Abstract
Infection with helminth parasites causes morbidity and mortality in billions of people and livestock worldwide. Where anthelmintic drugs are available, drug resistance is a major problem in livestock parasites, and a looming threat to public health. Monitoring the efficacy of these medicines and screening for new drugs has been hindered by the lack of objective, high-throughput approaches. Several cell monitoring technologies have been adapted for parasitic worms, including video-, fluorescence-, metabolism enzyme- and impedance-based tools that minimize the screening bottleneck. Using the xCELLigence impedance-based system we previously developed a motility-viability assay that is applicable for a range of helminth parasites. Here we have improved substantially the assay by using diverse frequency settings, and have named it the xCELLigence worm real-time motility assay (xWORM). By utilizing strictly standardized mean difference analysis we compared the xWORM output measured with 10, 25 and 50 kHz frequencies to quantify the motility of schistosome adults (human blood flukes) and hatching of schistosome eggs. Furthermore, we have described a novel application of xWORM to monitor movement of schistosome cercariae, the developmental stage that is infectious to humans. For all three stages, 25 kHz was either optimal or near-optimal for monitoring and quantifying schistosome motility. These improvements in methodology sensitivity should enhance the capacity to screen small compound libraries for new drugs both for schistosomes and other helminth pathogens at large.
Keywords: Helminths, Viability, xCELLigence, Schistosome, Strictly standardized mean difference (SSMD), High-throughput
Graphical abstract
Highlights
-
•
25 kHz on the xCELLigence system dramatically improves the schistosome xWORM assay.
-
•
xWORM assay can efficiently determine viability of Schistome adults or eggs.
-
•
First time cercariae have been incorporated into an automated viability assay.
-
•
Other helminth monitoring may benefit from alternate xCELLigence frequency options.
1. Introduction
In excess of one billion people are infected with helminths in developing countries, where these diseases cause substantial morbidity and hundreds of thousands of deaths annually (May, 2007; Albonico et al., 2008; Hotez, 2011; Bardosh, 2014). Helminths also plague livestock in developing and developed countries, with the global anthelmintic market for livestock and companion animals valued at $US 3.7 billion in 2002 (Evans and Chapple, 2002). Whereas chemotherapy is available for infection with most parasitic helminths, widespread use of anthelmintics in agriculture has resulted in the emergence of drug-resistant parasites (James et al., 2009; Molento, 2009). Concern remains about the emergence of drug resistance in human helminth parasites, and as mass drug administration campaigns increase this worrisome prospect becomes more likely (Abdul-Ghani et al., 2009; Abdulla et al., 2009; Cioli et al., 2014; Falzon et al., 2014). Despite the impact of helminths on public health and economies, the anthelmintic pharmacopoeia is not extensive. This is due in part to the high cost and limited financial return from drug development, particularly for pathogens of medical importance. A decade ago, the major component of this cost limitation was the lack of objective high-throughput screening methods for assessing drug effectiveness (Kotze et al., 2006; Abdul-Ghani et al., 2009; Keiser, 2009). The gold standard for measuring drug effectiveness for helminth parasites was the costly, laborious and subjective in vitro assessment of worm motility, as measured visually via microscopy and larval development assays (Kotze et al., 2004; Abdulla et al., 2009; Keiser, 2009). Indeed, there are multiple requests in the peer-reviewed literature for high-throughput screening methods to facilitate drug development and to detect emerging resistance (Morel, 2003; Sommerfeld and Oduola, 2007; Keiser, 2009). In response, the Tropical Diseases Research network (TDR) of the WHO (http://apps.who.int/tdr/) developed an international resistance screening network, but due to the limitations of available techniques, screening deployed to date has utilized only low-to medium-throughput approaches (Hopkins et al., 2007; Abdulla et al., 2009).
Recently, a range of screening techniques have emerged, often adapted from methods to monitor cultured cells, which allow monitoring of several helminth parasite species and developmental stages by video (Paveley et al., 2012), impedance (Smout et al., 2010), enzymatic (Mansour and Bickle, 2010), colorimetric (Tritten et al., 2012), fluorescence (Peak et al., 2010) and other mechanisms (Howe et al., 2015; Lalli et al., 2015). We have previously adapted the impedance-based real time cell assay – the xCELLigence system – to monitor the viability of a range of human and livestock parasitic helminths (Smout et al., 2010). We herein rename this assay xWORM for xCELLigence worm real-time motility assay. The xCELLigence unit conventionally measures cell growth relaying on gold electrodes embedded in the base of tissue culture microplates that monitor changes in conductivity due to contact of the cells with the electrodes (Vistejnova et al., 2009; Smout et al., 2011). With many species of parasitic helminths, the wild type phenotype is motile in tissue culture, and the movement of the worm is detected by change in the conductivity on the electrodes. The xWORM approach is simple, objective, high-throughput, relatively inexpensive, and applicable to many species of parasitic worms (Smout et al., 2010). The technique has been favorably received in the field for its sensitivity, broad applicability and adaptability (Peak and Hoffmann, 2011; Silbereisen et al., 2011; Tritten et al., 2012; You et al., 2013; Zeraik et al., 2014). However, alternate viability monitoring methods, such as energy metabolism and membrane permeability, have been shown to be more suitable for specific life cycle stages, notably the intra-mammalian larval stages of the human schistosome blood flukes (Mansour and Bickle, 2010; Peak et al., 2010; Howe et al., 2015; Lalli et al., 2015).
xWORM has been adapted for a range of applications that rely on measurement of a phenotypic change, including responses of genetically manipulated parasites (You et al., 2013). Moreover, developmental stages that originally were not envisioned suitable for the assay, such as eggs of schistosomes that require fresh water to hatch, have been successfully employed (Zeraik et al., 2014). These new applications were successful but required further optimization as a low concentration buffered salt solution of 0.1× phosphate buffered saline (PBS) was necessary for egg hatching. In addition the signal: noise ratio at the default 10 kHz frequency was sub-optimal for sensitive detection. Discussions with the developers of the xCELLigence system (ACEA), revealed alternate frequency settings of 25 and 50 kHz available for monitoring cells – or in our case monitoring parasitic helminths.
In order to improve the sensitivity of the xWORM assay we employed strictly standardized mean difference prime (SSMD′) statistical analysis to compare different monitoring frequencies, i.e. the default 10 kHz and alternate 25 kHz and 50 kHz. Monitoring of egg hatching and adult motility was substantially improved at 25 kHz. In addition, low concentration salt motility assay facilitated accurate measurement of motility of cercariae, the infective stage of the schistosome for humans and other mammals.
2. Materials and methods
2.1. Ethics statement
Mice infected with Schistosoma mansoni were obtained from the Biomedical Research Institute (BRI), Rockville, MD and housed at the Animal Research Facility of the George Washington University Medical School, which is accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC no. 000347) and has an Animal Welfare Assurance on file with the National Institutes of Health, Office of Laboratory Animal Welfare, OLAW assurance number A3205-01. All procedures employed were consistent with the Guide for the Care and Use of Laboratory Animals. Maintenance of the mice and recovery of schistosomes were approved by the Institutional Animal Care and Use Committee of the George Washington University.
2.2. Schistosomes
Biomphalaria glabrata snails and Swiss-Webster mice infected with the NMRI (Puerto Rican) strain of S. mansoni were supplied by the Biomedical Research Institute, Rockville, Maryland USA under NIH-NIAID contract HHSN272201000005I. Four developmental stages collected and maintained as described (Mann et al., 2010) were investigated: adults, eggs/miracidia, cercariae and schistosomula.
2.3. Establishment of xWORM for measuring motility
The motility assay was performed using the xCELLigence DP platform (ACEA Biosciences, San Diego, CA) at 10 kHz as described (Zeraik et al., 2014), and with the addition of 25 and 50 kHz frequencies. All motility index readings were monitored with sweeps at intervals of 15 s by the RTCA software. Prior to the addition of parasites to E-plates, baseline motility using media alone was determined overnight in E-plate wells containing 100 μl of relevant media but without parasites (see Sections 2.4–2.7 below). Treatments of forchlorfenuron in DMSO (FCF, Sigma–Aldrich) or DMSO (untreated vehicle controls) were included when specified for the different life cycle stages. Developmental stages of S. mansoni were heat killed, 80 °C for 15 min, immediately before the assay.
2.4. Establishment of xWORM for measuring egg hatching
Eggs were collected from livers of experimentally infected mice (Mann et al., 2010), and egg hatching/miracidia motility was assessed as described (Zeraik et al., 2014). Briefly 5000 eggs were seeded into 180 μl of 0.1× PBS, pH 7.2 (13.7 mM NaCl, 0.27 mM KCl, 1 mM NaH2PO4/Na2HPO4, 0.2 mM KH2PO4) per well and supplemented with 20 μl of DMSO vehicle control, 50 μM or 200 μM of FCF (final concentration) in duplicate. The eggs were induced to hatch under bright light at 23 °C for ∼16 h. A replicate of the experiment was run in parallel in a 96-well microtitre plate for direct visual observation of the egg hatching using an Axio Observer A.1 inverted microscope fitted with an AxioCam ICc3 camera (Zeiss).
2.5. Cercariae
Cercariae shed from B. glabrata snails infected with S. mansoni in water under bright light for 2 h at room temperature were washed three times in 0.1× PBS and 2% antibiotic/antimycotic (Life Technologies), transferred to 0.1× PBS and dispensed in duplicate to E-plate wells in two-fold decreasing serial dilution of cercariae from 9000-281 cercariae/well. Dead (heat-killed) cercariae were included as controls. The motility of the larvae was monitored overnight for 20 h at 23 °C.
2.6. Schistosomula
Schistosomula were obtained by mechanical transformation of cercariae (Mann et al., 2010) and cultured in modified Basch's medium under 5% CO2 at 37 °C (Dalton et al., 1997; Mann et al., 2010; Zeraik et al., 2014). Serial two-fold dilutions of 5000-78 schistosomula in 100 μl of modified Basch's medium were added to E-plate wells containing 100 μl of the same medium. Two wells containing 200 μl of modified Basch's medium alone were included as empty well controls. The parasites were monitored every 15 s at 37 °C under 5% CO2 for ∼16 h. The experiment was performed in duplicate.
2.7. Adult schistosomes
Adult worms were recovered from the hepatic veins of infected mice by portal perfusion (Mann et al., 2010; Zeraik et al., 2014). Thereafter, adult worms were washed and cultured in Dulbecco's modified Eagle's medium (DMEM-Life Technologies), supplemented with 10% fetal bovine serum and 2% antibiotic/antimycotic (Invitrogen, Carlsbad, CA, catalogue no. 15240-062). Individual adult schistosomes were dispensed into wells of E-plates and the pre-treatment motility of flukes was registered for a minimum of 6 h before addition of FCF to 1 mM or DMSO vehicle control with 6–8 replicates per condition.
2.8. Motility analysis employing strictly standardized mean difference (SSMD)
The motility index for each E-plate well, i.e. individual flukes, 5000 eggs, schistosomula or cercariae, was calculated in Excel (Microsoft) as the standard deviation over 800 data points of the cell index (CI) difference from the rolling average over 30 data points as described (Smout et al., 2010). The absolute SSMD value is the ratio of the difference of the sample mean to the standard deviation of the sample data and was generated as described by Zhang (2011). Treatment and negative control samples (untreated parasites) for egg hatch and cercariae motility have mean values designated as and with standard deviations of and respectively. For egg hatch and cercariae motility, the total motility index over 16 and 20 h respectively was used for graphing and SSMD calculations.
SSMD prime (SSMD′) is based upon the positive/negative controls and replaces with the heat-killed parasite positive control. Outliers encountered with the adult fluke assay require the use of robust SSMD value (SSMD*), which uses a similar SSMD formula as shown below, where the mean and standard deviations are replaced with median and median absolute deviation (MAD) values . Adult worm motility used the average motility index over 5 h for generation of SSMD* and SSMD*′ scores.
The heat-killed parasites used as a positive control were an “extremely strong control” and used the most stringent category, shown in Table 1, for determination of assay quality. Treatment magnitude in Table 2 delineates SSMD ranges for effect quality as recommended by Zhang (2011).
Table 1.
Assay quality SSMD′ ranges with heat killed schistosomes based upon the findings of Zhang (2011).
| Assay quality | Absolute SSMD′ or SSMD*′ |
|---|---|
| Excellent | >7 |
| Good | 5 to 7 |
| Inferior | 3 to 5 |
| Poor | 0–3 |
Table 2.
Classification of treatment hits using SSMD values based upon Zhang (2011).
| Hit strength category | Absolute SSMD or SSMD* |
|---|---|
| Extremely strong | >5 |
| Very strong | 3–5 |
| Strong | 2–3 |
| Fairly strong | 1.645–2 |
| Moderate | 1.28–1.645 |
| Fairly moderate | 1–1.28 |
| Fairly weak | 0.75–1 |
| Weak | 0.5–0.75 |
| Very weak | 0.25–0.5 |
| Extremely weak | 0–0.25 |
| No effect | 0 |
3. Results
3.1. The xWORM S. mansoni egg hatch assay is most sensitive at 25 kHz frequency
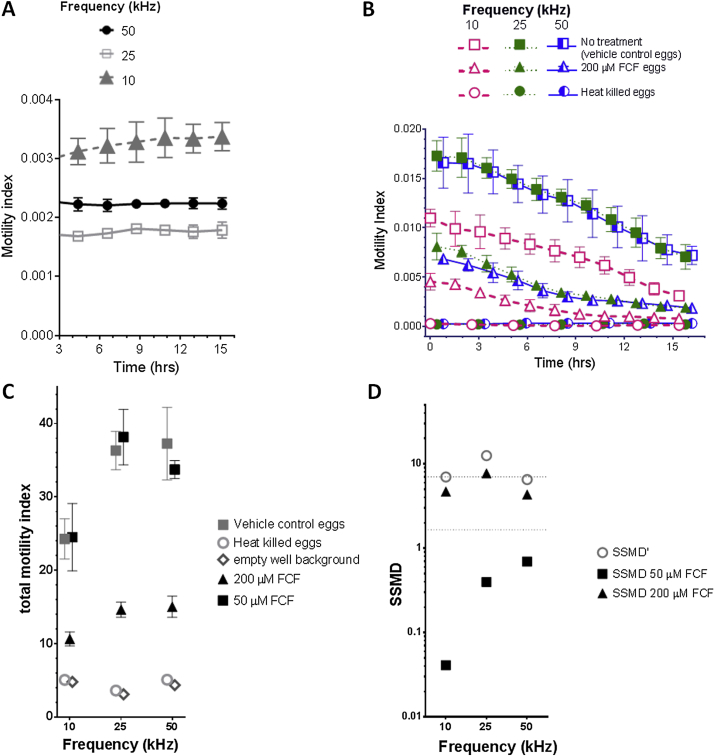
The xCELLigence motility assay was developed (Smout et al., 2010) for measuring motility of hookworms and adult blood flukes (schistosomes) (Fig. 1), and adapted later to several helminth species and developmental stages (Silbereisen et al., 2011; Tritten et al., 2012; You et al., 2013; Zeraik et al., 2014). The schistosome egg hatch assay requires 0.1× PBS as an approximation for the salinity of fresh water (Zeraik et al., 2014); tests using buffer alone (0.1× PBS) at 25 kHz and 50 kHz showed both a lower and more temporally stable background motility index than the default 10 kHz xCELLigence frequency output (Fig. 2A). Assessment of the three frequencies with egg hatching showed an improvement for both 25 kHz and 50 kHz frequencies compared to 10 kHz (68.9% and 70.4% total motility index respectively), although 50 kHz resulted in higher variability (Fig. 2B). Furthermore, inhibition of egg hatching with 200 μM FCF, reported by Zeraik et al. (2014) measured at 10 kHz, resulted in a similar ∼60% reduction of signal across all frequencies (Fig. 2C). Although it is worth noting the readings obtained at the two higher frequencies, 25 and 50 kHz, demonstrated marginally steeper slopes than that at 10 kHz (Fig. 2B). This may represent the frequency influencing hatching rates and miracidial motility, and/or the higher sensitivity of the assay reflects improved detection resolution of normal hatching.
Fig. 1.
Schistosome adult male and female pair in the well of an xCELLigence E-plate. Representative picture of a male and female pair in a well of an E-plate, the consumable component of the xWORM assay. The rows of small dark circles are the gold electrodes embedded in the plate that measure the motility of the worm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
xWORM optimization of the schistosome egg hatch assay. Background motility index measurements over time at frequencies of 10, 25 and 50 KHz, generated from xCELLigence wells with 200 μl 0.1× PBS without parasites (Panel A). Egg motility index curves blanked against 0.1× PBS alone controls (Panel B). Total motility index over 16 h (Panel C). Strictly standardized mean difference (SSMD) for the three frequencies (Panel D). An SSMD′ score above 7 indicates an “excellent assay”, and a treatment SSMD score above 1.645 is equivalent obtains a significance level of P ≤ 0.05 and represents a positive hit. For all panels: the mean of biological duplicate experiments is plotted with standard error of the mean (SEM) error bars.
These xWORM experiments were designed to determine which frequency generates the highest quality control parameters – that is the largest difference between positive and negative controls relative to the variation within each group. Comparing assay quality using classic null hypothesis statistical significance tests with P-values is problematic (Cohen, 1994; Kirk, 1996) given that the magnitude of the difference between groups is not measured, and only confirms that the difference is not zero (Zhang, 2007; Goktug et al., 2012). A range of statistical assessments has been developed for high-throughput assays, many compatible with xWORM analysis (Birmingham et al., 2009; Kozak, 2012). Z factor was initially used for quality control, but over the past decade the more meaningful strictly standardized mean difference (SSMD) parameter has become more commonplace (Zhang, 2007; Goktug et al., 2012). The SSMD value is the difference between groups relative to the variance within each group. Hence a high SSMD value represents a low variance within each group relative to the difference between the groups. This allows the output to measure the magnitude of impact more effectively that other metrics based on null hypothesis (Zhang, 2007; Birmingham et al., 2009; Kozak, 2012).
When used to evaluate the assay quality, the SSMD prime (SSMD′) value is generated to compare the positive and negative control groups, i.e. heat killed and untreated parasites, respectively. Zhang and colleagues determined SSMD′ ranges to asses assay quality based upon the predicted effect size of the positive control (Zhang, 2011). As heat-killed control parasites were expected to be completely immobile, the strictest assay criteria were selected, and a summary is shown in Table 1 (Zhang, 2011). An absolute SSMD′ of 5–7 is considered a “good assay” rating, and values >7 indicate an “excellent assay” meaning that the difference in motility between groups is >7-fold the square root of sum of the variance of the groups. The 10 and 50 kHz frequencies generated “good assay” SSMD′ scores of 5–7 (Fig. 2D). Although the 25 and 50 kHz groups had similar total motility indices (Fig. 2C), the lower variation obtained with the 25 kHz frequency resulted in an “excellent assay” SSMD′ score of >7 (Fig. 2D).
Screening of schistosome eggs in the presence of different compounds to detect a significant reduction in hatching/motility relies on the comparison of untreated negative control and treatment groups, in this case the drug FCF. A positive hit for a significant motility reduction from a treatment is any SSMD >1.645, as mathematically this is the equivalent of the conventional t-test p < 0.05 (Zhang et al., 2010; Zhang, 2011). We observed that the SSMD values for parasites treated with 200 μM FCF were significant (>1.645) at every frequency; the 10 and 50 kHz SSMD scores of 3–5 are considered “strong hits” (Table 2) and the 25 kHz score (>5) was considered an “extremely strong hit” (Fig. 2D). Comparison of control flukes and those treated with 50 μM FCF did not reach significance at any frequency (Fig. 2D) as previously noted at 10 kHz by Zeraik et al. (2014).
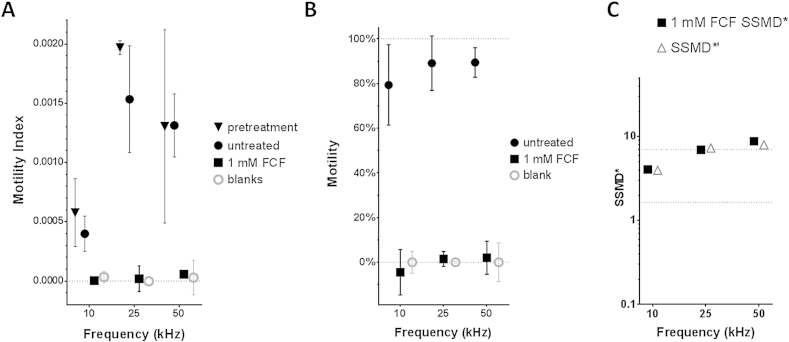
3.2. xWORM performance improvement for S. mansoni adults when frequencies of 25 or 50 kHz were employed
Compared to the egg hatch assay (Fig. 2), the adult motility index score showed greater variability within groups at all the tested frequencies (Fig. 3A), probably due to differences in size/activity levels among individual worms. As each worm generally displays similar motility index scores over time, this variability was reduced when post-treatment motility index scores were converted to percentages of pre-treatment values as previously described (Fig. 3B) (Smout et al., 2010). Because the occasional parasite was overly active or subdued in the untreated groups we utilized median scores to counter outliers. The subsequent SSMD values generated with median values have been designated robust SSMD (Zhang, 2011) and distinguished with an asterisk (SSMD* and SSMD*′). While the 10 kHz frequency assay reached the “good assay” (SSMD*′ 3–5) category with a score of 4.0, both the 25 and 50 kHz frequency reached the “excellent assay” range (SSMD*′ >7) with 7.3 and 7.9 scores respectively (Fig. 3C). The complete ablation of schistosome movement when flukes were cultured in 1 mM FCF was observed at all three frequencies in Fig. 3B. Both the 25 and 50 kHz frequencies scored “extremely strong hits” (SSMD* > 5) with SSMD* scores of 6.9 and 8.8 respectively, while the 10 kHz frequency scored SSMD* = 4.1 and is categorized a “very strong hit” (SSMD* = 3–5).
Fig. 3.
Improved xWORM assay for adult schistosomes. Average motility index across 3 frequencies (Panel A). Post-treatment motility index as a percentage of pre-treatment (Panel B). Robust strictly standardized mean difference (SSMD*) for the 3 frequencies (Panel C). For all panels: the median of 5–8 biological replicates is plotted with median absolute deviation (MAD) bars.
3.3. xWORM measures the viability of S. mansoni infectious stage cercariae, but not schistosomula
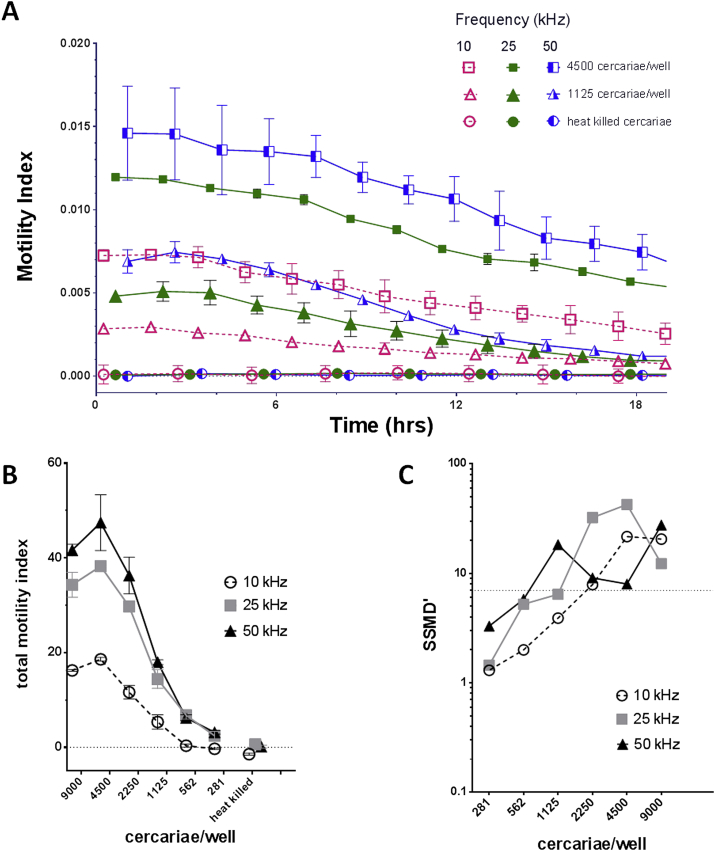
Given the success of xWORM for measuring egg hatch in low salt conditions, we also tested the assay with the highly motile cercariae of S. mansoni, the fork-tailed, free-swimming larval stage that is infective to humans. Normally, when maintained in fresh water this developmental stage has minimal contact with the gold electrodes at the base of the wells, since it actively swims in the water column, and hence does not generate a substantial signal. However, when cultured in 0.1× PBS rather than water, the cercariae still twitched rapidly (as seen during swimming), but sunk to the base of the wells making contact with the sensors of the E-plate. Optimal numbers of cercariae per well, 281–9000/well, were assessed at several frequencies. As with hatching of schistosome eggs, the motility was initially high but slowly declined over time (Fig. 4A). The area under the curve was employed to generate a total motility index (Fig. 4B), further emphasizing the higher scores generated at 25 or 50 kHz compared to 10 kHz. As with the egg hatching, the downward motility index slope tended to increase as frequency was raised (Fig. 4A) and might reflect the parasites responding to varied conditions.
Fig. 4.
Motility frequency optimization of schistosome cercariae. Motility index curves for two quantities of cercariae (1125 and 4500) per well, at three frequencies (Panel A). Total motility index curves of cercariae (Panel B). Strictly standardized mean difference prime (SSMD′) data for cercariae (Panel C). For all panels: mean data from biological duplicate experiments was plotted with SEM bars.
Analyzing assay quality with SSMD′ scores (Fig. 4C) we further demonstrated that 25 kHz with 4500 cercariae/well scored the highest SSMD′ value of 42.5 – substantially greater than the “excellent assay” SSMD′ threshold. Indeed, all three frequencies tested with 2250–9000 cercariae/well all scored above the “excellent assay” threshold (Table 2). Whereas the highest SSMD score would be optimal for applications requiring high sensitivity levels, a high-throughput screen that seeks to test thousands of samples would ideally consume fewer parasites/well. Our data showed that a “good assay” score in the assay (SSMD′ 5–7) was achievable at 562 cercariae/well with 25 and 50 kHz frequencies.
Schistosomula of S. mansoni move in the bottom of the well by an expansion and contraction process rather than the period of rapid twitching exhibited by the cercariae in the water column since the tails of schistosomula were shed during the mechanical transformation. Therefore, their movement was below the detection threshold of the xCELLigence monitoring frequencies. Testing of 150–5000 schistosomula at the three discrete frequencies was unsuccessful, with minimal signals detected (not shown). The majority of the conditions tested showed quality ratings of “inferior assay” (SSMD′ < 3) and only 625 schistosomula at 25 kHz stimulated a score of 3.03, the “poor assay” category (SSMD′ = 3–5). This score did allow for detection of motility, but not at levels for reliable testing (data not shown).
4. Discussion
We previously developed a motility assay for adult and larval parasitic helminths (Smout et al., 2010), and here have named the method the xWORM assay. Our assay has been adapted by a number of other laboratories for a range of anthelmintics screens and other explorations of drug–helminth interactions (Mansour and Bickle, 2010; Silbereisen et al., 2011; Tritten et al., 2012; You et al., 2013; Zeraik et al., 2014). More recently the assay was adapted to monitor hatching of eggs of the human schistosome, S. mansoni, measuring a combination of direct miracidial electrode contact, the erratic movement of eggs induced by miracidial hatching as well as agitation of eggs by the highly motile hatched miracidia in the microplate well (Zeraik et al., 2014). A low salt buffer is essential for schistosome egg hatching, as it mimics the natural fresh water body where hatching occurs. However, xWORM requires buffered salts for conductivity measurements by the xCELLigence system. The alternate frequency option of 25 kHz and 0.1× PBS was verified by SSMD′ comparisons as the optimal combination to measure egg hatching using xWORM. When assessing the utility of xWORM to monitor motility of cercariae, the 25 kHz frequency was determined to be optimal with 4500 cercariae/well; with “good assay” motility scores with as few as 562 cercariae/well. Although studying cercariae out of the natural fresh water environment is artificial, the motility assay in 0.1× PBS with the xCELLigence system represents a surrogate approach to monitor motility of this developmental stage. Despite this, caution should be taken as the artificial conditions may confound interpretation of results. Despite the improvements to the xWORM sensitivity, schistosomula were unable to be adequately monitored. The range of xCELLigence systems that incorporate the three frequency options (10, 25 and 50 kHz) required for this optimized xWORM assay span from small lab scale (Dual-plate unit – 3 × 16 wells and Single Plate unit – 96 wells) to larger systems (Multi-plate unit – 6 × 96 well and High-throughput unit – 384 wells).
The xWORM assay is a robust yet sensitive technique that can now be predicted to be widely applicable across a range of species and life cycle stages, but recent progress has greatly expanded the available options to researchers seeking reliable methods to monitor parasitic helminths (Peak and Hoffmann, 2011). Initially, employing the model free-living helminth Caenorhabditis elegans, methods that allowed microfluidics-based detection of motility from an individual worm were developed (Rohde et al., 2007; Tong et al., 2013). Subsequent approaches were adapted from techniques originally designed to count mammalian cells – either based on mitochondrial enzymes (Lai et al., 2014) or computer algorithms that automated detection in bright field images (White et al., 2013). These algorithmic and enzymatic techniques have been shown to be efficacious for monitoring the viability of schistosomula, the ideal developmental stage for high-throughput anthelmintic drug screening. A range of cell health indicators are now available for monitoring schistosomula viability, such as the colorimetric Alamar blue (Mansour and Bickle, 2010) and the dual fluorogenesis assay using propidium iodide and fluorescein diacetate (Peak et al., 2010; Marxer et al., 2012; Rinaldi et al., 2012). Progress has been accomplished in developing assays to detect and quantify metabolism, notably luminescent quantitation of ATP (Lalli et al., 2015) and lactate – the end product of glycolysis (Howe et al., 2015). These provide a low-cost approach to screening schistosomula with a standard microplate reader, and while effective at detecting reduced metabolism or impaired cellular membranes, these assays have difficulty in detecting phenotypic or motility distortion (Marxer et al., 2012; Paveley et al., 2012). This impediment was overcome by the development of algorithms that detected schistosomula movement or phenotypic change by using automated image acquisition systems (Paveley et al., 2012). Progress towards the screening of other schistosome life cycle stages is less advanced, and the detection of lactate (Howe et al., 2015) is the only non-observational method that is also applicable to the adult schistosome. In terms of other flatworm genera, there has been some progress, such as screening for secreted enzymes in tapeworms (Mahanty et al., 2013), but the limited penetration of current dyes has hindered efforts. So while xWORM is not suitable for measuring the motility of the schistosomula, our assay complements the current range of schistosomula viability assays with wide applicability for the remaining major aspects of the schistosome life cycle (eggs, cercariae, adults). The widespread use of high-throughput methods to screen for new therapeutics or emerging drug resistance will facilitate greater capacity to drug screens to parse small compound libraries for novel interventions.
5. Conclusions
Monitoring the efficacy of anthelmintic medicines and screening for new drugs was previously hindered by the lack of objective, high-throughput approaches. Here we present a more sensitive xWORM assay for S. mansoni adults and egg hatch monitoring and expanded capabilities to monitor cercariae. We focused on S. mansoni, but the xWORM assay is envisaged to be applicable for the majority of helminth species and developmental stages where egg hatch assays and/or motility are deployed presently to evaluate parasite viability. Additionally, this assay further improves high-throughput helminth viability screening and will be an asset in the fight against the wide range of biomedical and veterinary helminths.
Contributions of authors
GR helped conceive, design and perform the bulk of experiments. MJS helped conceive, design and analyze experiments, provided project oversight and wrote the manuscript. JI provided technical assistance and provided raw data for blank well frequency comparisons. AL and PJB provided project oversight. All authors edited and proofed drafts of the manuscript.
Conflicts of interest
GR and MJS have received travel funds to present at an xCELLigence workshop, and the 2014 The International Congress of Parasitology meeting through the competitive ACEA travel award program, respectively. JI is an employee of ACEA, the developers of the xCELLigence system. ACEA played no role in performing the experiments, analyzing the data, or developing the manuscript other than providing raw data for Fig. 2A and technical assistance from author JI.
Acknowledgments
We thank Victoria H. Mann and Hong-Bin Yan for assistance with developmental stages of schistosomes. This work was supported in part by the National Institute of Allergy and Infectious Diseases, awards R21 AI109532 and R01AI072773, and a National Health and Medical Research Council program grant (APP1037304) and Principal Research Fellowship to A. L. (APP1020114)
References
- Abdul-Ghani R.A., Loutfy N., Hassan A. Experimentally promising antischistosomal drugs: a review of some drug candidates not reaching the clinical use. Parasitol. Res. 2009;105(4):899–906. doi: 10.1007/s00436-009-1546-2. [DOI] [PubMed] [Google Scholar]
- Abdulla M.H., Ruelas D.S., Wolff B., Snedecor J., Lim K.C., Xu F., Renslo A.R., Williams J., Mckerrow J.H., Caffrey C.R. Drug discovery for schistosomiasis: hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl. Trop. Dis. 2009;3(7):e478. doi: 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albonico M., Allen H., Chitsulo L., Engels D., Gabrielli A.F., Savioli L. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl. Trop. Dis. 2008;2(3):e126. doi: 10.1371/journal.pntd.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardosh K. Global aspirations, local realities: the role of social science research in controlling neglected tropical diseases. Infect. Dis. Poverty. 2014;3(1):35. doi: 10.1186/2049-9957-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham A., Selfors L.M., Forster T., Wrobel D., Kennedy C.J., Shanks E., Santoyo-Lopez J., Dunican D.J., Long A., Kelleher D., Smith Q., Beijersbergen R.L., Ghazal P., Shamu C.E. Statistical methods for analysis of high-throughput RNA interference screens. Nat. Methods. 2009;6(8):569–575. doi: 10.1038/nmeth.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioli D., Pica-Mattoccia L., Basso A., Guidi A. Schistosomiasis control: praziquantel forever? Mol. Biochem. Parasitol. 2014;195(1):23–29. doi: 10.1016/j.molbiopara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Cohen J. The Earth is round (p<0.05) Am. Psychol. 1994;49(12):997–1003. [Google Scholar]
- Dalton J.P., Day S.R., Drew A.C., Brindley P.J. A method for the isolation of schistosome eggs and miracidia free of contaminating host tissues. Parasitology. 1997;115(Pt 1):29–32. doi: 10.1017/s0031182097001091. [DOI] [PubMed] [Google Scholar]
- Evans T., Chapple N. The animal health market. Nat. Rev. Drug Discov. 2002;1(12):937–938. doi: 10.1038/nrd975. [DOI] [PubMed] [Google Scholar]
- Falzon L.C., O'neill T.J., Menzies P.I., Peregrine A.S., Jones-Bitton A., Vanleeuwen J., Mederos A. A systematic review and meta-analysis of factors associated with anthelmintic resistance in sheep. Prev. Vet. Med. 2014;117(2):388–402. doi: 10.1016/j.prevetmed.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Goktug A.N., Ong S.S., Chen T. GUItars: a GUI tool for analysis of high-throughput RNA interference screening data. PLoS One. 2012;7(11):e49386. doi: 10.1371/journal.pone.0049386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A.L., Witty M.J., Nwaka S. Mission possible. Nature. 2007;449(7159):166–169. doi: 10.1038/449166a. [DOI] [PubMed] [Google Scholar]
- Hotez P. Enlarging the “Audacious Goal”: elimination of the world's high prevalence neglected tropical diseases. Vaccine. 2011;29(Suppl. 4):D104–D110. doi: 10.1016/j.vaccine.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Howe S., Zophel D., Subbaraman H., Unger C., Held J., Engleitner T., Hoffmann W.H., Kreidenweiss A. Lactate as a novel quantitative measure of viability in Schistosoma mansoni drug sensitivity assays. Antimicrob. Agents Chemother. 2015;59(2):1193–1199. doi: 10.1128/AAC.03809-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C.E., Hudson A.L., Davey M.W. Drug resistance mechanisms in helminths: is it survival of the fittest? Trends Parasitol. 2009;25(7):328–335. doi: 10.1016/j.pt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Keiser J. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology. 2009;137(3):589–603. doi: 10.1017/S0031182009991739. [DOI] [PubMed] [Google Scholar]
- Kirk R.E. Practical significance: a concept whose time has come. Educ. Psychol. Meas. 1996;56(5):746–759. [Google Scholar]
- Kotze A.C., Clifford S., O'grady J., Behnke J.M., Mccarthy J.S. An in vitro larval motility assay to determine anthelmintic sensitivity for human hookworm and Strongyloides species. Am. J. Trop. Med. Hyg. 2004;71(5):608–616. [PubMed] [Google Scholar]
- Kotze A.C., Le Jambre L.F., O'grady J. A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet. Parasitol. 2006;137(3–4):294–305. doi: 10.1016/j.vetpar.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Kozak K. Springer; Verlag Berlin Heidelberg: 2012. The Use of Design Specificity in Standardized Mean Difference for Analysis of High Throughput RNA Interference Screens. [Google Scholar]
- Lai Y., Xiang M., Liu S., Li E., Che Y., Liu X. A novel high-throughput nematicidal assay using embryo cells and larvae of Caenorhabditis elegans. Exp. Parasitol. 2014;139:33–41. doi: 10.1016/j.exppara.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Lalli C., Guidi A., Gennari N., Altamura S., Bresciani A., Ruberti G. Development and validation of a luminescence-based, medium-throughput assay for drug screening in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015;9(1):e0003484. doi: 10.1371/journal.pntd.0003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S., Madrid E.M., Nash T.E. Quantitative screening for anticestode drugs based on changes in baseline enzyme secretion by Taenia crassiceps. Antimicrob. Agents Chemother. 2013;57(2):990–995. doi: 10.1128/AAC.01022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann V.H., Morales M.E., Rinaldi G., Brindley P.J. Culture for genetic manipulation of developmental stages of Schistosoma mansoni. Parasitology. 2010;137(3):451–462. doi: 10.1017/S0031182009991211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour N.R., Bickle Q.D. Comparison of microscopy and Alamar blue reduction in a larval based assay for schistosome drug screening. PLoS Negl. Trop. Dis. 2010;4(8):e795. doi: 10.1371/journal.pntd.0000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxer M., Ingram K., Keiser J. Development of an in vitro drug screening assay using Schistosoma haematobium schistosomula. Parasites Vectors. 2012;5:165. doi: 10.1186/1756-3305-5-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R.M. Parasites, people and policy: infectious diseases and the Millennium Development Goals. Trends Ecol. Evol. 2007;22(10):497–503. doi: 10.1016/j.tree.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Molento M.B. Parasite control in the age of drug resistance and changing agricultural practices. Vet. Parasitol. 2009;163(3):229–234. doi: 10.1016/j.vetpar.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Morel C.M. Neglected diseases: under-funded research and inadequate health interventions. Can we change this reality? EMBO Rep. 2003;4(Spec. no):S35–S38. doi: 10.1038/sj.embor.embor851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paveley R.A., Mansour N.R., Hallyburton I., Bleicher L.S., Benn A.E., Mikic I., Guidi A., Gilbert I.H., Hopkins A.L., Bickle Q.D. Whole organism high-content screening by label-free, image-based Bayesian classification for parasitic diseases. PLoS Negl. Trop. Dis. 2012;6(7):e1762. doi: 10.1371/journal.pntd.0001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak E., Chalmers I.W., Hoffmann K.F. Development and validation of a quantitative, high-throughput, fluorescent-based bioassay to detect schistosoma viability. PLoS Negl. Trop. Dis. 2010;4(7):e759. doi: 10.1371/journal.pntd.0000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak E., Hoffmann K.F. Cross-disciplinary approaches for measuring parasitic helminth viability and phenotype. Ann. Acad. Bras. Cienc. 2011;83(2):649–662. doi: 10.1590/s0001-37652011000200024. [DOI] [PubMed] [Google Scholar]
- Rinaldi G., Suttiprapa S., Tort J.F., Folley A.E., Skinner D.E., Brindley P.J. An antibiotic selection marker for schistosome transgenesis. Int. J. Parasitol. 2012;42(1):123–130. doi: 10.1016/j.ijpara.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde C.B., Zeng F., Gonzalez-Rubio R., Angel M., Yanik M.F. Microfluidic system for on-chip high-throughput whole-animal sorting and screening at subcellular resolution. Proc. Natl. Acad. Sci. U. S. A. 2007;104(35):13891–13895. doi: 10.1073/pnas.0706513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereisen A., Tritten L., Keiser J. Exploration of novel in vitro assays to study drugs against Trichuris spp. J. Microbiol. Methods. 2011;87(2):169–175. doi: 10.1016/j.mimet.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Smout M.J., Kotze A.C., Mccarthy J.S., Loukas A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl. Trop. Dis. 2010;4(11):e885. doi: 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout M.J., Mulvenna J.P., Jones M.K., Loukas A. Expression, refolding and purification of Ov-GRN-1, a granulin-like growth factor from the carcinogenic liver fluke, that causes proliferation of mammalian host cells. Protein Expr. Purif. 2011;79(2):263–270. doi: 10.1016/j.pep.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Sommerfeld J., Oduola A.M. Health-related biotechnologies for infectious disease control in Africa: Ethical, Legal and Social Implications (ELSI) of transfer and development. Afr. J. Med. Med. Sci. 2007;36(Suppl. 1–5) [PubMed] [Google Scholar]
- Tong J., Rezai P., Salam S., Selvaganapathy P.R., Gupta B.P. Microfluidic-based electrotaxis for on-demand quantitative analysis of Caenorhabditis elegans' locomotion. J. Vis. Exp. 2013;(75):e50226. doi: 10.3791/50226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritten L., Braissant O., Keiser J. Comparison of novel and existing tools for studying drug sensitivity against the hookworm Ancylostoma ceylanicum in vitro. Parasitology. 2012;139(3):348–357. doi: 10.1017/S0031182011001934. [DOI] [PubMed] [Google Scholar]
- Vistejnova L., Dvorakova J., Hasova M., Muthny T., Velebny V., Soucek K., Kubala L. The comparison of impedance-based method of cell proliferation monitoring with commonly used metabolic-based techniques. Neuro Endocrinol. Lett. 2009;30(Suppl. 1):121–127. [PubMed] [Google Scholar]
- White A.G., Lees B., Kao H.L., Cipriani P.G., Munarriz E., Paaby A.B., Erickson K., Guzman S., Rattanakorn K., Sontag E., Geiger D., Gunsalus K.C., Piano F. DevStaR: high-throughput quantification of C. elegans developmental stages. IEEE Trans. Med. Imaging. 2013;32(10):1791–1803. doi: 10.1109/TMI.2013.2265092. [DOI] [PubMed] [Google Scholar]
- You H., Mcmanus D.P., Hu W., Smout M.J., Brindley P.J., Gobert G.N. Transcriptional responses of in vivo praziquantel exposure in schistosomes identifies a functional role for calcium signalling pathway member CamKII. PLoS Pathog. 2013;9(3):e1003254. doi: 10.1371/journal.ppat.1003254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeraik A.E., Galkin V.E., Rinaldi G., Garratt R.C., Smout M.J., Loukas A., Mann V.H., Araujo A.P., Demarco R., Brindley P.J. Reversible paralysis of Schistosoma mansoni by forchlorfenuron, a phenylurea cytokinin that affects septins. Int. J. Parasitol. 2014;44(8):523–531. doi: 10.1016/j.ijpara.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.D. A new method with flexible and balanced control of false negatives and false positives for hit selection in RNA interference high-throughput screening assays. J. Biomol. Screen. 2007;12(5):645–655. doi: 10.1177/1087057107300645. [DOI] [PubMed] [Google Scholar]
- Zhang X.D. Illustration of SSMD, z score, SSMD*, z* score, and t statistic for hit selection in RNAi high-throughput screens. J. Biomol. Screen. 2011;16(7):775–785. doi: 10.1177/1087057111405851. [DOI] [PubMed] [Google Scholar]
- Zhang X.D., Lacson R., Yang R., Marine S.D., Mccampbell A., Toolan D.M., Hare T.R., Kajdas J., Berger J.P., Holder D.J., Heyse J.F., Ferrer M. The use of SSMD-based false discovery and false nondiscovery rates in genome-scale RNAi screens. J. Biomol. Screen. 2010;15(9):1123–1131. doi: 10.1177/1087057110381919. [DOI] [PubMed] [Google Scholar]