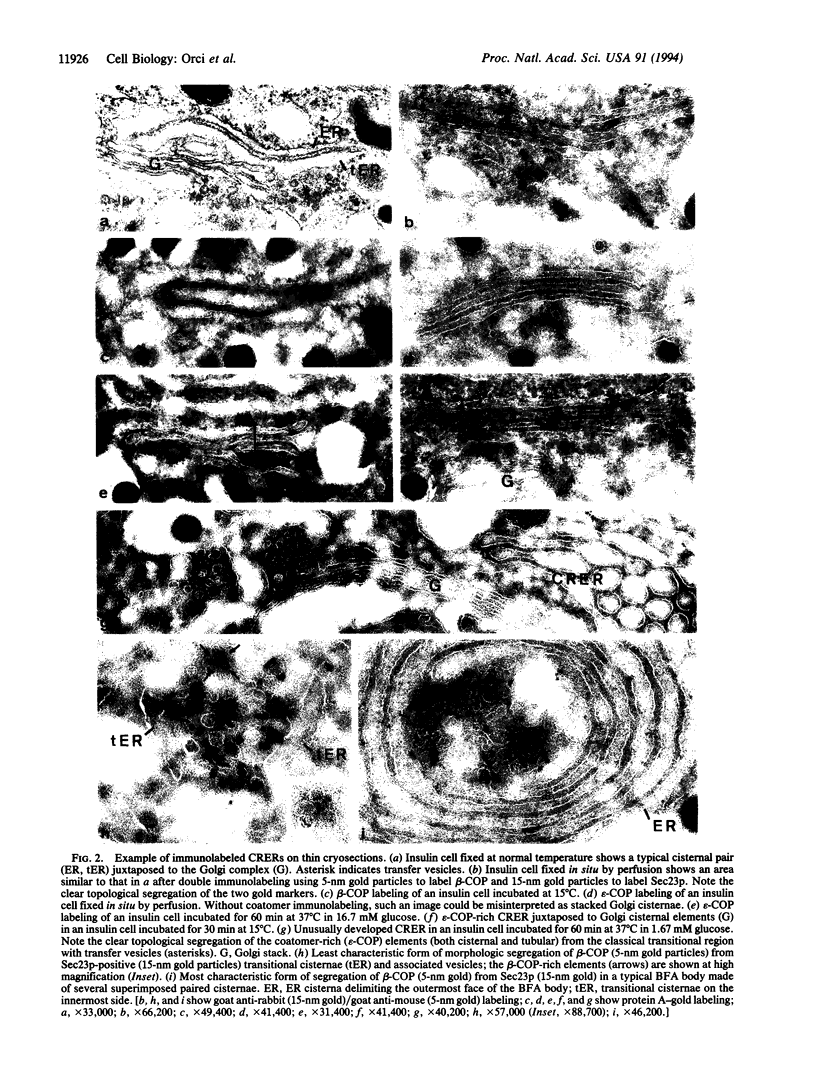
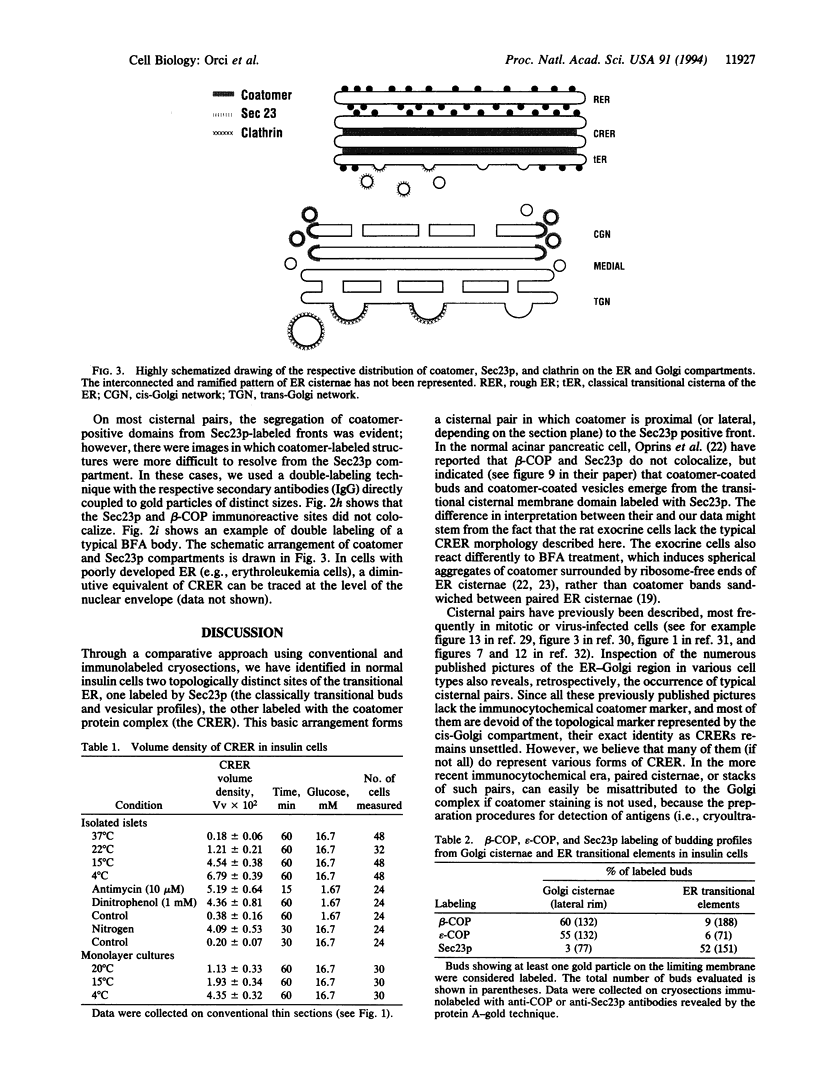
Abstract
We identify in normal cells the existence of two distinct sites of the transitional endoplasmic reticulum (ER), one housing the Sec23p protein complex (the classical transitional element), the other the coatomer protein complex (the coatomer-rich ER). Experimental conditions that reduce transport from the ER to the Golgi complex lead to the overexpression of this newly defined coatomer-rich ER.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M. F., Ravazzola M., Amherdt M., Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994 Jun 17;77(6):895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Cosson P., Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994 Mar 18;263(5153):1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Duden R., Griffiths G., Frank R., Argos P., Kreis T. E. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991 Feb 8;64(3):649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Hara-Kuge S., Kuge O., Orci L., Amherdt M., Ravazzola M., Wieland F. T., Rothman J. E. En bloc incorporation of coatomer subunits during the assembly of COP-coated vesicles. J Cell Biol. 1994 Mar;124(6):883–892. doi: 10.1083/jcb.124.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks L. C., McCaffery M., Palade G. E., Farquhar M. G. Disruption of endoplasmic reticulum to Golgi transport leads to the accumulation of large aggregates containing beta-COP in pancreatic acinar cells. Mol Biol Cell. 1993 Apr;4(4):413–424. doi: 10.1091/mbc.4.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi M., Kreis T., Schekman R. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992 Dec 10;360(6404):603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge O., Hara-Kuge S., Orci L., Ravazzola M., Amherdt M., Tanigawa G., Wieland F. T., Rothman J. E. zeta-COP, a subunit of coatomer, is required for COP-coated vesicle assembly. J Cell Biol. 1993 Dec;123(6 Pt 2):1727–1734. doi: 10.1083/jcb.123.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V., Serafini T., Orci L., Shepherd J. C., Rothman J. E. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989 Jul 28;58(2):329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Oprins A., Duden R., Kreis T. E., Geuze H. J., Slot J. W. Beta-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993 Apr;121(1):49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Glick B. S., Rothman J. E. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986 Jul 18;46(2):171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Orci L., Like A. A., Amherdt M., Blondel B., Kanazawa Y., Marliss E. B., Lambert A. E., Wollheim C. B., Renold A. E. Monolayer cell culture of neonatal rat pancreas: an ultrastructural and biochemical study of functioning endocrine cells. J Ultrastruct Res. 1973 May;43(3):270–297. doi: 10.1016/s0022-5320(73)80039-5. [DOI] [PubMed] [Google Scholar]
- Orci L., Malhotra V., Amherdt M., Serafini T., Rothman J. E. Dissection of a single round of vesicular transport: sequential intermediates for intercisternal movement in the Golgi stack. Cell. 1989 Feb 10;56(3):357–368. doi: 10.1016/0092-8674(89)90239-0. [DOI] [PubMed] [Google Scholar]
- Orci L., Perrelet A., Ravazzola M., Wieland F. T., Schekman R., Rothman J. E. "BFA bodies": a subcompartment of the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11089–11093. doi: 10.1073/pnas.90.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Meda P., Holcomb C., Moore H. P., Hicke L., Schekman R. Mammalian Sec23p homologue is restricted to the endoplasmic reticulum transitional cytoplasm. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8611–8615. doi: 10.1073/pnas.88.19.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcl L., Palmer D. J., Amherdt M., Rothman J. E. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993 Aug 19;364(6439):732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Orci L., Tani K., Amherdt M., Ravazzola M., Elazar Z., Rothman J. E. Stepwise assembly of functionally active transport vesicles. Cell. 1993 Dec 3;75(5):1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Palisano J. R. The absence of confronting cisternae following thymidine-induced synchronization in HeLa cells. Cell Biol Int. 1993 Jul;17(7):653–664. doi: 10.1006/cbir.1993.1115. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Robinson M. S. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Pepperkok R., Scheel J., Horstmann H., Hauri H. P., Griffiths G., Kreis T. E. Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993 Jul 16;74(1):71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Peter F., Plutner H., Zhu H., Kreis T. E., Balch W. E. Beta-COP is essential for transport of protein from the endoplasmic reticulum to the Golgi in vitro. J Cell Biol. 1993 Sep;122(6):1155–1167. doi: 10.1083/jcb.122.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryer N. K., Wuestehube L. J., Schekman R. Vesicle-mediated protein sorting. Annu Rev Biochem. 1992;61:471–516. doi: 10.1146/annurev.bi.61.070192.002351. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Sato S., Murphy G. F., Harrist T. J. The genesis of paired cisternae during the mitotic cycle. Am J Pathol. 1982 May;107(2):150–160. [PMC free article] [PubMed] [Google Scholar]
- Serafini T., Stenbeck G., Brecht A., Lottspeich F., Orci L., Rothman J. E., Wieland F. T. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature. 1991 Jan 17;349(6306):215–220. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- Stenbeck G., Harter C., Brecht A., Herrmann D., Lottspeich F., Orci L., Wieland F. T. beta'-COP, a novel subunit of coatomer. EMBO J. 1993 Jul;12(7):2841–2845. doi: 10.1002/j.1460-2075.1993.tb05945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A., Warren G. Laminated cisternae of the rough endoplasmic reticulum induced by coronavirus MHV-A59 infection. Eur J Cell Biol. 1985 Jan;36(1):108–115. [PubMed] [Google Scholar]
- Walter R. J., Tandler B. Viruses and annulate lamellae in Friend erythroleukemia cells. J Submicrosc Cytol Pathol. 1989 Jan;21(1):93–101. [PubMed] [Google Scholar]
- Waters M. G., Serafini T., Rothman J. E. 'Coatomer': a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991 Jan 17;349(6306):248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]