mutations in 4 genes (RYR2, COL22A1, ADAMTS12 and ZFPM2) than patients < 65 years; in HPR, only ACVR2A had more mutations in stages III–IV cancers than stages I–II tumors, and ADCY7 had a higher mutation rate in patients ≥ 65 years than those < 65 years (Table S7 sheet 4). These results demonstrate the genotoxic effect of air pollution and the urgent need to attenuate pollution.
PAHs are important carcinogens in PM2.5 and PM10 (Zielinska et al., 2010, Mumford et al., 1987). A variety of enzymes metabolize PAHs to more polar and water-soluble metabolites to be excreted from the body. However, during the course of metabolism, some unstable and reactive intermediates are formed, which can bind to DNA to form bulky DNA adducts (Hecht, 2012, DeMarini et al., 2001). At the same time, the cells constantly deal with the formation of DNA adducts by DNA repair processes to eliminate these alterations so that mutation does not occur (Irigaray and Belpomme, 2010). We showed that in the WGS NSCLCs, genes responsible for PAH detoxification (GSTM1, GSTP1, GSTT1) were mainly copy loss (Fig. S5B) or down-regulated (Fig. S5C), while genes involved in PAH activation (CYP1B1 in particular; Fig. S5C) were mainly up-regulated. DNA repair genes were mainly copy loss or mutated (Table S3). Mutations in DNA repair pathways have also been implicated in the production of chromosomal translocations (Aplan, 2006). Therefore, the events in PAH metabolism and DNA repair genes may pave the way to genomic mutations and chromosomal translocations, and may represent an essential step to allow accumulation of significant mutations to initiate malignant transformation.
PAHs are associated with the C:G → A:T transversions in nucleotides (Ruggeri et al., 1993, Eisenstadt et al., 1982), and recent studies in cell lines showed that BaP can induce this type of nucleotide substitutions (Olivier et al., 2014). We found that the C:G → A:T substitutions were the most frequent nucleotide substitutions in 12/14 patients (Fig. S8), and exome sequencing confirmed the prevalent of C:G → A:T transversions in HPR NSCLCs (Table 2), indicating that PAHs were the main carcinogens for these patients. However, in 2/14 cases the most frequent nucleotide changes were A:T → T:A transitions (Fig. S8), suggesting that there might be other pollutants that caused this signature in the genomes.
Some genes, e.g., TP53, EGFR, and KRAS, have high frequency of mutations in lung cancer (The Cancer Genome Atlas Research Network, 2012, Imielinski et al., 2012), and ethnic and sex-related differences in mutation spectrum are noted (Dearden et al., 2013, Kosaka et al., 2004). We showed that the mutation pattern of TP53, EGFR, and KRAS in CR NSCLCs (Figs. 2D and 3) was in consistence with previous report in Asian patients (Dearden et al., 2013), and HPR patients also had high mutation rates in these genes (Fig. 2D). Some genes, e.g., COL22A1, PAPPA2, TNR, TMEM132C, ADAMTS20, BAI3, CPS1, and OTOA, had high mutation frequencies in both regions (Table S7). Mutations in most genes, e.g., TP53, COL22A1, PAPP2A, CACNA1E, MYH3, NRXN2, RYR2, XIRP2, and TMEM132C, distributed throughout the entire genes were either missense or nonsense in nature; on the contrary, some genes, e.g., EGFR and KRAS, had mutation hot spots (Fig. 3). The results indicated that although NSCLCs from HPR and CR had distinct mutation patterns in many genes, they did show some similar points in some somatic mutations.
Alterations in Ca2 + TRP, ORAI1 and RYR channels have been identified in cancer (Monteith et al., 2012, Ho et al., 2013, Love et al., 2012), and overexpression of CACNA1E are correlated with relapse in Wilms' tumors (Natrajan et al., 2006), while CACNA2D1 plays a role in maintaining the properties of tumor-initiating cells in hepatocellular carcinoma (Zhao et al., 2013). Interestingly, Olivier et al. (2014) found that treatment of cells with BaP for 6 days leads to mutations in CACNA1C and CACNA1G. We showed that in the 79 HPR NSCLCs, calcium signaling-related genes RYR2, RYR1, XIRP2, CACNA1E and ANK2 had high frequency mutations (29.1%–17.7%), compared to 1.2%–8.2% mutation rates in CR patients. In HPR NSCLCs, RYR1 and RYR2 had mutations of loss of function patterns, because the mutations were distributed throughout the entire genes and were either missense or nonsense in nature. CACNA1B-FAF1 fusion (Fig. S6D) could also damage CACNA1B's Ca2 + channel function, because its C-terminal ion transmission and calcium channel domains were deleted. The 23 CACNA1E mutations in 15/79 (19%) HPR lung cancers were missense mutations and distributed throughout the entire gene. Among them, 10/23 (43.5%) mutations were found in the amino acid 119–546 region, and 10/15 (67%) patients had one mutation in ion transmission, PKD channel or calcium channel domains (Fig. 3B). These mutations may interfere with the function of the calcium channel and the intracellular Ca2 + concentration, the essential second messenger that can regulate nearly every aspect of cellular functions. Further investigation should be conducted to characterize the “driver mutation” aspects of these genes.
The following are the supplementary data related to this article.
Supplementary materials.
Clinical characteristics of tumors used in whole genome sequencing and summary of sequencing results of each tumor DNA.
Chromosomal rearrangements in the whole genome sequencing patients.
Somatic copy number alterations in the patients.
The mutated genes found in whole genome sequencing.
Mutations in 2010 genes in 164 non-small cell lung cancers.
Author Contributions
Study concept and design: G.B.Z. Biospecimens provided by: Y.C.H., Z.S.W., G.F.L., H.B.C., Z.P.H., and P.W. Experiments conducted by: X.J.Y., L.C.W., G.Z.W., B.Z., X.C., L.M., Y.D.Z., X.L.W., W.J.M., C.M.P., H.K., S.T.C., Y.Q.Z., W.Y.G., H.D. Data analysis: S.Y.W., J.Y.H., Z.H.G., M.J.Y., L.C.W., G.B.Z., Y.C., S.J.C. Assessment of exposure and response: L.W.T. Drafting of the manuscript: G.B.Z.
Competing Interests
The authors have declared that no competing interests exist.
Acknowledgment
This project has been funded by the National Natural Science Funds for Distinguished Young Scholar to G.B.Z. (81425025), National Key Program for Basic Research (2012CB910800), National Natural Science Foundation of China (81171925, 81201537), and grant from the Shanghai Municipal Science and Technology Commission (13431902000). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Sai-Juan Chen, Email: sjchen@stn.sh.cn.
Yi Cao, Email: caoy@mail.kiz.ac.cn.
Sheng-Yue Wang, Email: wangsychgc@gmail.com.
Guang-Biao Zhou, Email: gbzhou@ioz.ac.cn.
References
- Anenberg S.C., Horowitz L.W., Tong D.Q., West J.J. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environ. Health Perspect. 2010;118:1189–1195. doi: 10.1289/ehp.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplan P.D. Causes of oncogenic chromosomal translocation. Trends Genet. 2006;22:46–55. doi: 10.1016/j.tig.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla E., Travis W.D., Colby T.V., Corrin B., Shimosato Y. The new World Health Organization classification of lung tumours. Eur. Respir. J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- Cao Y., Gao H. Prevalence and causes of air pollution and lung cancer in Xuanwei City and Fuyuan County, Yunnan Province, China. Front. Med. 2012;6:217–220. doi: 10.1007/s11684-012-0192-8. [DOI] [PubMed] [Google Scholar]
- Chen Z. Peking Union Medical College Press; Beijing: 2008. The Third Chinese National Retrospective Surveys for the Causes of Death. [Google Scholar]
- Dearden S., Stevens J., Wu Y.L., Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap) Ann. Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarini D.M., Landi S., Tian D., Hanley N.M., Li X., Hu F., Roop B.C., Mass M.J., Keohavong P., Gao W., Olivier M., Hainaut P., Mumford J.L. Lung tumor KRAS and TP53 mutations in nonsmokers reflect exposure to PAH-rich coal combustion emissions. Cancer Res. 2001;61:6679–6681. [PubMed] [Google Scholar]
- Eisenstadt E., Warren A.J., Porter J., Atkins D., Miller J.H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc. Natl. Acad. Sci. 1982;79:1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstraw P., Crowley J., Chansky K., Giroux D.J., Groome P.A., Rami-Porta R., Postmus P.E., Rusch V., Sobin L. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Thorac. Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- Govindan R., Ding L., Griffith M., Subramanian J., Dees N.D., Kanchi K.L., Maher C.A., Fulton R., Fulton L., Wallis J., Chen K., Walker J., McDonald S., Bose R., Ornitz D., Xiong D., You M., Dooling D.J., Watson M., Mardis E.R., Wilson R.K. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer. 2012;131:2724–2732. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A.S., Kannan K., Roy D.M., Morris L.G.T., Ganly I., Katabi N., Ramaswami D., Walsh L.A., Eng S., Huse J.T., Zhang J., Dolgalev I., Huberman K., Heguy A., Viale A., Drobnjak M., Leversha M.A., Rice C.E., Singh B., Iyer N.G., Leemans C.R., Bloemena E., Ferris R.L., Seethala R.R., Gross B.E., Liang Y., Sinha R., Peng L., Raphael B.J., Turcan S., Gong Y., Schultz N., Kim S., Chiosea S., Shah J.P., Sander C., Lee W., Chan T.A. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 2013;45:791–798. doi: 10.1038/ng.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imielinski M., Berger A.H., Hammerman P.S., Hernandez B., Pugh T.J., Hodis E., Cho J., Suh J., Capelletti M., Sivachenko A., Sougnez C., Auclair D., Lawrence M.S., Stojanov P., Cibulskis K., Choi K., de Waa L., Sharifnia T., Brooks A., Greulich H., Banerji S., Zander T., Seidel D., Leenders F., Ansén S., Ludwig C., Engel-Riedel W., Stoelbenh E., Wolf J., Goparju C., Thompson K., Winckler W., Kwiatkowski D., Johnson B.E., Jänne P.A., Miller V.A., Pao W., Travis W.D., Pass H.I., Gabriel S.B., Lander E.S., Thomas R.K., Garraway L.A., Getz G., Meyerson M. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irigaray P., Belpomme D. Basic properties and molecular mechanisms of exogenous chemical carcinogens. Carcinogenesis. 2010;31:135–148. doi: 10.1093/carcin/bgp252. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Yatabe Y., Endoh H., Kuwano H., Takahashi T., Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- Lan Q., Chapman R.S., Schreinemachers D.M., Tian L., He X. Household stove improvement and risk of lung cancer in Xuanwei, China. J. Natl. Cancer Inst. 2002;94:826–835. doi: 10.1093/jnci/94.11.826. [DOI] [PubMed] [Google Scholar]
- Lanner J.T., Georgiou D.K., Joshi A.D., Hamilton S.L. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.H., Zhang Y.S., Li Y., Yin G.Q., Li Y.B., Ning B.F., Guo J.M. Descriptive study on the epidemiology of lung cancer in coal-producing area in eastern Yunnan, China. Chin. J. Lung Cancer. 2011;14:107–119. doi: 10.3779/j.issn.1009-3419.2011.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis D., Grosse Y., Lauby-Secretan B., Ghissassi F.E., Bouvard V., Benbrhim-Tallaa L., Guha N., Baan R., Mattock H., Straif K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–1263. doi: 10.1016/s1470-2045(13)70487-x. [DOI] [PubMed] [Google Scholar]
- Love C., Sun Z., Jima D., Li G., Zhang J., Miles R., Richards K.L., Dunphy C.H., Choi W.W.L., Srivastava G., Lugar P.L., Rizzieri D.A., Lagoo A.S., Bernal-Mizrachi L., Mann K.P., Flowers C.R., Naresh K.N., Evens A.M., Chadburn A., Gordon L.I., Czader M.B., Gill J.I., Hsi E.D., Greenough A., Moffitt A.B., McKinney M., Banerjee A., Grubor V., Levy S., Dunson D.B., Dave S.S. The genetic landscape of mutations in Burkitt lymphoma. Nat. Genet. 2012;44:1321–1325. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith G.R., Davis F.M., Roberts-Thomson S.J. Calcium channels and pumps in cancer: changes and consequences. J. Biol. Chem. 2012;287:31666–31673. doi: 10.1074/jbc.R112.343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford J.L., He X.Z., Chapman R.S., Cao S.R., Harris D.B., Li X.M., Xian Y.L., Jiang W.Z., Xu C.W., Chuang J.C. Lung cancer and indoor air pollution in Xuan Wei, China. Science. 1987;235:217–220. doi: 10.1126/science.3798109. [DOI] [PubMed] [Google Scholar]
- Natrajan R., Little S.E., Reis-Filho J.S., Hing L., Messahel B., Grundy P.E., Dome J.S., Schneider T., Vujanic G.M., Pritchard-Jones K., Jones C. Amplification and overexpression of CACNA1E correlates with relapse in favorable histology Wilms' tumors. Clin. Cancer Res. 2006;12:7284–7293. doi: 10.1158/1078-0432.CCR-06-1567. [DOI] [PubMed] [Google Scholar]
- Olivier M., Weninger A., Ardin M., Huskova H., Castells X., Vallee M.P., McKay J., Nedelko T., Muehlbauer K.R., Marusawa H., Alexander J., Hazelwood L., Byrnes G., Hollstein M., Zavadil J. Modelling mutational landscapes of human cancers in vitro. Sci. Rep. 2014;4:4482. doi: 10.1038/srep04482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallone T., Khurana S., Cao C. Voltage-gated calcium channels: structure and function (CACNA) In: Choi S., editor. Encyclopedia of Signaling Molecules. Springer; New York: 2012. pp. 1984–1992. [Google Scholar]
- Raaschou-Nielsen O., Andersen Z.J., Beelen R., Samoli E., Stafoggia M., Weinmayr G., Hoffmann B., Fischer P., Nieuwenhuijsen M., Brunekreef B., Xun W., Katsouyanni K., Dimakopoulou K., Sommar J., Forsberg B., Modig L., Oudin A., Oftedal B., Schwarze P., Per Nafstad P., De Faire F., Pedersen N.L., Ostenson C., Fratiglioni L., Penell J., Korek M., Pershagen G., Eriksen K., Sorensen M., Tjonneland A., Ellermann T., Eeftens M., Peeters P., Meliefste K., Wang M., Bueno-de-Mesquita B., Key T., de Hoogh K., Concin H., Nagel G., Vilier A., Grioni S., Krogh V., Tsai M., Ricceri F., Sacerdote C., Galassi C., Migliore E., Ranzi A., Cesaroni G., Badaloni C., Forastiere F., Tamayo I., Amiano P., Dorronsoro M., Trichopoulou A., Bamia C., Vineis P., Hoek G. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- Ruggeri B., DiRado M., Zhang S.Y., Bauer B., Goodrow T., Klein-Szanto A.J. Benzo[a]pyrene-induced murine skin tumors exhibit frequent and characteristic G to T mutations in the p53 gene. Proc. Natl. Acad. Sci. 1993;90:1013–1017. doi: 10.1073/pnas.90.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinton J.E., Smith K.R., Hu H.S., Liu J.Z. World Health, Organization; Geneva (Switzerland): 1995. Indoor Air Pollution Database for China. (WHO/EHG/95.8) [Google Scholar]
- Soong T.W., Stea A., Hodson C.D., Dubel S.J., Vincent S.R., Snutch T.P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- Sullvivan J.B., Krieger G.R. Lippincott Williams & Wilkins; Philadelphia, PA: 2014. Clinical Environmental Health and Toxic Exposures. [Google Scholar]
- The Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van D.A., Martin R.V., Brauer M., Kahn R., Levy R., Verduzco C., Villeneuve P.J. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: development and application. Environ. Health Perspect. 2010;118:847–855. doi: 10.1289/ehp.0901623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Mullighan C.G., Easton J., Roberts S., Heatley S.L., Ma J., Rusch M.C., Chen K., Harris C.C., Ding L., Holmfeldt L., Payne-Turner D., Fan X., Wei L., Zhao D., Obenauer J.C., Naeve C., Mardis E.R., Wilson R.K., Downing J.R., Zhang J. CREST maps somatic structural variation in cancer genomes with base-pair resolution. Nat. Methods. 2011;8:652–654. doi: 10.1038/nmeth.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization International Agency for Research on Cancer . vol. 95. 2010. Household use of solid fuels and high-temperature frying. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans). ( http://monographs.iarc.fr/ENG/Monographs/vol95/) [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Shao Y., Yu X., Zhou G. The epidemic status and risk factors of lung cancer in Xuanwei City, Yunnan Province, China. Front. Med. 2012;6:388–394. doi: 10.1007/s11684-012-0233-3. [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang L., Han H., Jin K., Lin N., Guo T., Chen Y., Cheng H., Lu F., Fang W., Wang Y., Xing B., Zhang Z. 1B50-1, a mAb raised against recurrent tumor cells, targets liver tumor-initiating cells by binding to the calcium channel alpha2delta1 subunit. Cancer Cell. 2013;23:541–556. doi: 10.1016/j.ccr.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Zielinska B., Samy S., McDonald J.D., Seagrave J. Atmospheric transformation of diesel emissions. Res. Rep. Health Eff. Inst. 2010;147:5–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.
Clinical characteristics of tumors used in whole genome sequencing and summary of sequencing results of each tumor DNA.
Chromosomal rearrangements in the whole genome sequencing patients.
Somatic copy number alterations in the patients.
The mutated genes found in whole genome sequencing.
Mutations in 2010 genes in 164 non-small cell lung cancers.