Summary
A 21-year-old woman presented with amenorrhea, bilateral galactorrhea and fatigue. Visual acuity and visual fields were normal. Laboratory examination demonstrated hyperprolactinemia. Magnetic resonance imaging (MRI) of the pituitary showed a 19×17×12-mm sellar mass with supra- and parasellar extension, causing compression of the pituitary stalk and optic chiasm. Further examinations confirmed mild hyperprolactinemia, strongly elevated TSH (>500 mU/l), low free thyroxine (FT4), hypogonadotropic hypogonadism and secondary adrenal insufficiency. Hydrocortisone and l-T4 replacement therapy was started. Three months later, the galactorrhea had disappeared, thyroid function was normalized and MRI revealed regression of the pituitary enlargement, confirming the diagnosis of pituitary hyperplasia (PH) due to primary hypothyroidism. Subsequently, the menstrual cycle returned and the hypocortisolism normalized. This case demonstrates that severe primary hypothyroidism may have an unusual presentation and should be considered in the differential diagnosis of pituitary enlargement associated with moderate hyperprolactinemia.
Learning points
One should always try to find one etiology as the common cause of all the clinical findings in a pathologic process.
Amenorrhea, galactorrhea and fatigue may be the only presenting clinical manifestations of primary hypothyroidism.
Not every patient with galactorrhea, hyperprolactinemia and a pituitary mass has a prolactinoma.
Primary hypothyroidism should always be considered in the differential diagnosis of hyperprolactinemia associated with pituitary enlargement and pituitary hormone(s) deficiency(ies).
When PH due to primary hypothyroidism is suspected, thyroid hormone replacement should be started and only regression of pituitary enlargement on MRI follow-up can confirm the diagnosis.
Examination of thyroid function in patients with a pituitary mass may avoid unnecessary surgery.
Background
Amenorrhea, galactorrhea and hyperprolactinemia associated with pituitary enlargement can be a rare presentation of primary hypothyroidism. Elevated levels of prolactin (PRL) are found in one-third of patients with primary hypothyroidism (1) (2) (3), while amenorrhea and galactorrhea are less frequent (1) (2).
The association between primary hypothyroidism and pituitary gland enlargement was first described in 1851 by Niepce during autopsy on patients with cretinism (4). Several case reports of pituitary hyperplasia (PH) due to primary hypothyroidism in adults (5) (6) (7) (8) (9) (10) (11) and children have been published (12) (13) (14).
Long-standing primary hypothyroidism results in thyrotroph hyperplasia with consequent enlargement of the pituitary gland (15). The lack of negative feedback from thyroxine (T4) leads to elevated levels of TRH that stimulates both pituitary thyrotroph and lactotroph cells (16), subsequently resulting in the increased secretion of thyroid stimulating hormone (TSH) and PRL (17).
According to published work, the frequency of PH in patients with primary hypothyroidism ranges from 25 to 81% (18). A correlation between the magnitude of the increase in size of the sella turcica and the increase in the serum level of TSH has been demonstrated by Yamada et al. (19). More recently, Khawaja et al. (20) have reported the presence of pituitary enlargement in 70% of patients with TSH levels ≥50 μIU/ml, along with a statistically significant positive correlation between the size of the enlarged pituitary gland and TSH levels.
We report a case of a young woman with amenorrhea, galactorrhea and hyperprolactinemia whose MRI revealed a lesion suggestive of a pituitary macroadenoma. Further studies and careful clinical management finally lead to the correct diagnosis and adequate treatment.
Case presentation
A 21-year-old woman was referred to a residential hospital because of secondary amenorrhea and galactorrhea that had started 6 and 2 months earlier respectively. Besides autism, there was no relevant medical history. She had a normal puberty, menarche was at the age of 14 and her menstrual cycle was persistently irregular, for which an oral contraceptive was started in 2007. In 2011, this was discontinued and persistent amenorrhea in combination with bilateral spontaneous galactorrhea were present. The patient also reported fatigue. She had no complaints of visual impairment, headaches, slow behavior, weight gain, hair loss or constipation. There was no history of smoking, alcohol, drug consumption nor the use of medication. Her family history was unremarkable.
On physical examination, BMI was 27.8 kg/m2, arterial blood pressure 104/60 mmHg, heart rate 78 BPM and temperature 36.4 °C. Neck palpation revealed an enlarged right thyroid lobe. Spontaneous bilateral galactorrhea was present. Visual field examination was normal.
Initial evaluation revealed hyperprolactinemia (1830 mIU/l) and a pituitary adenoma was suspected. Therefore, magnetic resonance imaging (MRI) of the pituitary was performed and it showed a 19×17×12-mm sellar mass with suprasellar and parasellar extension (Fig. 1). It was isointense in T1 and T2, with homogeneous enhancement after the administration of gadolinium. The suprasellar extension of the lesion caused compression of the pituitary stalk and optic chiasm. The patient was referred to our pituitary clinic for pituitary surgery.
Figure 1.
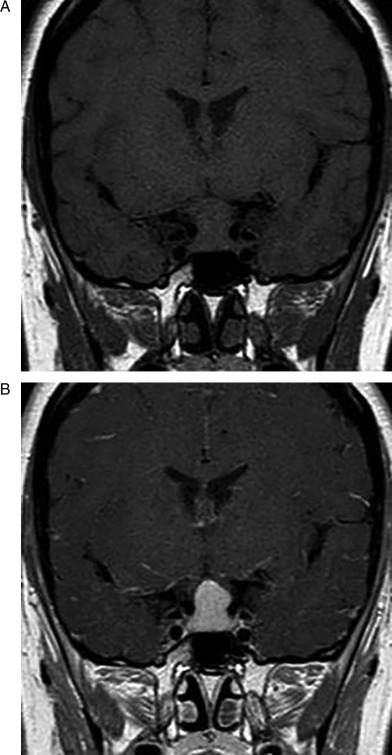
Initial pituitary MRI revealing pituitary gland enlargement. (A) unenhanced coronal image showing an enlarged pituitary gland with supra- and perisellar expansion and compression of the optic chiasm. (B) Homogeneous enhancement of the pituitary lesion after Gd-DTPA.
Investigation
After referral to our clinic, additional studies confirmed slightly elevated PRL levels, strongly elevated TSH and low free T4 (FT4), hypogonadotropic hypogonadism and secondary adrenal insufficiency (Table 1). The thyroid peroxidase antibodies were negative (Table 1).
Table 1.
Initial laboratorial evaluation
| Test (units) | Result (reference range) |
|---|---|
| Prolactin (mIU/l) | 1000 (0–700) |
| TSH (mU/l) | >500 (0.4–4.3) |
| FT4 (pmol/l) | 6.0 (11–25) |
| FSH (U/l) | 5.0 (1.0–8.0) |
| LH (U/l) | 1.0 (2.0–8.0) |
| Estradiol (pmol/l) | 99 |
| IGF1 (nmol/l) | 11 (15–47) |
| Cortisol (nmol/l) | 331 (200–700) |
| Metyrapone stimulation test: 11-deoxycortisol (nmol/l) | 257 (>290) |
| Thyroid peroxidase antibodies (iU/ml) | Negative (<100) |
The differential diagnosis between non-functioning pituitary macroadenoma causing mild hyperprolactinemia due to pituitary stalk compression with concomitant primary hypothyroidism was raised against the rare diagnosis of PH resulting from primary hypothyroidism.
On MRI, compression of the optic chiasm was demonstrated but ophthalmologic evaluation showed no visual field defects and visual acuity was normal.
Three months after the initiation of hormone replacement therapy (see below), hormonal evaluation revealed normalization of both TSH (2.31 mU/l) and FT4 (18.6 pmol/l). Pituitary MRI showed a significant reduction of the sellar mass, measuring 8×8 mm (Fig. 2).
Figure 2.
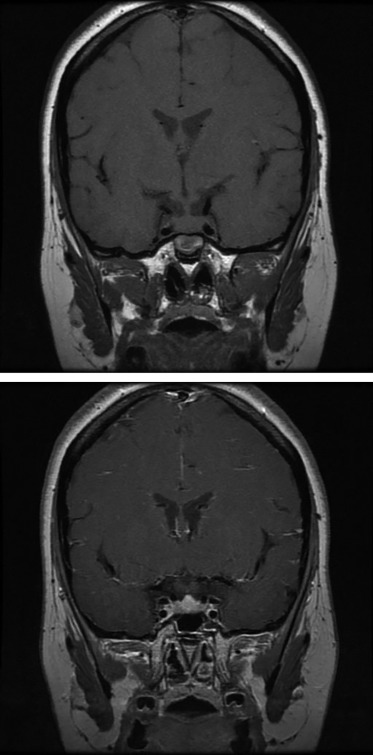
Gd-DTPA enhanced pituitary MRI 3 months after the start of thyroid hormone replacement showing a significant reduction in pituitary size and absent compression of the optic chiasm.
Treatment
Hydrocortisone replacement therapy was started (20 mg/day divided over three doses), 3 days later followed by the start of 50 μg of l-T4, which was progressively increased to 100 μg daily.
Outcome and follow-up
The patient continued treatment with l-T4 and hydrocortisone. On follow-up, 4 months after starting medication, energy levels had increased progressively, galactorrhea completely disappeared and menstrual cycles had resumed. Six months after initiation of therapy, she was biochemically euthyroid (TSH 0.427 mU/l FT4 15.2 pmol/l) while treated with 75 μg l-T4 daily. Meanwhile, hydrocortisone was gradually withdrawn. A metyrapone test demonstrated complete recovery of the pituitary adrenal axis (11-deoxycortisol 537 nmol/l). Currently, she is asymptomatic.
Discussion
This case demonstrates that primary hypothyroidism may present only with amenorrhea, galactorrhea and fatigue. Nevertheless, the presence of autism in our patient might have contributed to the paucity of hypothyroid symptoms. Amenorrhea and galactorrhea apparently result from hyperprolactinemia. Prolactin levels >1087 mIU/l were found in about 10% of patients with primary hypothyroidism (1). On the other hand, the most frequent causes of pathological hyperprolactinemia are pituitary tumors and drugs with dopamine antagonist properties, whereas primary hypothyroidism is a less common cause. However, macroprolactinomas usually present with much higher prolactin levels than those measured in our patient.
Hyperprolactinemia in primary hypothyroidism is caused by several mechanisms. First, the low levels of T4 induce a compensatory increase in TRH secretion from the hypothalamus, which stimulates not only TSH but also PRL synthesis from the anterior pituitary. Moreover, pituitary cells have a decreased sensitivity to the inhibitory action of dopamine possibly at receptor or post-receptor levels (6) (21). On the other hand, 3,5,3' triiodothyronine (T3) was shown to decrease PRL mRNA levels in pituitary cells. Therefore, reduced thyroid hormone levels will lead to more PRL synthesis (8). Also, in hypothyroidism PRL clearance from the circulation is decreased (22). In our patient, an additional mechanism might be involved. The PH caused compression of the pituitary stalk, potentially leading to less dopamine inhibitory action on PRL secretion.
At first presentation, a non-functioning pituitary macroadenoma with concomitant primary hypothyroidism was considered. Despite the advances in imaging techniques, MRI and CT still have low sensitivity to distinguish a pituitary macroadenoma from PH. The MRI characteristics of a macroadenoma include homogeneous enlargement of the gland with a height >10 mm, with or without perisellar extension and deviation of the pituitary stalk. These overlap with the finding of a diffusely enlarged pituitary gland in PH (17). Thus, pituitary imaging alone is not capable of making a definite diagnosis and further examinations are essential.
PH in primary hypothyroidism may be more common than clinically apparent. Long-standing PH may result in irreversible pituitary injury, which can lead to a deficiency in one or more pituitary hormones (23), including hypopituitarism (24). In our patient, ACTH deficiency was transient.
The differential diagnosis of a pituitary lesion and such alterations in pituitary function also include lymphocytic hypophysitis and TSH-producing adenomas. Hypophysitis is a rare diagnosis and can only be histologically confirmed. TSH-producing pituitary adenomas present with inappropriately normal or elevated TSH levels with elevated thyroid hormone levels.
Although there was evidence of compression of the optic chiasm on MRI, our patient did not present with any visual field defects. Surgery in PH has been used for the decompression of the optic chiasm when vision is compromised or in cases where a diagnosis is needed because of progression or absent response of a pituitary mass after the initiation of thyroid hormone replacement (23).
Regression of pituitary enlargement on l-T4 treatment as documented on MRI confirms the diagnosis of PH (2) (16). On follow-up MRI, 85% of patients with PH have shown a decrease in the size of the pituitary gland with thyroid hormone replacement therapy (20). The time of response is variable and varies from 1 week (15) to months (5) (6) (7) (8) (9) (11). In our patient, a MRI response was observed after 3 months, but earlier studies had not been performed.
In conclusion, in a female with amenorrhea, galactorrhea, fatigue and hyperprolactinemia in association with a pituitary enlargement, primary hypothyroidism should be considered and excluded. Follow-up of the pituitary size by MRI with l-T4 replacement is essential for a correct diagnosis, and unnecessary pituitary surgery can be avoided.
Patient consent
Written informed consent was obtained.
Author contribution statement
C P Neves has written this case report during an internship in the Endocrinology Department in Erasmus Medical Center and she has the permission of the physician who was responsible for the patient. E T Massolt is one of the physicians who followed the case while the patient was in the ward. R P Peeters is the head of the Rotterdam Thyroid Center and supervisor of the endocrinology ward at the time our patient was admitted. S J Neggers is the physician who followed the patient after she was discharged from the ward, in the outpatient clinic. W W de Herder is the clinical head of the department of endocrinology where the patient was admitted. He was also the supervisor of the first author's activities during her internship in the Erasmus MC.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1. Honbo KS, van Herle AJ & Kellett KA. 1978. Serum prolactin levels in untreated primary hypothyroidism. American Journal of Medicine 64 782–787. 10.1016/0002-9343(78)90517-X [DOI] [PubMed] [Google Scholar]
- 2. Poretsky L, Garber J & Kleefield J. 1986. Primary amenorrhea and pseudoprolactinoma in a patient with primary hypothyroidism. Reversal of clinical, biochemical, and radiologic abnormalities with levothyroxine. American Journal of Medicine 81 180–182. 10.1016/0002-9343(86)90207-X [DOI] [PubMed] [Google Scholar]
- 3. Edwards CR, Forsyth IA & Besser GM. 1971. Amenorrhea, galactorrhea and primary hypothyroidism with high circulating levels of prolactin. BMJ 3 462–464. 10.1136/bmj.3.5772.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niepce B. 1851 Traité du goitre et du crétinisme, dans le bassin de l'Isère. Baillière, Paris.
- 5. Groff TR, Shulkin BL, Utiger RD & Talbert LM. 1984. Amenorrhea-galactorrhea, hyperprolactinemia, and suprasellar pituitary enlargement as presenting features of primary hypothyroidism. Obstetrics and Gynecology 63 86–88S. [PubMed] [Google Scholar]
- 6. Grubb MR, Chakeres D & Malarkey WB. 1987. Patients with primary hypothyroidism presenting as prolactinomas. American Journal of Medicine 83 765–769. 10.1016/0002-9343(87)90911-9 [DOI] [PubMed] [Google Scholar]
- 7. Wolansky LJ, Leavitt GD, Elias BJ, Lee HJ, Dasmahapatra A & Byrne W. 1996. MRI of pituitary hyperplasia in hypothyroidism. Neuroradiology 38 50–52. 10.1007/BF00593219 [DOI] [PubMed] [Google Scholar]
- 8. Kroese JM, Grootendorst AF & Schelfhout LJ. 2004. Postpartum amenorrhoea–galactorrhoea associated with hyperprolactinaemia and pituitary enlargement in primary hypothyroidism. The Netherlands Journal of Medicine 62 28–30. [PubMed] [Google Scholar]
- 9. Betônico CC, Rodrigues R, Mendonça SC & Jorge PT. 2004. Primary hypothyroidism mimicking pituitary macroadenoma. Arquivos Brasileiros de Endocrinologia e Metabologia 48 423–426. [DOI] [PubMed] [Google Scholar]
- 10. Joshi AS & Woolf PD. 2005. Pituitary hyperplasia secondary to primary hypothyroidism: a case report and review of the literature. Pituitary 8 99–103. 10.1007/s11102-005-3281-8 [DOI] [PubMed] [Google Scholar]
- 11. Passeri E, Tufano A, Locatelli M, Lania AG, Ambrosi B & Corbetta S. 2011. Large pituitary hyperplasia in severe primary hypothyroidism. Journal of Clinical Endocrinology and Metabolism 96 22–23. 10.1210/jc.2010-2011 [DOI] [PubMed] [Google Scholar]
- 12. Eom KS, See-Sung C, Kim JD, Kim JM & Kim TY. 2008. Primary hypothyroidism mimicking a pituitary macro adenoma: regression after thyroid hormone replacement therapy. Pediatric Radiology 39 164–167. 10.1007/s00247-008-1012-9 [DOI] [PubMed] [Google Scholar]
- 13. Franceschi R, Rozzanigo U, Failo R, Bellizzi M & Di Palma A. 2011. Pituitary hyperplasia secondary to acquired hypothyroidism: case report. Italian Journal of Pediatrics 37 1–15. 10.1186/1824-7288-37-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papakonstantinou O, Bitsori M, Mamoulakis D, Bakantaki A, Papadaki E & Gourtsoyiannis N. 2000. MR imaging of pituitary hyperplasia in a child with growth arrest and primary hypothyroidism. European Radiology 10 516–518. 10.1007/s003300050087 [DOI] [PubMed] [Google Scholar]
- 15. Sarlis N, Brucker-Davis F, Doppman J & Skarulis M. 1997. MRI-demonstrable regression of a pituitary mass in a case of primary hypothyroidism after a week of acute thyroid hormone therapy. Journal of Clinical Endocrinology and Metabolism 82 808–811. 10.1210/jcem.82.3.3796 [DOI] [PubMed] [Google Scholar]
- 16. Jawadi MH, Ballcnoff LB, Stears JC & Katz FH. 1978. Primary hypothyroidism and pituitary enlargement. Radiological evidence of pituitary regression. Archives of Internal Medicine 138 1555–1557. 10.1001/archinte.1978.03630350079021 [DOI] [PubMed] [Google Scholar]
- 17. Valenta L, Tamkin J, Sostrin R, Elias A & Eisenberg H. 1983. Regression of a pituitary adenoma following levothyroxine therapy of primary hypothyroidism. Fertility and Sterility 40 389–392. [DOI] [PubMed] [Google Scholar]
- 18. Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC & Weintraub BD. 1996. Thyrotropin-secreting pituitary tumors. Endocrine Reviews 17 610–638. [DOI] [PubMed] [Google Scholar]
- 19. Yamada T, Tsukui T, Ikejiri K, Yukimura Y & Kotani M. 1976. Volume of sella turcica in normal subjects and in patients with primary hypothyroidism and hyperthyroidism. Journal of Clinical Endocrinology and Metabolism 42 817–822. 10.1210/jcem-42-5-817 [DOI] [PubMed] [Google Scholar]
- 20. Khawaja NM, Taher BM, Taher BM, Barham ME, Naser AA, Hadidy AM, Ahmad AT, Hamamy HA, Yaghi NA & Ajlouni KM. 2006. Pituitary enlargement in patients with primary hypothyroidism. Endocrine Practice 12 29–34. 10.4158/EP.12.1.29 [DOI] [PubMed] [Google Scholar]
- 21. Foord SM, Peters JR, Dieguez C, Jasani B, Hall R & Scanlon MF. 1984. Hypothyroid pituitary cells in culture: an analysis of thyrotrophin and prolactin response to dopamine (DA) and DA receptor binding. Endocrinology 115 407–415. 10.1210/endo-115-1-407 [DOI] [PubMed] [Google Scholar]
- 22. Cave WT Jr & Paul MA. 1980. Effects of altered thyroid function on plasma prolactin clearance. Endocrinology 107 85–91. 10.1210/endo-107-1-85 [DOI] [PubMed] [Google Scholar]
- 23. Şimşek E, Şimşek T, Savaş-Erdeve S, Erdoğmuş B & Döşoğlu M. 2009. Pituitary hyperplasia mimicking pituitary macro adenoma in two adolescent patients with long-standing primary hypothyroidism: case reports and review of literature. Turkish Journal of Pediatrics 51 624–630. [PubMed] [Google Scholar]
- 24. Dutta D, Maisnam I, Ghosh S, Mukhopadhyay P & Mukhopadhyay S. 2012. Panhypopituitarism with empty sella a sequel of pituitary hyperplasia due to chronic primary hypothyroidism. Indian Journal of Endocrinology and Metabolism 16 S282–S284. 10.4103/2230-8210.103041 [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a