Abstract
This study reports a synthetic polymer functionalized with catechol groups as dental adhesives. We hypothesize that a catechol-functionalized polymer functions as a dental adhesive for wet dentin surfaces, potentially eliminating the complications associated with saliva contamination. We prepared a random copolymer containing catechol and methoxyethyl groups in the side chains. The mechanical and adhesive properties of the polymer to dentin surface in the presence of water and salivary components were determined. It was found that the new polymer combined with an Fe3+ additive improved bond strength of a commercial dental adhesive to artificial saliva contaminated dentin surface as compared to a control sample without the polymer. Histological analysis of the bonding structures showed no leakage pattern, probably due to the formation of Fe–catechol complexes, which reinforce the bonding structures. Cytotoxicity test showed that the polymers did not inhibit human gingival fibroblast cells proliferation. Results from this study suggest a potential to reduce failure of dental restorations due to saliva contamination using catechol-functionalized polymers as dental adhesives.
Introduction
Dental adhesives have been used widely in dental practice to improve the bonding quality between composite resin restorations and dentin, preventing bonding failure1 and reducing the risk of secondary caries2,3 and hypersensitivity.4 In general, dental adhesives are synthetic resins made from hydrophilic monomers, which provide better wettability to the relatively hydrophilic surface of dentin.5,6 Dentin consists primarily of hydroxyapatite, organic components such as collagen and water.7,8 Adhesive monomers are applied to the dentin surface and polymerized in situ, generating bonding interfaces consisting of an adhesive resin layer and a hybrid layer reinforced with collagen fibers (Figure 1). The surface of the adhesive resin layer provides chemical functionality (polymerizable vinyl groups) for the bonding to dental composites.9,10
Figure 1.
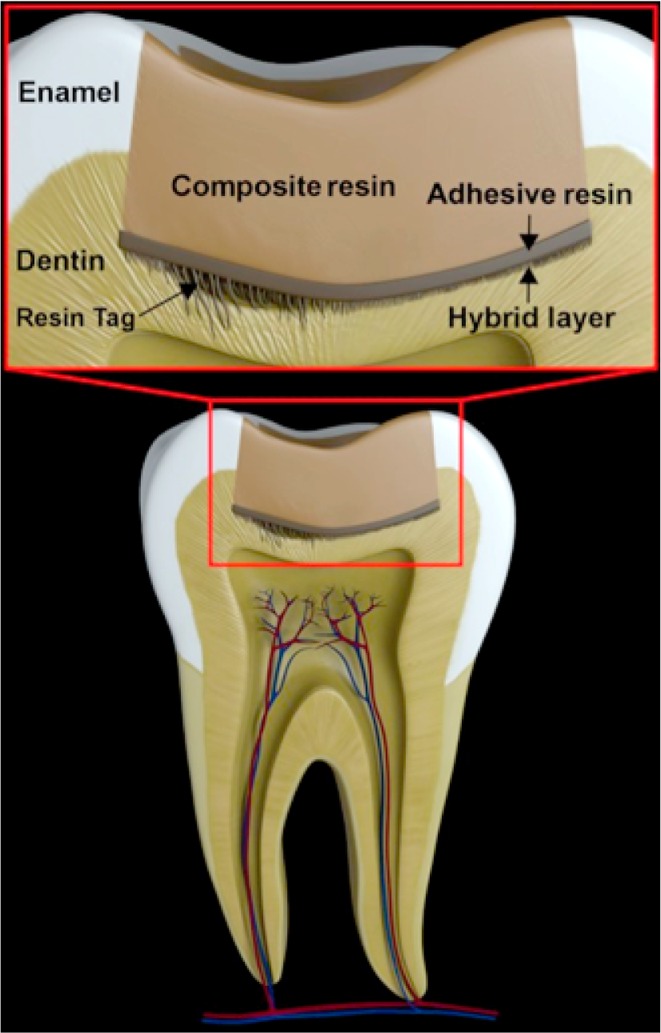
Schematic representation of the composite resin–dentin interface. Resin tags, the hybrid layer, and microleakage could be identified in the interface between dentin and adhesive resin.
The primary adhesion mechanism of the adhesive resin to the dentin surface is micromechanical by the interlocking of adhesive resin in rough microstructures of the dentin surface generated by phosphoric acid etching.9,11 The adhesive resin also penetrates into the dentin tubules generating “tags” of resins for mechanical retention (Figure 1). Furthermore, some chemical bonding of functional groups of adhesive monomers including phosphate and carboxylic groups to the organic or inorganic constituents of the dentin has been reported.9
In the bonding procedure, the dentin surface needs to be kept relatively dry and free from saliva contamination. Excessive water prevents the penetration of adhesive resin into the dentin surface microstructure reducing the mechanical bond strength.12 High water content in the adhesive may also cause precipitation or aggregation of resin polymers, compromising its mechanical strength. After saliva contamination, salivary components such as glycoproteins and mucins accumulate on the dentin surface, preventing the intimate interaction of monomers and dentin structure13 and also inhibiting polymerization chemical reactions.14
Several other factors can cause failure of the bonding between the adhesive resin and the dentin substrate.15 Setting shrinkage of resin composite restorations causes mechanical stress to the adhesion layer that could result in the breakage of adhesive resin. Structural defects reduce the bonding strength of adhesives, which may cause bond failure of restorations. Gaps between dentin surface and adhesive resin create the so-called microleakage patterns that act as channels for oral fluids and oral bacteria and nutrients, increasing the risk of secondary caries and dentinal sensitivity. Additionally, percolation of oral fluids accompanied by the shrinkage of restorations in response to temperature changes cause movement of fluid inside the dentinal tubules, which may result in hypersensitivity.
Accordingly, isolation of the area to be bonded from oral fluids is essential for high bonding quality. However, preventing oral fluid contamination completely under clinical conditions is frequently difficult because of the natural wetness of the oral environment. Development of dental adhesives that can effectively adhere to contaminated dentinal surfaces and provide adequate bond strength is of great interest and could improve dramatically the bonding quality of dental adhesives reducing failure of dental restorations.
This study investigated the potential of a new polymer functionalized with catechol groups as a dental adhesive, with an emphasis on adhesion to saliva-contaminated wet dentin surfaces. This synthetic polymer mimics mussel adhesive proteins that enable mussels to anchor to a variety of wet surfaces.16,17 The catechol groups of adhesion proteins displace tightly bound water molecules from substrates and form hydrogen bonding with surfaces such as titanium dioxide.16 The catechol groups also undergo cross-linking or polymerization, which immobilize proteins on substrate surfaces. In addition, the strong chelation of catechol groups with metal ions and metal oxides provides strong cross-linking effect for their adhesive capability.18−21 The unique properties of catechol groups and derivatives have been previously used to prepare new functional adhesive and coating materials17,22 including, for example, adhesives polymers,23−25 bone adhesives,26 hydrogel-based adhesives,27 coatings on yeast cells,28 and antifouling coatings.29,30 Catechol adsorbs onto hydroxyapatite more readily than other agents such as alcohol, amines, and carboxylic acids.31 Accordingly, we hypothesized that a catechol-functionalized polymer can function as a dental adhesive for wet dentin surfaces, potentially eliminating the complications associated with saliva contamination. It has been previously reported that plant-based polymers with catechol groups showed a good adhesive property to dry dentin surfaces in lap shear adhesion testing.32 To the best of our knowledge, our study is the first report of the bonding performance of a synthetic mimic of mussel adhesive proteins to saliva-contaminated wet dentin surfaces.
For the investigation, we prepared a random copolymer containing catechol and methoxyethyl groups in the side chains as previously reported by Lee et al.25 This polymer structure represents a simple model with only the essential components of the catechol functionality. We evaluated the mechanical and adhesive properties of the synthesized polymer to the dentinal surface in the presence of water and salivary components. The structures of bonding layers were further examined by hematoxylin and eosin (H&E) staining and scanning electron microscopy. The cytotoxicity of the polymer-coated surfaces was also evaluated.
Materials and Methods
Materials
t-Butyldimethylsilyl (TBDMS) ethers (Aldrich, >98%), triethylamine (Et3N; Acros organics, >98%), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU; Acros organics, purity >95%), dichloromethane (Fisher, >99.5%), acetonitrile (Fisher, >99.5%), toluene (Aldrich, >99%), diethyl ether (Aldrich, >98%), hexane (Aldrich, >99%), 2,2′-azobis(isobutyronitrile) (AIBN; Sigma, >98%), and 3-hydroxytyramine hydrochloride (dopamine; Acros organics purity >98%) were used without purification. Methacryloyl chloride (MEA; Acros organics, Inc., U.S.A.; >95%) were purified by distillation over calcium hydride before use. Water used in this work was deionized water from a Milli-Q (18 MΩ·cm) system. Commercial adhesives (BeautiBondR, Shofu Dental corp., Japan) and (Scotchbond Multi-PurposeR, 3 M ESPE, MN, U.S.A.) were purchased. The human gingival fibroblast cells were obtained from ATCC: hGF-1 (ATCC CRL2014). Porcine gastric mucin powder (American Laboratories, Inc. Omaha NE 68127, lot # 01490543, mucin content 68.5%) was purchased.
Synthesis of Catechol-Functionalized Polymer
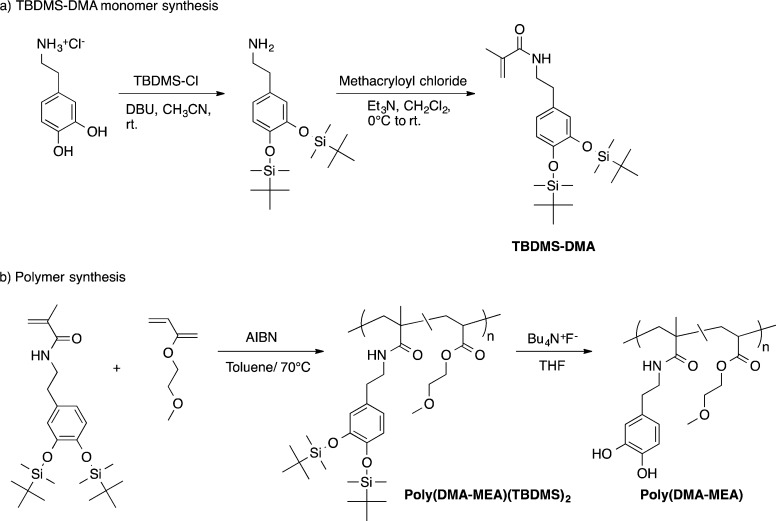
The hydroxyl groups of 3-hydroxytyramine were protected by reacting with t-butyldimethylsilyl (TBDMS) chloride to give TBDMS-dopamine according to the literature procedure33,34 (Figure 4). t-Butyldimethylsilyl chloride (6.25 g, 41.5 mmol) was dissolved in acetonitrile (50 mL) and bubbled by nitrogen gas for 20 min. After cooling this solution in an ice bath, 3-hydroxytyramine hydrochloride (2.72 g, 14.3 mmol) was added. Then, DBU (6.5 mL, 44 mmol) was added to the reaction mixture dropwise. The reaction mixture was stirred for 4 h in an ice bath and kept for 20 h at room temperature. The solution volume was reduced to 20 mL under reduce pressure using a rotary evaporator. The resultant white slurry product was treated twice with cold chloroform (50 mL) at 0 °C. The resulting TBDMS-dopamine solid was collected by vacuum filtration.
Figure 4.
Synthesis of catechol-functionalized methacrylate random copolymer. TBDMS-Cl, t-butyldimethylsiloxy chloride; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; AIBN, 2,2′-azobis(2-methylpropionitrile); DMA, dopamine-methacrylate; MEA, 2-methoxyetheyl acrylate.
To synthesize TBDMS-dopamine methacrylate (TBDMS-DMA) monomer, methacryloyl chloride (0.46 g, 4.40 mmol) was added to TBDMS-dopamine (1.13 g, 2.96 mmol) in dichloromethane (2.5 mL) containing triethylamine (0.4 g, 4.0 mmol) according to the previously described method with modification.35 The product was collected by precipitation.
Protected poly(DMA-MEA) was prepared by free radical polymerization of TBDMS-DMA. In a reaction flask, TBDMS-DMA (0.31 g, 0.69 mmol) with 2-methoxyetheyl acrylate (MEA; 0.41 g, 3.2 mmol), initiator 2,2′-azobis(isobutyronitrile) (AIBN; 66 mg, 0.40 mmol) are mixed in toluene (5 mL). The reaction mixture was heated at 65 °C for 12 h. The solvent was removed under reduced pressure, and the crude polymer was precipitated in hexane to remove unreacted monomers. After removing the solvent, the resultant was dissolved in methanol, and lyophilized obtaining a sticky paste. Yield: 81%. The molecular weight of the protected polymer was determined by gel permeation chromatography (Waters GPC with HT-4, HT-3, and HT-2 columns) in THF using polystyrene standards (Mn = 40800, Mw = 73900, PDI = 1.81). The polymer was characterized by 1H nuclear magnetic resonance spectroscopy (Varian 400 MHz, CD3OD) (Figure S1 for 1H NMR spectrum and assignments). The polymer contained 13 mol % of TBDMS-DMA unit, which was determined by comparing the integrated intensities of the 1H NMR resonances from the side chain of MEA unit relative to the phenyl groups of TBDMS-DMA.
The protecting TBDMS groups for hydroxyl group of polymer were removed using tetrabutylammonium fluoride (TBAF). A solution mixture containing the TBDMS-protected polymer (catechol = 1 mM) and TBAF (5 mM) in THF was stirred for 30 min, and the precipitate was collected by centrifugation for 5 min. The remaining colorless solid was washed three times with THF and dried under reduced pressure. The resultant polymer was characterized by 1H NMR (Varian 400 MHz, DMSO-d6; Figure S2 for 1H NMR spectrum and assignments) The polymer contained 15 mol % of DMA unit, which was determined by comparing the integrated intensities of the 1H NMR resonances from the side chain of MEA unit relative to the phenyl groups of DMA. The 1H NMR indicates that the resultant polymer contains 30 wt % of TBAF in the total amount of sample, which was used without further purification.
Preparation of Polymer Solution, Artificial Saliva, BSA and Fe3+ Solution
Polymer stock solution was prepared by dissolving the poly(DMA-MEA) (200 mg) in methanol (1 mL) or a mixture of deionized water and MeOH (1:4) to give a concentration of 200 mg/mL, which corresponds to a catechol concentration of 5 mmol. The methanol solution was used for the lap shear bond strength experiment for examining the effect of saliva on the strength and the water/methanol mixture solution was used for the lap shear bond strength for the effect of water on the strength and microtooth bond strength experiment. Artificial saliva (pH 7) consisted of purified water, sodium chloride (6.5 mM), calcium chloride (1.5 mM), potassium phosphate (5.4 mM), and potassium chloride (15.0 mM).36 To this artificial saliva (1 L), mucin powder (2.2 g) was added. For preparation of solutions containing ferric ions, Fe(NO3)3 was dissolved in deionized water or artificial saliva (80 mM). Bovine serum albumin (BSA) solution (35%, Sigma-Aldrich, Co., U.S.A.) was used as received.
Lap Shear Bond Strength Experiment (L-SBS)
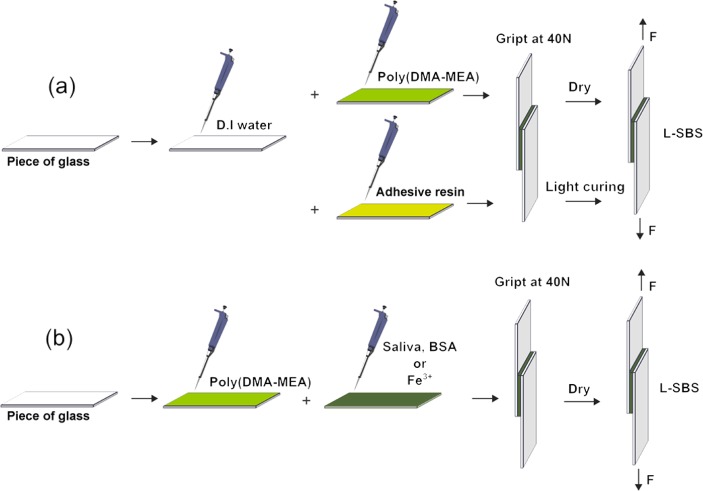
A microscope glass slide was cut to 5 mm × 20 mm × 1 mm using a cutting knife. The cut edge was polished with 400-grit silicon carbide paper to fabricate smooth and parallel sides. The surface to be used for the bonding experiment was cleaned with ethanol and acetone in an ultrasonic chamber for 15 min, rinsed with water, and dried under high vacuum for 12 h prior to the bonding procedures. The dimension of each stick was determined using digital calipers with resolution of 10 μm. Excess polymer at the margins of glass slides was removed with methanol. To evaluate the effect of mixing ratio with water, deionized water (0.5–4 μL) was dropped on the glass surface first, then the polymer solution in a water/methanol mixture (1:4; 2 μL) solution were mixed on the glass for 5 s by pipetting. Another glass substrate was then placed over the first glass to obtain a bonding area of 0.25 cm2 (Figure 2a; see Table S1 in Supporting Information for the conditions).
Figure 2.
Schematic representation of the lap shear bond strength experiment. (a) Effect of water and (b) additives (saliva, BSA, or Fe3+) on the bonding strength of poly(DMA-MEA). The bonding area is 0.25 cm2. The samples with commercially available adhesive resins were light-cured for 20 s with curing light.
To evaluate the effect of the additive, polymer solution in methanol (4 μL) was dropped on the glass surface first. The amount of the polymer solution was increased from 2 to 4 μL to increase the shear bond strength of polymer so that the differences in the shear bond strength of tested samples in different conditions are substantial, and thus the effect of salivary components and additives on the strength is clear, facilitating the data analysis. To the glass surfaces with the polymer solution, the artificial saliva, BSA (35% in water), or Fe3+ (80 mM) solution (2 μL) was added, depending on the group. Solutions were mixed on the glass for 5 s by pipetting and another glass substrate was then placed over the first glass to obtain a bonding area of 0.25 cm2 (Figure 2b; see Table S2 in Supporting Information for detailed information on the conditions).
Each sample was tightened using a binder clip with a maximum binding force of 40 N for 2 mm thickness of two glass slides. The bonded glass samples were cured under high vacuum at room temperature for 72 h. A commercially available adhesive resin (Scotchbond Multi-PurposeR, 3 M ESPE, MN, U.S.A.) was also tested as a control. The adhesive resin agent was diluted with methanol to give the same concentration of 200 mg/mL with the poly(DMA-MEA) solution. Testing of the control adhesive as described above with light-curing for 20 s with a curing light (Ivoclar vivadent AG, Schaan, Liechtenstein). The light intensity of the curing light was verified to be over a 400 mW/cm2 before curing. The glass surfaces were not acid-etched before use. After curing of the adhesive, samples were dried under high vacuum for 72 h.
Specimens were positioned in a microtensile testing machine equipped with a force gauge (BSP-SINGLE SPEED PUMP, Braintree Sci., Inc., MA, U.S.A./COMPACT GAUGE, Dillon, Quantrol, Co., MN, U.S.A.) applying a tensile force parallel to the long axis of each specimen at a crosshead speed of 0.1 mm/min. The shear bond strength of the samples was measured at room temperature.
Preparation for Micro-Tooth Bond Strength (μ-TBS)
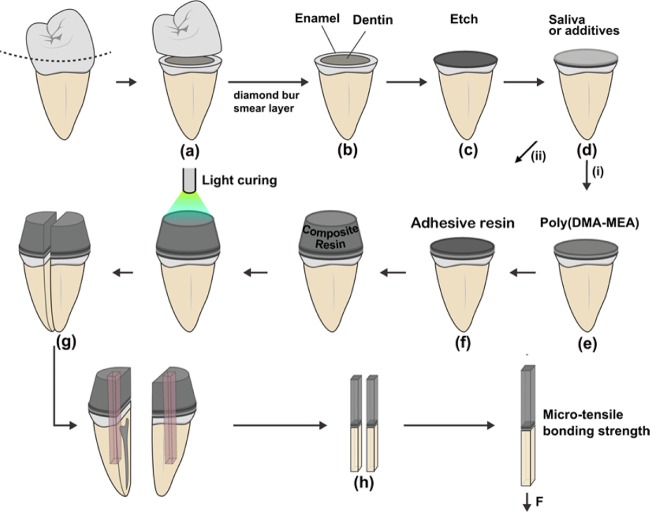
Unidentifiable extracted human third molars were used for this experiment (Protocol was IRB exempted by University of Michigan IRB board). A flat midcoronal dentin surface was prepared perpendicular to the longitudinal axis of each molar using an Isomet saw (Buehler Ltd., Lake Bluff, IL, U.S.A.) under water-cooling (Figure 3).
Figure 3.
Schematic representation of tooth specimen preparation for μ-TBS. (a) Human third molars were cut and (b) prepared for dentin bonding. (c) The surfaces of some specimens were treated with 37% phosphoric acid for etching. (d) The dentin surfaces were further treated by saliva or additives and then with either of the two difference pretreatments; (e) poly(DMA-MEA) or (f) adhesive resin prior to composite resin addition (Filtek Flow). (g) The teeth-composite sets were vertically sectioned into 1 × 0.2 mm thick beams. (h) The beams were trimmed to a flat shape (h).
The teeth were randomly divided into two groups according to the demineralizing method for the commercial adhesive systems used after the application of poly(DMA-MEA): (1) The bonding surface of dentin was polished with a 600-grit silicon carbide paper before using the self-etching commercial adhesive resin (BeautiBondR, Shofu Dental corp., Japan), or (2) etched with 37% H3PO4 for 15 s, rinsed with water, and air-dried for 5 s prior to the bonding procedure with the other commercial adhesive resin (Scotchbond Multi-PurposeR, 3 M ESPE, MN, U.S.A.). After these treatments, the polymer was applied to the tooth surfaces, followed by the commercial adhesive resins. A polymer solution (200 mg/mL) in a water/methanol mixture (1:4), which corresponds to a catechol concentration of 5 mmol, was used for this microtooth bond strength testing.
Each group was divided into five subgroups (n = 10). The first subgroup was pretreated with artificial saliva with mucin prior to adhesive application. The second subgroup was pretreated with artificial saliva without mucin, while the third group was not treated (control). For the saliva subgroups, the acid-etched dentin for Scotchbond Multi-Purpose or nonetched polished dentin for BeautiBond (19.6 mm2) was pretreated with artificial saliva (10 μL) for 20 s and air-dried for 3 s. The polymer solution in a water/methanol mixture (1:4; 200 mg/mL, 20 μL) was dispensed and air-dried for 5 s to two independent preparations of 10 teeth for each condition (Figure 3, Table S3). The commercial adhesive resins were added onto the surface by the following 2-step bonding procedure. For Scotchbond, first, a primer (10 μL) was applied to the dentin surface using a microbrush, and after 30 s, the surface was dried by gentle air-blowing. This air-blowing step was repeated until the surface showed a glossy appearance. Second, the adhesive resin (20 μL) was applied, thinned by gentle air-blowing, and light-cured for 10 s. For BeautiBond, the same procedure was used except a primer.
After all bonding steps, a commercial flowable composite resin (Filtek Flow, 3 M ESPE) was placed on each treated dentin surface and light-cured for 40 s (Ivoclar vivadent AG, Schaan, Liechtenstein). After curing of the composite resin, a D.I. water-soaked cotton gauze was placed over the specimens to maintain high humidity conditions. The specimens were stored at 37 °C for 72 h.
Testing Procedures for μ-TBS
The bonded tooth and composite resin specimens were cut to 1.0 ± 0.2 mm longitudinal sections using an Isomet saw (Buehler Ltd., Lake Bluff, IL, U.S.A.) under water cooling. A minimum of two sections per tooth was obtained (Figure 3). The dimension of each section and the thickness of the bonded layer were determined by a microscope equipped with a digital micrometer. The dentin-adhesive-composite beams were fixed to glass plates using cyanoacrylate glue. The specimen sets were positioned in a microtensile testing machine equipped (BSP-SINGLE SPEED PUMP, Braintree Sci., Inc., MA, U.S.A./COMPACT GAUGE, Dillon, Quantrol, Co., MN, U.S.A.). Microtensile force was applied parallel to the long axis of each specimen at a crosshead speed of 0.1 mm/min. The mode of failure for the adhesive interfaces was analyzed under a stereomicroscope at 25× magnification.
Histological Staining
The tooth/adhesive/composite specimens were dehydrated in ethanol, embedded in methacrylate resin, and sectioned in the buccol-lingual plane using a diamond saw. The central section from each hybrid layer was reduced to a final thickness of 50 μm by microgrinding and polishing with a cutting and grinding device (Exakt, Apparatebau GmbH, Norderstedt, Germany). The sections were stained with hematoxylin and eosin stains. Histologic analyses were performed using a polarized light microscope (BX51 Microscope, Olympus Research Systems, Tokyo, Japan) and a personal computer-based image analysis system (Image-Pro Plus, Media Cybernetics, Silver Spring, MD).
Cytotoxicity Test
Cytotoxicity test was carried out using commercially available human gingival fibroblast cells (HGF-1, ATCC CRL2014; ATCC, U.S.A.) with passages between 5 and 7. Wells of standard 96-well culture plate was coated with poly(DMA-MEA) and commercial adhesive resin Scotchbond in MeOH where wells in control plate were coated with MeOH only. Polymers coated flasks were freeze-dried and sterilized with ethylene oxide. A total of 1 × 104 cells were then cultured on coated and uncoated wells in 5% CO2 and 37 °C. Viable cells were determined using water-soluble tetrazolium (WST, EZ-Cytotox, Dae-il Lab, Korea) assay which was added to each well after 24 h of culture and read at 450 nm. The results for each of test groups were expressed as the percentage to the optical absorbance of control group. Additional staining with calcein AM/ethidium homodimer-1 (Invitrogen, U.S.A.) for observation under the confocal laser microscope (LSM700, Carl-Ziess, U.S.A.) was carried out to confirm the results that showed viable cells as green and dead cells as red.
Scanning Electron Microscopy
The specimens prepared for SEM were treated with 5 N HCl for 30 s followed by 5% NaOCl for 30 min. After rinsing and drying in air, the breached specimens were mounted on 12 mm aluminum stubs and sputter coated with platinum. The specimens were examined at various magnifications.
Statistical Analysis
The data were analyzed by ANOVA analysis using SPSS software (version 10.1, SPSS Inc., Chicago, IL, USA). Since the values were normally distributed, the data were analyzed with a one-way ANOVA. When statistical differences were found, post hoc multiple comparisons were performed using Tukey’s test. Statistical significance was set at 5% (α = 0.05).
Results and Discussion
Syntheses of Catechol Functionalized Adhesive Polymer
The catechol-functionalized polymer was prepared by free radical copolymerization of the TBDMS-protected dopamine methacrylate (TBMDS-DMA) with methoxyethyl acrylate (MEA; Figure 4). The protection of hydroxyl groups of catechol groups prevent undesired oxidation and polymerization of catechol groups as well as facilitate preparation and characterization of polymer in nonpolar organic solvents. The number-average (Mn) and weight-average (Mw) molecular weights of polymer are 41800 and 73900, respectively, giving a polydispersity index of 1.81, based on gel permeation chromatography (GPC) analysis. The TBDMS groups were removed by treating the polymer using TBAF. The 1H NMR spectrum of polymer indicated that the catechol groups were quantitatively deprotected. The polymer contained 15 mol % of DMA, relative to the total amount of monomers in a polymer chain. As the monomer reactivities of DMA and MEA are likely different, one of the monomers reacted first, and then the other monomer was incorporated into the polymer chains, resulting in the formation of DMA segment. The high density of catechol groups along a polymer chain might enhance the adhesion of polymers to substrate surfaces or enhance the complexation with Fe3+ as discussed below. In addition, the composition of DMA in a polymer would be also an important factor to control the adhesion of polymer to dental surfaces. Although it is beyond the scope of this study, the effects of DMA distribution and contents in polymer chains on the adhesion properties of polymers would be the subjects of the future investigations for chemical optimization toward implementation of polymer to dental applications. In addition, the polymer sample contains a TBAF salt as impurity. The chemical and synthetic optimizations for improving polymer purities and manufacturing would be also investigated in future studies. The resultant polymer poly(DMA-MEA) was used for the following adhesion tests.
Effect of Water and Salivary Components on Lap Shear Bond Strength
Prior to testing poly(DMA-MEA) for its adhesiveness to dentin surfaces, we evaluated the adhesiveness of the polymer onto glass surfaces in the presence of water. A glass surface provides a defined surface, eliminating variations of surface properties, as compared to dentin. Polymer solution in methanol was placed between two slide glass plates (Figure 2) and dried under vacuum at room temperature for 72 h to remove methanol and water before the shear bond strength of the samples was measured. It should be noted that this dry condition was aimed at avoiding potential variations in the adhesive strength due to remaining solvents although the dry condition may not reflect the wet oral environment and actual dental procedure. We will examine the effect of saliva on the adhesion performance of the polymer under a wet condition in the tensile bond testing discussed later. In addition, there is a concern for the use of methanol to deliver the polymer regarding the potential toxicity to oral tissues. However, adhesives would not directly contact with pulpal and gingival tissues, and some commercial dental adhesives also contain methanol in their compositions. Therefore, the use of methanol may not be of significant concern for the patients’ health although more comprehensive toxicity testing would be necessary for implementation of this polymer. To determine the effect of contamination of the surface by water on the adhesiveness of the polymer, the glass surface was wet by water prior to the addition of polymer solution. As an adhesive resin control, commercially available Scotchbond Multi-Purpose (3 M EPSE) was used. The adhesive resin agent was diluted with methanol to give the same concentration with the poly(DMA-MEA) solution. The adhesive resin samples were prepared by the same method for poly(DMA-MEA) and light-cured. The glass slides were not treated by acid (acid etching) or primers.
The shear strength of polymer treated specimens increased from 100 kPa to 1.2 MPa as the amount of water increased and leveled off above 2.5 μL of water (Figure 5). This finding contrasts with the significant reduction in the shear strength of the control adhesive resin in the presence of water. It is not clear at this point why the shear strength of polymer increased in the presence of water. It has been reported that the carbonyl groups of the polymer side chains form hydrogen bonding with water.37 We speculate that the water–polymer interaction provided high hydrophilicity and possibly expanded polymer chains in a methanol–water mixture although the polymer is insoluble to 100% water. The expansion of polymer chains would increase the wettability of the polymer to the glass surfaces, increasing the effective bonding area to improve the interfacial bonding. This effect could also increase the possibility of catechol groups to generate strong bonding to the glass surfaces through the formation of hydrogen bonds to the hydroxyl groups of the glass surfaces,38,39 increasing the interfacial adhesion of the polymer. The extension of polymer chains could also facilitate physical cross-linking of multiple polymer chains by entanglement. In addition, the catechol groups may undergo coupling chemical reactions to cause cross-linking of polymer chains,39 although there is no direct evidence to indicate the catechol reaction in this adhesive. These effects would increase the mechanical strength of polymer layer, which increases cohesive bonding. These effects of enhanced interfacial and cohesive bonding would be more effective to increase the shear strength when the solvent was removed from the adhesive after drying than the wet condition.
Figure 5.
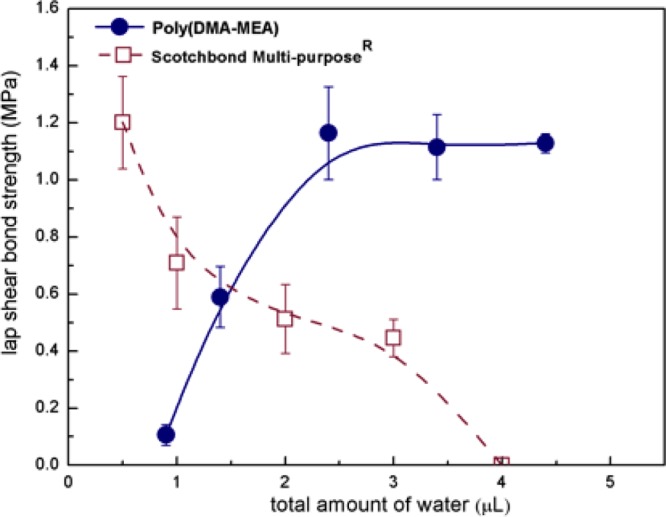
Bond strength of poly(DMA-MEA) vs control adhesive resin in the presence of water.
On the other hand, the addition of water to the control adhesive resin decreased the monomer concentration, which may have resulted in incomplete polymerization, decreasing the mechanical strength of the bond. The interfaces between resin and glass surface might have been also compromised by the water layer preventing the wetting of the hydrophobic adhesive resin to the glass surface structure, reducing the adhesiveness of the resin to the glass surface.
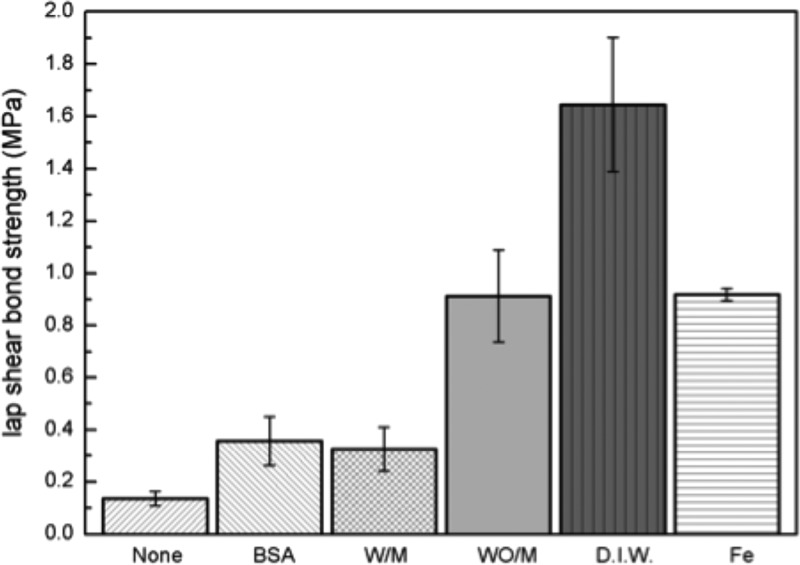
We further tested the polymer for the shear bond strength in the presence of salivary components. Human saliva is composed of more than 99% water and other minor organic components like protein, enzymes, mucins as well as inorganic salts such as calcium, potassium and bicarbonate.40,41 To mimic some of the properties of saliva, we used an artificial saliva commonly used in laboratory studies.42 Glycosylated proteins (mucins, 2.2 wt %) were added to the artificial saliva, and bovine serum albumin (BSA, 35%) solution was also used to examine the effect of salivary proteins on the polymer adhesion. Similar to the shear bond strength testing described above, the artificial saliva or BSA solution was dispensed onto the glass surface, and the polymer solution in methanol was added to the artificial saliva. The shear strength of the polymer without any salivary components and water was 136 kPa and increased significantly to 1.6 MPa in the presence of DI water (Figure 6). However, the shear strength was reduced in artificial saliva without mucins to 0.9 MPa. The shear strength was further reduced to ∼300 kPa in the presence of mucin in saliva or BSA.
Figure 6.
Bond performance of the poly(DMA-MEA) against a glass surface in the presence of various contaminants. Bovine serum albumin (BSA), artificial saliva with and without mucin (W/M and WO/M), and deionized water (D.I.W.) solution were used to examine the effect of saliva components on the polymer adhesion.
These results suggest that salivary components reduce shear bond strength possibly due to reducing cross-linking of catechol groups or to physically interfering with polymer to the glass surface. In addition to the salivary components, we also tested ferric ion (Fe3+) as an additive. Fe3+ has been reported to increase the mechanical strength of catechol-functionalized polymers by forming a complex with three catechol groups in water, resulting in cross-linking of the polymer chains.18,20,21 In our experiments, the addition of Fe3+ in DI water significantly reduced the shear strength of the polymer to 920 kPa. Although more detailed investigation is necessary, it may be possible that the binding of Fe3+ to catechol groups causes cross-linking of polymer chains, which increases the cohesive strength of polymer layer, but concurrently decreases the number of catechol groups interacting with glass surfaces, which reduces the interfacial adhesion, resulting in low ultimate shear bonding strength.
Microtensile Bond Strength of Polymer Adhesives to Dentin
We first determined the microtensile bond strength of commercially available adhesive resins to dentin surface in the presence of salivary components (Figure 7). The adhesive resins were one that requires preliminary acid-etching (AE) and one that is self-etching (SE). The basic concept of bonding to enamel and dentin is essentially an exchange process involving replacement of minerals removed from the dentin by resin monomers, which, upon setting, become micromechanically interlocked in the created porosities. The AE adhesive resin involves a separate etch and rinse process, whereas the SE approach is based on the use of acidic monomer that eliminates the etching process. Both systems are popular in dental practice. For the study, two widely used adhesive resins (Scotchbond Multi-Purpose, 3 M EPSE, U.S.A., and Beautibond, Shofu, Co., Japan) with published literature on evaluation of micro tensile bonding strength to dentin were selected.14,43−49
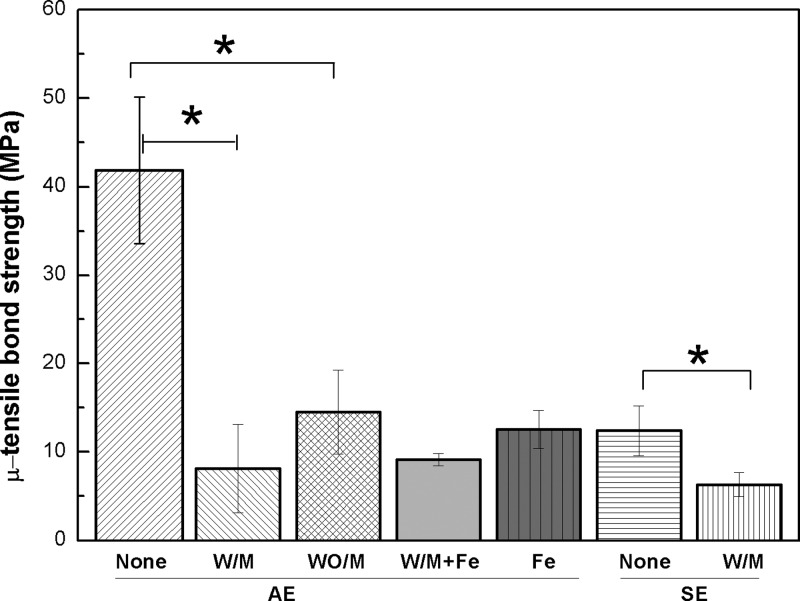
Figure 7.
Bond strength of the commercial dental adhesive resins with various additives against the dentin surface. Saliva with mucin (W/M) or without mucin (WO/M), saliva with Fe3+ and mucin (Fe/W/M) and Fe3+ in D.I. water (Fe) were used; *p < 0.05.
The preparation procedure for the dentin surface is depicted in Figure 3. The bonding surface of dentin was polished with silicon carbide paper for the SE adhesive resin or etched with 37% H3PO4 for the AE bonding procedure. Prior to adhesive application, the bonding surface was treated with artificial saliva with or without mucin and quickly dried in air. The commercial adhesive resins were added onto the surface by the following 2-step bonding procedure: the primer (10 μL) was applied to the dentin surface, and then the adhesive resin (20 μL) was placed and set by light-curing. After the bonding application, a flowable composite resin (Filtek Flow, 3 M ESPE) was placed on each treated dentin surface and light-cured as the clinical restoration procedure in general dental practice. The tooth-composite specimens were cut to create longitudinal sections of 1.0 ± 0.2 mm in thickness that were used for tensile-bond strength testing. To mimic the dental procedure and wet oral environment, the specimens were not dried under vacuum and stored under moisture.
The bond strength of the AE adhesive resin was 42 MPa without any saliva contamination. The addition of artificial saliva with mucin reduced the tensile-bond strength significantly to 8 MPa. In general, tensile-bond strength of ∼15 MPa is necessary for adequate bond strength in restorations under clinical conditions.50,51 Salivary contamination reduced bond strength below this minimum value suggesting potential clinical failure. On the other hand, the tensile-bond strength of the SE adhesive resin without saliva was 12 MPa, and the addition of artificial saliva also reduced the strength to 6 MPa. These results overall indicate that salivary contamination significantly compromises bonding performance of commercial adhesive resins.
We next determined the effect of poly(DMA-MEA) on the tensile-bond strength of the AE adhesive resin to the contaminated dentin surfaces. To simulate the salivary contamination in the bonding procedure in clinical situations, the polymer solution in water/methanol mixture was applied to the bonding dentin surface treated by artificial saliva, and then the AE adhesive resin was added. The tensile-bond strength of the AE adhesive resin on the polymer-treated dentin surface without any artificial saliva was 8.4 MPa (Figure 8), which is significantly lower than that of the AE adhesive resin alone (42 MPa; Figure 7). The addition of artificial saliva with mucin slightly increased (12.9 MPa) the tensile bond strength of the adhesive resin. The catechol groups of polymer chains may bind to dentin surfaces by hydrogen bonding or chelating with calcium in hydroxyapatite minerals.52,53 Therefore, the polymer was expected to increase the bonding of the adhesive resin to dentin in the presence of water, similar to the result of shear bonding strength. However, the results suggest that the polymer itself reduces the bonding of adhesive resin to dentin, and the effect of polymer on the bonding strength to dentin is also not significant as compared to bonding to glass surface. This may indicate that the polymer did not make strong bonding to dentin surfaces or other factors in the adhesive structures might be determinants in the bonding strength.
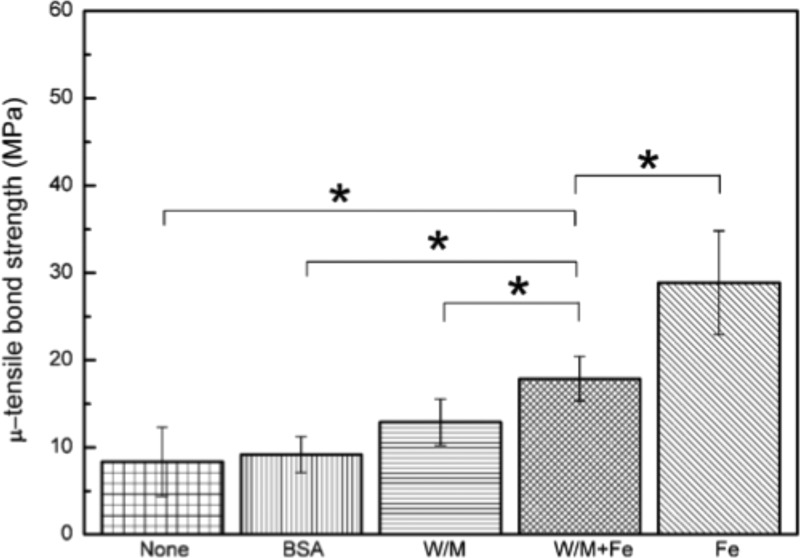
Figure 8.
Bond performance of the poly(DMA-MEA) with various additives against the dentin surface. Bovine serum albumin (BSA; 35%) solution, artificial saliva with mucin (W/M), Fe3+ in artificial saliva with mucin (W/M+Fe) or Fe3+ in D.I. water (Fe) was dispensed onto the dentin surface, and then poly(DMA-MEA) was applied; *p < 0.05.
On the other hand, the tensile bond strength of the AE adhesive resin on the polymer-treated surface with Fe3+ in water or in artificial saliva further increased the bond strength, 29 or 18 MPa, respectively (p < 0.05, Figure 8). This finding indicates that pretreatment of bonding dentin surfaces with Fe3+ solution increased the tensile bond strength of the adhesive resin. Fe3+ did not improve the tensile bond strength of saliva-contaminated AE adhesive resin without the polymer (W/M vs W/M/Fe in Figure 7; ∼8 MPa), indicating that the polymer and Fe3+ are both necessary to improve the bond strength of contaminated adhesive resin. The increased tensile-bond strength to dentin surfaces by the polymer with Fe3+ is opposite to the result of reduced shear strength to glass surfaces (Figure 6). According to the bonding structures examined by histological images in the next section, this contradiction likely resulted from the differences in the adherent substrates and bonding mechanisms of the polymer in the presence of Fe3+, which will be discussed in detail later.
Microscopic Analysis of Leakage Patterns of Adhesive Resins
To investigate the effect of poly(DMA-MEA) and salivary components on the bonding of the adhesive resin to dentin, we characterized the structure of the bonding interface regions by histological H&E staining. The bonding region consisted of four layers: composite resin, adhesive resin layer, hybrid layer, and dentin (Figure 9). The adhesive resin alone showed no indication of structural defects or leakage pattern in the bonded interface (Figure 9a). However, in the presence of artificial saliva, the adhesive resins had a leakage pattern between the hybrid layer and the adhesive resin layer (Figure 9b) and large defects or delamination (Figure 9c). In corroboration with the tensile strength testing (Figure 7), the leakage pattern could be responsible for the reduction in the tensile-bond strength of boding agent contaminated by saliva. This result also indicates that the interface between the hybrid layer, and adhesive resin layer is the weakest structure in the boding region and, likely, the most prone to crack formation by mechanical stress, while the hybrid layer is reinforced by collagen fibers from the dentin.
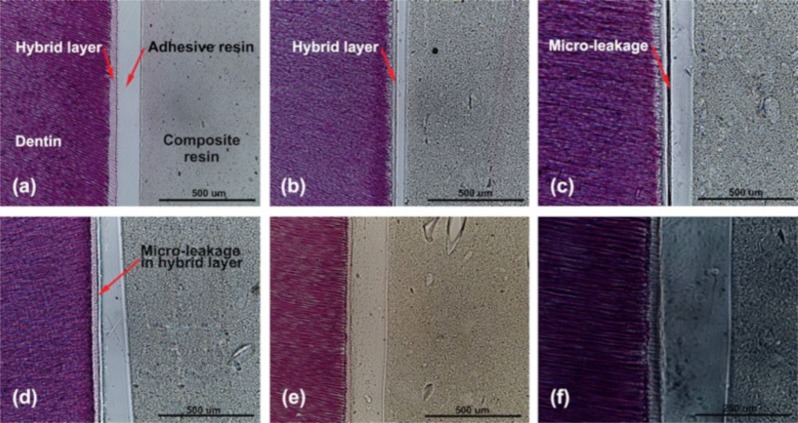
Figure 9.
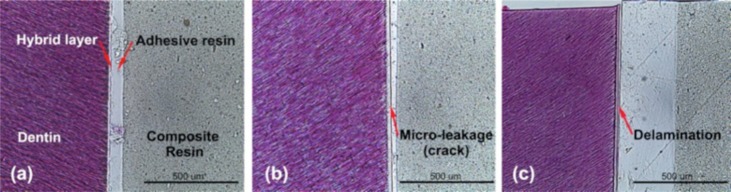
Histological inspections of interface regions between dentin and dentin adhesive resin with various additives (H&E staining). No additive treatment (a), artificial saliva with mucin (b), and artificial saliva without mucin (c) were used to examine the effect of saliva components on the dentin adhesive resin bond. Bar = 500 μm.
We also examined the bonding interface regions of the specimens pretreated by poly(DMA-MEA). The adhesive resin with the polymer appears to have no leakage pattern (Figure 10a). However, addition of water caused a crack in the hybrid layer rather than in the interface with the adhesive resin layer (Figure 10d), which contrasts with the results using saliva. It appears that addition of water may cause precipitation of the commercial resin polymers on the dentin surfaces, reducing the formation of collagen reinforced polymer matrix in the hybrid layer resulting in a weaker bond and the formation of defects. Because the interface between the hybrid layer and adhesive resin is relatively stronger than the hybrid layer itself, the hybrid layer is more prone to defect formation. On the other hand, the AE adhesive resin with the polymer in the presence of BSA (35%) solution or artificial saliva with mucin showed separation in the width of a few micrometers at the interface between the adhesive resin layer and the hybrid layer (Figure 10b,c). These findings indicate that salivary proteins reduce the bond strength between hybrid and adhesive resin layers rather than compromise the mechanical property of the hybrid layer. We speculate that the proteins might aggregate with the polymer, which reduce the effective concentration of polymer that reinforces the adhesive resin structures at the interface, resulting in the formation of microleakage patterns. These results suggest that the polymer is not effective in preventing the formation of leakage patterns at the layer interface of the saliva-contaminated dentin surface, which is likely responsible for the reduced bond strength of AE adhesive resin.
Figure 10.
Histological inspection of interface regions between dentin and poly(DMA-MEA) with various additives by H&E staining. No additive treatment (a), bovine serum albumin (35%) solution (b), artificial saliva with mucin (c), D.I. water (d), Fe3+ solution (e), and high magnification of Fe3+ solution (f) were used to examine the effect of saliva components on the dentin adhesive resin bond. Bar = 500 μm (a–e) and 250 μm (f).
On the other hand, the adhesive resin with the poly(DMA-MEA) on the dentin surfaces pretreated by Fe3+ in artificial saliva did not show any leakage pattern (Figure 10e,f). These results indicate that the polymer improved the bond quality of the interface between the hybrid and the adhesive resin layers. It has been previously reported that catechol groups are capable of forming Fe3+–catechol complexes in the presence of water and cross-linking of polymer chains.18,20,21 In the lap shear strength experiment (Figure 6), addition of Fe3+ reduced the lap shear strength to glass surfaces, possibly because the Fe3+–catechol complex reduces the number of catechol groups that adhere to glass surfaces, resulting in low interfacial adhesion of polymer. However, the histological images (Figure 10) of adhesive resin on dentin surfaces suggest that leakage patterns or defects in the hybrid layer and at the interface between the hybrid and adhesive resin layers could be responsible for the low bonding strength of adhesive resins, rather than the adhesion of polymer and adhesive resin to the dentin surface. In addition, the polymer was likely mixed with the adhesive resin on the dentin surfaces and set with the adhesive resin matrix. Therefore, the polymer might work cooperatively with the adhesive resin. The Fe-cross-linked polymer chains could reinforce the structures of adhesive resins in the hybrid layer and at the interface between the hybrid and adhesive resin layers, reducing the defect formation by increasing the cohesive bonding strength of adhesive resin. Similar to the lap shear strength, the Fe-cross-linking of polymer chains may reduce the number of catechol groups that potentially adhere to dentin surfaces. However, the increased tensile bonding strength by the polymer with Fe3+ suggests that the reduction of defect formation in the adhesive structure is more effective to improve the tensile strength rather than lower interfacial bonding to dentin. Therefore, the contradiction between the effects of Fe3+ on the shear and tensile bonding strengths is due to the Fe3+-cross-linked polymers that reduced the interfacial bonding of polymer to glass surfaces (Figure 6) or increased the cohesive bonding of adhesive resin to dentin surface (Figure 8).
Fine Structure of Bonding Interfaces
We further examined the fine structure of bonding interfaces by scanning electron microscopy (SEM). The interface between the hybrid layer and adhesive resin layer has some defects at the localized region (Figure 11a), similar to the results previously reported.54 In contrast, the adhesive resin placed after contamination with artificial saliva showed a narrow gap structure or leakage patterns spanning through the interface (Figure 11b). However, the addition of poly(DMA-MEA) with Fe3+ in artificial saliva did not show any leakage pattern in the microstructure of the bonding interface (Figure 11c). These results also support the conclusion that poly(DMA-MEA) with Fe3+ prevents the formation of defects at the interface between the hybrid layer and the adhesive resin layer on the saliva contaminated dentin surface.
Figure 11.

SEM micrographs of dentin specimens bonded with various adhesives. The specimens of only adhesive resin (Scotchbond) (a), adhesive resin after contamination with artificial saliva (b), and adhesive resin and poly(DMA-MEA) with Fe3+ in artificial saliva (c) were examined.
Cytotoxicity
As a first assessment of cytotoxicity of polymer to oral tissues toward implementation, cell adhesion and viability of human gingival fibroblast cells HGF-1 was examined. In general, fibroblast cells undergo cell adhesion processes of (1) substrate attachment, (2) spreading, and (3) cytoskeleton development. Morphology of the cells on the poly(DMA-MEA)-coated surface and controls after 24 h was evaluated by fluorescent images (Figure 12). The spreading of cells on the polymer-coated surface and development of cell cytoskeleton (Figure 12b) were enhanced as compared to the control (unmodified cell culture plate; Figure 12a). A plate surface was also treated by solvent methanol which was used for polymer casting (Figure 12c). The methanol-treated surface did not show any difference in the cell morphology from the control, indicating that the poly(DMA-MEA) is responsible for the enhanced cell adhesion. As a comparison, the morphology of cells on a commercial adhesive resin AE (Scotchbond) coated surface (Figure 12c) was similar to that of the control surface. The cells adhered on the polymer-coated surface also exhibited well-stretched actin bundles (Figure 12b). These results suggest that the cells underwent the aforementioned general adhesion processes. In addition, when cells were cultured on the polymer-coated substrates, there was no significant difference in the cell viability from the control, methanol-treated surface, and the commercial adhesive resin AE (Scotchbond) coated surfaces (Figure 12e), suggesting that the polymer surface did not cause any toxic effect to the fibroblast cells. The results of mechanical bonding experiments indicated that Fe3+ increased the tensile bond strength of polymer in the presence of saliva. At this point, the biocompatibilities of polymer surfaces containing Fe3+ and other additives have not been determined. More comprehensive cytotoxicity testing of polymer samples with additives would be necessary for further development and clinical implementation of the proposed polymer adhesives.
Figure 12.
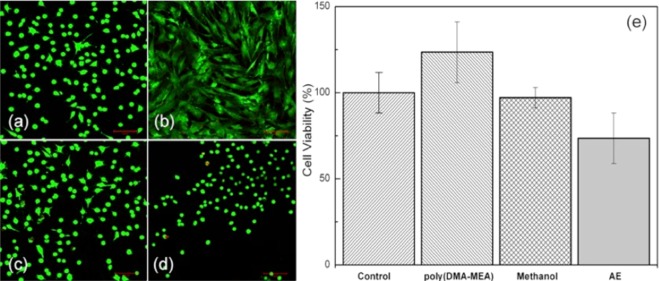
Effect of poly(DMA-MEA) on cell viability of human gingival fibroblast cells HGF-1 after 24 h incubation. The cells were cultured on a culture plate control (a), poly(DMA-MEA)-coated surface (b), methanol-treated surface (c), or an adhesive resin (Scotchbond) coated surface (d) for 1 day. In these fluorescence images (a–d), live or dead cells were stained in green or red, respectively. The cell viability of these cell cultures was determined by the WST cell viability assay (e). The scale bar represents 100 μm.
Conclusion
In summary, we evaluated the potential of catechol-functionalized polymer poly(DMA-MEA) as a dental adhesive resistant to contamination by oral fluids. The polymer with Fe3+ additive improved the bond strength of commercial adhesive resin to the saliva contaminated dentin surface. We hypothesize this is because of the formation of Fe–catechol complexes, which reinforce the bonding structures at the interface between the hybrid and boding resin layers, preventing the formation of leakage patterns. These results support our hypothesis that a catechol-functionalized polymer would function as a dental adhesive for contaminated dentin surfaces. In addition, the polymers did not inhibit proliferation of human gingival fibroblast cells. Although more detailed studies are needed, the polymer adhesives could be used for dental implant coatings, where good biocompatibility and good cell adhesion are required. The results of this investigation suggest that the polymer is effective in improving the properties of the interface between the hybrid layer and adhesive resin in the boding region, but it is still not clear if the polymer is able to form any bonding with the dentin surface. Moreover, the addition of polymer to the adhesive resin significantly reduced its bond strength. Despite the potential of the catechol-functionalized polymer as a dental adhesive, it is clear that the polymer needs further chemical modifications and optimization to improve the bonding to the adhesive resin and the dentin surface. This presented work provides new insights into the function of catechol-functionalized polymers on the biological surface of dentin and their potential applications in dentistry.
Acknowledgments
This research was supported by the Dental Device Testing and Evaluation Center, College of Dentistry, Yonsei University, NIH R21DE020908 (to K.K.) and U01DE023771 (to K.K.). We thank Ms. Susan Flannagan, University of Michigan, Cariology research laboratory, for providing the mucin and artificial saliva, Prof. Mathilde Peters, Cariology research laboratory in University of Michigan for use of the microtensile testing devices, Mr. Sang-Hyun Park and Dr. Jae-Sung Kwon, College of Dentistry, Yonsei University for use of histological evaluation devices and cytotoxicity testing.
Supporting Information Available
1H NMR spectra of polymers, lap shear strength data, and micro tensile bond strength (μ-TBS) data. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biomac.5b00451.
The authors declare no competing financial interest.
Supplementary Material
References
- Erfan M.; Jafarzadeh-Kashi T. S.; Ghadiri M.; Rakhshan V. J. Adv. Prosthodont. 2014, 6, 333–45 10.4047/jap.2014.6.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes Porto I. C.; Honorio N. C.; Amorim D. A.; de Melo Franco A. V.; Penteado L. A.; Parolia A. J. Conservative Dent. 2014, 17, 65–9 10.4103/0972-0707.124151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz Y.; Ozalp M.; Attar N. J. Contemp. Dent. Pract. 2008, 9, 57–64. [PubMed] [Google Scholar]
- Pinna R.; Bortone A.; Sotgiu G.; Dore S.; Usai P.; Milia E. Clin. Oral Investig. 2015, 10.1007/s00784-014-1390-3. [DOI] [PubMed] [Google Scholar]
- Finger W. J.; Inoue M.; Asmussen E. Am. J. Dent. 1994, 7, 35–8. [PubMed] [Google Scholar]
- Nishitani Y.; Yoshiyama M.; Donnelly A. M.; Agee K. A.; Sword J.; Tay F. R.; Pashley D. H. J. Dent. Res. 2006, 85, 1016–21 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen E.; Uno S. Oper Dent. 1992, Suppl 5, 68–74. [PubMed] [Google Scholar]
- Perdigao J.; Reis A.; Loguercio A. D. J. Esthet. Restor. Dent. 2013, 25, 219–41 10.1111/jerd.12016. [DOI] [PubMed] [Google Scholar]
- Moszner N.; Salz U.; Zimmermann J. Dent. Mater. 2005, 21, 895–910 10.1016/j.dental.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Milia E.; Cumbo E.; Cardoso R. J.; Gallina G. Curr. Pharm. Des. 2012, 18, 5542–52 10.2174/138161212803307491. [DOI] [PubMed] [Google Scholar]
- Kubo S.; Finger W. J.; Muller M.; Podszun W. J. Esthet. Dent. 1991, 3, 62–9 10.1111/j.1708-8240.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen T.; Soderholm K. J. Dent. Mater. 1995, 11, 132–136 10.1016/0109-5641(95)80048-4. [DOI] [PubMed] [Google Scholar]
- Chung C. W. M.; Yiu C. K. Y.; King N. M.; Hiraishi N.; Tay F. R. J. Dent. 2009, 37, 923–931 10.1016/j.jdent.2009.07.007. [DOI] [PubMed] [Google Scholar]
- el-Kalla I. H.; Garcia-Godoy F. Am. J. Dent. 1997, 10, 83–7. [PubMed] [Google Scholar]
- Miyazaki M.; Tsubota K.; Takamizawa T.; Kurokawa H.; Rikuta A.; Ando S. Jpn. Dent. Sci. Rev. 2012, 48, 53–60 10.1016/j.jdsr.2011.11.002. [DOI] [Google Scholar]
- Lee H.; Scherer N. F.; Messersmith P. B. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 12999–13003 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. P.; Messersmith P. B.; Israelachvili J. N.; Waite J. H. Annu. Rev. Mater. Res. 2011, 41, 99–132 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten-Andersen N.; Harrington M. J.; Birkedal H.; Lee B. P.; Messersmith P. B.; Lee K. Y. C.; Waite J. H. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 2651–2655 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.; Policastro G. M.; Hua G.; Guo K.; Zhou J.; Wesdemiotis C.; Doll G. L.; Becker M. L. J. Am. Chem. Soc. 2014, 136, 16357–16367 10.1021/ja508946h. [DOI] [PubMed] [Google Scholar]
- Wilker J. J. Angew. Chem., Int. Ed. 2010, 49, 8076–8078 10.1002/anie.201003171. [DOI] [PubMed] [Google Scholar]
- Zeng H.; Hwang D. S.; Israelachvili J. N.; Waite J. H. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 12850–12853 10.1073/pnas.1007416107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedó J.; Saiz-Poseu J.; Busqué F.; Ruiz-Molina D. Adv. Mater. 2013, 25, 653–701 10.1002/adma.201202343. [DOI] [PubMed] [Google Scholar]
- Matos-Pérez C. R.; White J. D.; Wilker J. J. J. Am. Chem. Soc. 2012, 134, 9498–9505 10.1021/ja303369p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; Deming T. J. Macromolecules 1998, 31, 4739–4745 10.1021/ma980268z. [DOI] [PubMed] [Google Scholar]
- Lee H.; Lee B. P.; Messersmith P. B. Nature 2007, 448, 338–41 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- Nordberg A.; Antoni P.; Montañez M. I.; Hult A.; Von Holst H.; Malkoch M. ACS Appl. Mater. Interfaces 2010, 2, 654–657 10.1021/am100002s. [DOI] [PubMed] [Google Scholar]
- Skelton S.; Bostwick M.; O’Connor K.; Konst S.; Casey S.; Lee B. P. Soft Matter 2013, 9, 3825–3833 10.1039/c3sm27352k. [DOI] [Google Scholar]
- Yang S. H.; Kang S. M.; Lee K.-B.; Chung T. D.; Lee H.; Choi I. S. J. Am. Chem. Soc. 2011, 133, 2795–2797 10.1021/ja1100189. [DOI] [PubMed] [Google Scholar]
- Gong Y.-K.; Liu L.-P.; Messersmith P. B. Macromol. Biosci. 2012, 12, 979–985 10.1002/mabi.201200074. [DOI] [PubMed] [Google Scholar]
- Li G.; Cheng G.; Xue H.; Chen S.; Zhang F.; Jiang S. Biomaterials 2008, 29, 4592–4597 10.1016/j.biomaterials.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Chirdon W. M.; O’Brien W. J.; Robertson R. E. J. Biomed. Mater. Res. 2003, 66B, 532–538 10.1002/jbm.b.10041. [DOI] [PubMed] [Google Scholar]
- Hiraishi N.; Kaneko D.; Taira S.; Wang S.; Otsuki M.; Tagami J. J. Investig. Clin. Dent 2015, 6, 59–62 10.1111/jicd.12054. [DOI] [PubMed] [Google Scholar]
- Nguyen H. G. T.; Weston M. H.; Farha O. K.; Hupp J. T.; Nguyen S. T. CrystEngComm 2012, 14, 4115–4118 10.1039/c2ce06666a. [DOI] [Google Scholar]
- Sever M. J.; Wilker J. J. Tetrahedron 2001, 57, 6139–6146 10.1016/S0040-4020(01)00601-9. [DOI] [Google Scholar]
- Chen C.; Ahmed I.; Fruk L. Nanoscale 2013, 5, 11610–11614 10.1039/c3nr03563h. [DOI] [PubMed] [Google Scholar]
- Leung V. W. H.; Darvell B. W. J. Dent. 1997, 25, 475–484 10.1016/S0300-5712(96)00068-1. [DOI] [PubMed] [Google Scholar]
- Li G. F.; Ye S.; Morita S.; Nishida T.; Osawa M. J. Am. Chem. Soc. 2004, 126, 12198–12199 10.1021/ja046183x. [DOI] [PubMed] [Google Scholar]
- Lin Q.; Gourdon D.; Sun C.; Holten-Andersen N.; Anderson T. H.; Waite J. H.; Israelachvili J. N. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 3782–6 10.1073/pnas.0607852104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Keijsers J.; van Heek M.; Stuiver A.; Stuart M. A. C.; Kamperman M. Polym. Chem. 2015, 6, 3121–3130 10.1039/C4PY01790K. [DOI] [Google Scholar]
- Humphrey S. P.; Williamson R. T. J. Prosthet. Dent. 2001, 85, 162–169 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- Papacosta E.; Nassis G. P. J. Sci. Med. Sport 2011, 14, 424–434 10.1016/j.jsams.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabezas C.; Jiang H.; Fontana M.; Eckert G. J. Dent. 2012, 40, 522–526 10.1016/j.jdent.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Villela-Rosa A. C. M.; Goncalves M.; Orsi I. A.; Miani P. K. Braz. Oral. Res. 2011, 25, 143–149 10.1590/S1806-83242011005000008. [DOI] [PubMed] [Google Scholar]
- Sauro S.; Toledano M.; Aguilera F. S.; Mannocci F.; Pashley D. H.; Tay F. R.; Watson T. F.; Osorio R. Dent. Mater. 2011, 27, 563–572 10.1016/j.dental.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Ritter A. V.; Bertoli C.; Swift E. J. J. Dent. Res. 2000, 79, 375–375. [Google Scholar]
- Nakajima M.; Kanemura N.; Pereira P. N. R.; Tagami J.; Pashley D. H. Am. J. Dent. 2000, 13, 324–328. [PubMed] [Google Scholar]
- Hegde M.; Manjunath J. Oper. Dent. 2011, 36, 169–176 10.2341/10-55-L. [DOI] [PubMed] [Google Scholar]
- Gallo J. R.; Henderson M.; Burgess J. O. Am. J. Dent. 2000, 13, 267–270. [PubMed] [Google Scholar]
- Dalkilic E. E.; Genc O.; Ozcopur B.; Belli S.; Eskitascioglu G.; Ozcan M. Dent. Mater. J. 2012, 31, 758–764 10.4012/dmj.2011-273. [DOI] [PubMed] [Google Scholar]
- Kiremitci A.; Yalcin F.; Gokalp S. Quintessence Int. 2004, 35, 367–70. [PubMed] [Google Scholar]
- Hegde M. N.; Bhandary S. J. Conservative Dent. 2008, 11, 71–5 10.4103/0972-0707.44054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Park C. B. Biomaterials 2010, 31, 6628–6634 10.1016/j.biomaterials.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Ryu J.; Ku S. H.; Lee M.; Park C. B. Soft Matter 2011, 7, 7201–7206 10.1039/c1sm05307h. [DOI] [Google Scholar]
- Breschi L.; Mazzoni A.; Ruggeri A.; Cadenaro M.; Di Lenarda R.; Dorigo E. D. Dent. Mater. 2008, 24, 90–101 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.