Abstract
Thoracic spine pain is as disabling as neck and low back pain without receiving the same level of attention in the scientific literature. Among the different structures that can refer pain to the thoracic spine, muscles often play a relevant role. Trigger points (TrPs) from neck, shoulder and spinal muscles can induce pain in the region of the thoracic spine. There is a lack of evidence reporting the presence of TrPs in the region of the thoracic spine, but clinical evidence suggests that TrPs can be a potential source of thoracic spine pain. The current paper discusses the role of TrPs in the thoracic spine and dry needling (DN) for the management of TrPs in the thoracic multifidi and longissimus thoracis. This paper also includes a brief discussion of the application of DN in other tissues such as tendons, ligaments and scars.
Keywords: Dry needling, Thoracic spine, Pain, Trigger points, Muscle pain
Introduction
Back pain represents a major public health issue for society. Thoracic spine pain has not received as much attention as the cervical and lumbar spine in the scientific literature, yet it can be just as disabling. In fact, whereas several epidemiological studies have investigated the prevalence of neck and low back pain, there are few prevalence studies on thoracic spine pain. The prevalence of thoracic spine pain varied considerably depending on the definition criteria. Briggs et al. found that the 1-year prevalence of thoracic spine pain ranged from 3.0 to 55.0% in an adult working population.1 In a systematic review, the same authors identified that the point prevalence for thoracic pain ranged from 4 to 72%, a 1-month prevalence from 1.4 to 34.8%, a 1-year prevalence from 3.5 to 34.8%, and a lifetime prevalence from 15.6 to 19.5%.2 This review also reported a 1-year incidence of 3.8 to 35.3% for thoracic pain.2 The prevalence of thoracic spine pain is higher in women than in men with a female-to-male ratio of 2:1.3
Considering the impact of thoracic spine pain on society, determining the most appropriate treatment strategies for this area is essential, especially because having pain in the thoracic spine is a predictor of not returning to work in men.4 Because thoracic pain can be due to several different factors, knowing the potential causes of nociception and resultant pain is of importance to ensure that a proper and thorough examination are performed.
The thoracic spine is a site of musculoskeletal pain of systemic origin and patients with thoracic spine pain are more likely to have serious pathology compared to patients with lumbar or cervical pain.5 Several visceral structures can refer to different levels of the thoracic spine, including the heart, aorta, lungs, oesophagus, stomach, duodenum, pancreas, gall bladder, liver, kidney and ureter. Several inflammatory and infectious disorders induce pain into the thoracic spine including rheumatoid arthritis, ankylosing spondylitis, osteomyelitis, Pott's disease or spinal infection.6 Primary cancer, such as osteoid osteoma, can cause thoracic spine pain and impairments in the spinal canal and spinal nerve roots, including foraminal stenosis, arthrosis, diffuse idiopathic skeletal hyperostosis or zygapophyseal joint cysts.7 The thoracic spine can also harbour metastases from breast cancer and other primary cancer sources, including the lungs, thyroid, oesophagus and skin. Other conditions that may contribute to thoracic spine pain include acromegaly, osteoporosis, Paget's disease and sickle cell disease.6 Screening for potential underlying medical conditions is important to establish the proper diagnosis and develop the optimal plan of care. The paper on screening and imaging in this special issue will cover this topic in detail.
From a musculoskeletal perspective, thoracic spine pain is often thought to be due to the facet joints, because of their innervation from the medial branch of the primary dorsal rami.8 Although facet joint pain is poorly defined in a clinical sense, the prevalence of thoracic pain from the facet joints is reported to range from 34 to 48%.9,10 Anaesthetic facet blocks are poorly correlated with observations based on imaging studies, e.g., MRI or CT. In addition, the effectiveness of facet joint nerve blocks is quite variable11,12 and it is therefore necessary to explore other potential sources of thoracic spine pain.
Muscle Pain and Thoracic Spine
Myofascial trigger points (TrPs)
The International Association for the Study of Pain recognises that muscles are a common source of musculoskeletal pain, including thoracic spine pain.13 According to Simons et al.,14 myofascial TrPs located in several muscles can refer to the thoracic spine region, including the lower cervical and thoracic multifidi, the thoracic longissimus and iliocostalis, psoas, latissimus dorsi, serratus posterior inferior, rectus abdominus, scalenes and rhomboid muscles.
A myofascial TrP is a hyperirritable nodule within a taut band of skeletal muscle fibres.14 It is important to realise that a TrP is not some kind of anatomical structure, but a muscle contracture palpable in a skeletal muscle. To be able to accurately find a TrP, tissues must be palpated perpendicular to the muscle fibres. This mechanical input can cause local and referred pain and may elicit a local twitch response depending on the muscle and the palpation technique. Myofascial TrPs can clinically be classified as either active or latent. An active myofascial TrP is spontaneously painful, generating both local and referred pain and does not require digital stimulation to cause pain symptoms. A latent myofascial TrP is not spontaneously painful and does require digital stimulation to induce local and referred pain, yet, it can display the other qualities of active myofascial TrPs.14 Active myofascial TrPs reproduce patient's symptoms and the patient usually recognises the pain as familiar pain, whereas latent myofascial TrPs do not reproduce a familiar pain for the individual. In fact, latent myofascial TrPs are also found in healthy asymptomatic individuals.14–16
Myofascial TrPs can contribute to impairments of the musculoskeletal system including decreased range of motion,17,18 decreased strength,19 altered muscle activation patterns,20 increased tissue stiffness,21,22 muscle fatigability23 and local and referred pain.24–27 Myofascial TrPs have also been associated with decreased joint mobility in the cervical spine,28 although this appears to be dependent on the sample size and the manual joint assessment technique.29
The most accepted hypothesis for the development of myofascial TrPs is called the ‘integrated hypothesis’,30 which stipulates that dysfunctional motor endplates release an excessive amount of non-quantal acetylcholine (ACh), leading to localised contractures. The contractures may cause decreased blood flow within the tissue,31,32 which in turn can lead to local ischaemia, a lowered tissue pH and a subsequent release of pro-inflammatory substances and some chemical mediators including bradykinin, cytokines, substance P, calcitonin gene-related peptide (CGRP), glutamate, tumour necrosis factor-α (TNF-α), cyclo-oxygenase-2 (COX-2), hypoxia-inducible factor 1-alpha (HIF-1α), inducible nitric oxide synthase (iNOS), and vascular endothelial growth factor (VEGF) among others.33,34 These chemical mediators can activate and maintain muscle nociceptors, resulting in the experience of pain and muscle tenderness. The acidic pH contributes to the inhibition of acetylcholinesterase (AChE) at the motor endplate, thereby preventing the breakdown of ACh in the synaptic cleft, but also activates acid sensing ion channels (ASIC 1 and ASIC 3), vanilloid receptors, especially the transient receptor potential cation channel subfamily V member 1 (TRPV1), and short transient receptor potential channel 4 and 5 (TRPC-4 and TRPC-5).34
At the motor endplate, CGRP contributes to the up-regulation of ACh receptors, increases the release of ACh from the motor nerve terminal and inhibits AChE, which contributes to an increased quantity of ACh in the synaptic cleft, an increase in the frequency of end-plate potentials, sarcomere contraction, and taut band formation.30 At the dorsal horn lamina, CGRP can potentiate substance P, activate second messenger pathways protein kinase A and C (PKA and PKC), and enhance the release of brain derived neurotrophic factor (BDNF), which all contribute to the experience of pain.
Active and latent myofascial TrPs have altered chemical milieus as compared to normal muscle.33,35,36 As described, the local biochemical changes may activate muscle nociceptors and contribute to peripheral and central sensitisation mechanisms and also to functional changes within the dorsal horn.37
Dry Needling (DN) Interventions for Tendons, Entheses and Scar Tissues
As early as 1979, Lewit described DN of multiple tissues in addition to targetting TrPs, including insertion points of ligaments, scar tissue, periosteum, muscle spasms, tendons, entheses and even joints.38 He observed immediate analgesia in 271 out of 312 painful structures following DN and suggested that the intensity of the painful stimulus was crucial. In addition, patients with myofascial pain, for example, following a whiplash accident, often present with painful entheses, which can indeed be treated effectively with DN.39 There is also evidence from the medical literature that DN of fascial tissue may be indicated for pain relief, which likely involves targetting fibroblasts.40–42
Overall, there is limited scientific support for DN of all these structures. It is, however, a defensible position that physical therapists should incorporate elements of the acupuncture literature,43 where needling procedures are described for a range of neuro-musculoskeletal diagnoses, e.g., osteoarthritis,44 low back pain45 and migraine,46 as long as the diagnoses and indications are within the scope of physical therapy practice. In the context of the current paper, we have opted to exclude the acupuncture literature and focus primarily on myofascial trigger point dry needling (TrP-DN).
Trigger Point Dry Needling
Dry needling is a ‘skilled intervention that uses a thin filiform needle to penetrate the skin and stimulate underlying TrPs, muscles and connective tissues for the management of both neuro-musculoskeletal pain and movement impairments’.47 In fact, the Physiotherapy Acupuncture Association of New Zealand separated acupuncture by physical therapist in three main categories: traditional acupuncture, western acupuncture and TrP-DN.48 They describe DN as a rapid, short-term needling to altered or dysfunctional tissues in order to improve or restore function. This may include (but is not limited to) needling of myofascial TrPs, periosteum and connective tissues. It may be performed with an acupuncture needle or any other injection needle without the injection of a fluid. This is a common practice utilised by both traditional and Western acupuncturists.47
Some researchers have suggested that in patients exhibiting central sensitisation, therapeutic interventions must be pain-free to avoid increase sensitisation mechanisms,49 which stands in direct opposition to Lewit's observations. There is emerging literature suggesting that a brief painful needle stick is insufficient to induce or maintain central sensitisation. The response in the pain neuromatrix is strongly influenced by the context in which the painful stimulus appears and within the context of a therapeutic encounter, DN may in fact trigger a conditioned pain modulation response by activating an endogenous pain inhibitory mechanism that inhibits early nociceptive processing.50,51
Effectiveness of TrP-DN
Trigger point dry needling for muscles of the thoracic spine is a technique that is often employed in clinical practice by a trained clinician to help in the management of pain and dysfunction, yet there is very limited research on DN in the thoracic region. The only research to date is a case series that describes the use of TrP-DN and intramuscular stimulation for non-specific thoracic spine pain in two patients.52 Both patients exhibited painful and altered movement patterns in addition to tissue hypertonicity in the thoracic paraspinal musculature. TrP-DN combined with electrical stimulation was performed to the affected thoracic multifidi and paraspinal muscles at the affected levels. After two sessions of TrP-DN and specific motor control exercises, both subjects demonstrated pain-free motion and a reduction in pain.
Despite the limited scientific evidence for TrP-DN in the thoracic spine region, there is growing support for the use of TrP-DN in other specific regions. Understanding TrP-DN research performed in these other areas can help to apply those concepts to the thoracic spine region until research is done in this region. For instance, it is known that TrP-DN to latent myofascial TrPs in shoulder musculature improves muscular activation patterns with elevation20,53,54 and muscle stiffness.55 Trigger point-DN can also improve range of motion of the cervical spine,56,57 temporomandibular joint (TMJ),17,18 and shoulder.58,59 A meta-analysis concluded that TrP-DN exhibits grade A evidence for reducing pain in upper quadrant syndrome at short-term, but given the small sample sizes of the included studies, these conclusions may be a bit premature and more research is clearly needed.60 Another meta-analysis found no differences between TrP-DN and lidocaine immediately after treatment.61
Looking at research detailed above and how TrP-DN influences different aspects of the musculoskeletal pain system, allows for understanding how TrP-DN in the work of Rock and Rainey had a positive effect on the reported patients.52 Both subjects had subjective reports of pain, pain with movement and altered movement patterns. In fact, we should also consider neurophysiological mechanisms of TrP-DN.62
Safety of TrP-DN in the thoracic spine
Prior to performing TrP-DN of the muscles related to thoracic spine pain, there are several factors that need to be considered. First and foremost, the clinician needs to have proper training in treating this region and the musculature, as there are some risks associated with poor needling technique including penetrating the lung fields, the spinal cord, peripheral nerves or blood vessels, or causing spinal infection.63–66
There is no available evidence of reported adverse events by physical therapists performing TrP-DN in the thoracic spine and trunk region. The only study to date that has explored the safety of TrP-DN by physical therapists has reported the technique to be safe. Because there were no documented serious adverse events in 7,629 TrP-DN treatments, the risk of occurrence of a significant adverse event was calculated to be < 0.04%.67 Although TrP-DN by physical therapists is safe, it cannot be implied that there is no risk of potentially serious complication. To optimise the safety of TrP-DN, adequate competency-based training is required.68
Even with training, if there is any hesitation or doubt about the ability to perform TrP-DN or the known benefit of applying the technique to a particular patient, TrP-DN should not be performed, as the risk to the patient may be greater than the benefit. Finally, all healthcare providers using solid filament needles, such as physical therapists, can learn from the experiences of acupuncturists to avoid placing the safety of patients at risk.
Precautions and contraindications to TrP-DN in the thoracic spine
Precautions and contraindications specific to TrP-DN in the thoracic spine region must be addressed. Contraindications include local or systemic infections, local skin lesions in the area requiring DN or an inability to communicate appropriately. Precautions include needle aversion, severe hyperalgesia or allodynia, or abnormal bleeding tendencies. A full detailed list of precautions and contraindications are detailed within the APTA description of TrP-DN in its clinical practice resource paper.47 An additional contraindication, not included in the APTA document, for performing TrP-DN in the thoracic spine region would be if the patient has a lipoma in the area to be treated. Lipomas can make it challenging to maintain appropriate landmarks for safety. It is not recommended that clinicians needle through a lipoma since muscles below cannot be adequately palpated. For areas other than the thoracic area, additional consideration needs to include implanted devices, pacemakers and cosmetic implants as performing TrP-DN in these areas is contraindicated.
TrP-DN of Thoracic Spine Musculature
In this section, we will discuss the application of TrP-DN into the main thoracic musculature including the thoracic multifidi and longissimus thoracis muscles.
Thoracic multifidi muscle
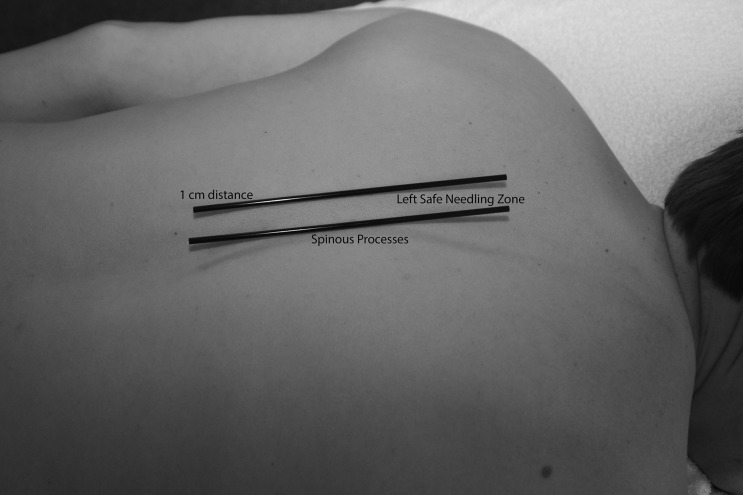
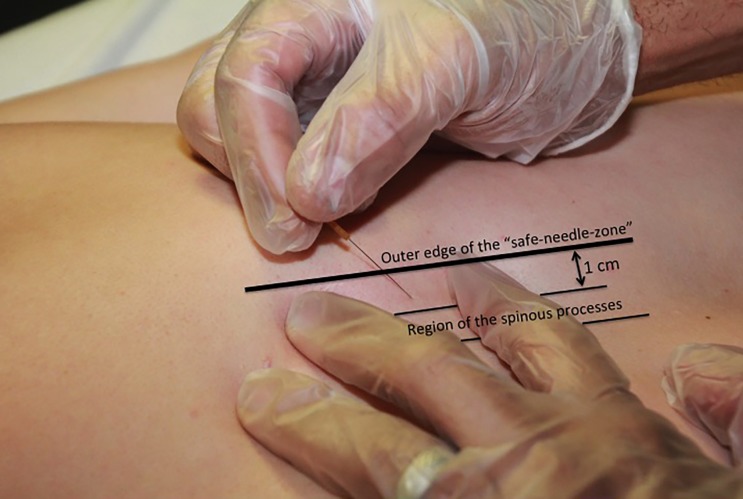
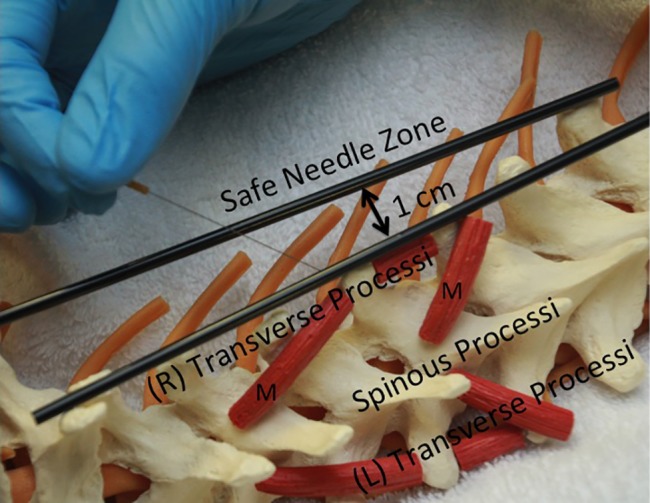
Trigger point dry needling of specific thoracic spine muscles requires proper knowledge of anatomy to ensure the patient's safety.69 The thoracic multifidi have attachments from the transverse processes to the base or top of the spinous process of the vertebrae above. The deepest layers attach to the adjacent vertebrae, the most superficial layers attach three to four levels above, and those in the middle layer attach two to three levels above. To properly palpate these muscles in the thoracic region, a finger must be placed immediately next to the spinous processes on either side. In this ‘gutter or valley’, TrPs in these muscles will typically feel like dense ‘speed bumps’ as the clinician palpates longitudinally down each level next to the spinous processes. To insert a needle safely into the thoracic multifidi muscle, there is a safe needle zone that must be adhered to, so that the needle does not puncture the lung cavity. The safe needle zone is one finger width (of the patient) immediately lateral to the spinous process, such that one side of the index finger is butting against the spinous process (Fig. 1). The needle is inserted perpendicular to the skin immediately inside the lateral borders of the index finger. Once the needle is tapped into the subcutaneous tissue, it is directed in a caudal medial direction towards the lamina (Fig. 2). In order to know that the multifidus muscle has been penetrated, the needle must come into contact with the vertebrae lamina. The direction of the needle should never be in a cranial medial direction as this can cause penetration of the spinal canal. Manipulation of the needle ‘in and out’ of the muscle should stay within the safe needle zone and maintain a caudal medial direction to ensure coming into contact with the lamina each time. For most patients, a 0.30 × 50 mm needle is used to ensure proper depth while also not permitting needle insertion completely up to the handle, as the needle has the potential to break at this junction.
Figure 1.
Safe zone on the thoracic spine for dry needling of the thoracic multifidi.
Figure 2.
Myofascial trigger point dry needling of the thoracic multifidi muscle. The needling is inserted into the safe zone of the thoracic spine, corresponding with the lamina of the thoracic vertebrae.
When the needle is inserted into a myofascial TrP in the thoracic multifidi, the patient will often complain of a deep aching cramp as a referred pain sensation; however, symptoms can also refer to the chest, along a rib (mimicking an intercostal neuralgia), or downward and/or outward several thoracic segments. From a clinical perspective, it has been observed that after treatment of the thoracic multifidi on both sides in areas of joint restriction, passive inter-vertebral mobility is improved. Often, if there is a restriction in forward bending, this will be improved as well.
In the upper thoracic region, down to T6 level specifically, needling of the thoracic multifidi may have an influence on semispinalis capitis, semispinalis cervicis, splenius capitis and splenius cervicis muscles since they originate from the thoracic spine. It has been shown clinically that performing TrP-DN in this area improves pain in the cervical spine region. Additionally, TrP-DN of the thoracic multifidi muscle can also influence other muscles including middle trapezius, rhomboid major, rhomboid minor, lower trapezius, latissimus dorsi and serratus posterior inferior muscles due to their attachments to their respective thoracic spinous processes. Therefore, these muscles can be influenced from a mechanical and/or a pain referral perspective. This improvement is potentially likely due to an indirect treatment of these cervical muscles, although formal research on this topic is needed.
Longissimus thoracis muscle
The longissimus thoracis muscle is immediately lateral to the thoracic multifidi on each side of the thoracic spine. It is the ‘hill’ muscle next to the thoracic multifidi. The muscle attaches from the tips of the transverse processes of all the thoracic vertebrae to ribs 3 or 4 through 12 between the angles and tubercles.69 Because there are no boney landmarks to use for TrP-DN of this muscle, a different technique from the multifidus is employed. After finding the myofascial TrP by palpating perpendicular to the muscle fibres, the TrP is marked with the guide tube by gently pressing down on the myofascial TrP. The needle is then inserted perpendicular to the skin just above the myofascial TrP, while the index and middle fingers are on each side of the needle and TrP (Fig. 3). After the needle is quickly tapped into the skin, the needle is directed at a shallow angle towards the myofascial TrP, going longitudinally along the length of the muscle. The depth of the needle can be gradually increased, but in order to maintain safety, it should not increase to more than 30–45° angle, depending on the muscular development of the patient (Fig. 4). This will ensure that the needle does not penetrate the lung. In addition to the needle being directed longitudinally into the muscle, it can have a slight medial direction, but never a lateral, as a lateral direction places the point of the needle towards the lung tissue.
Figure 3.
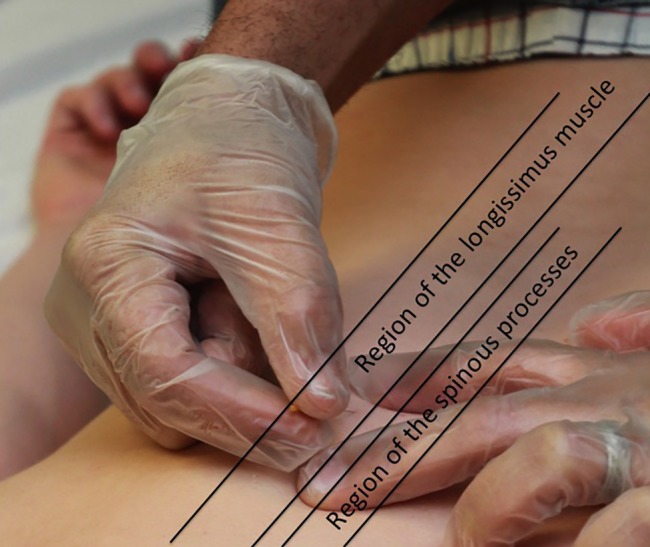
Myofascial trigger point dry needling of the longissimus thoracis muscle.
Figure 4.
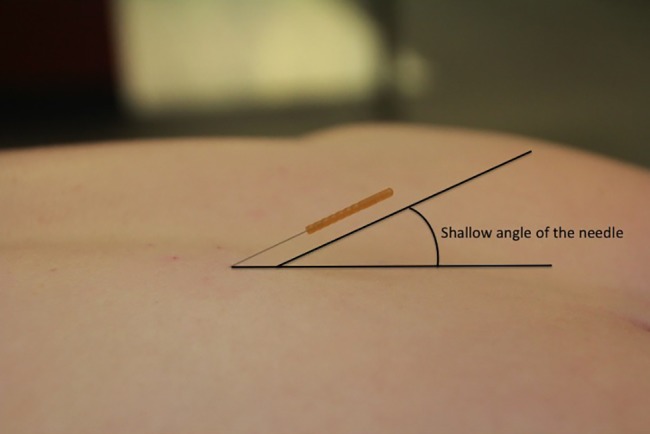
Angulation of the needle during dry needling of the longissimus thoracis.
When manipulating the needle ‘in and out’ of the muscle, the direction should always stay caudal or caudal medial. For most patients, a 0.30 × 50 mm needle is used to ensure proper depth and coverage, while also not permitting needle insertion completely up to the handle, as the needle has the potential to break at this junction. For patients with smaller musculature, a 0.30 × 30 mm needle is appropriate.
The presence of a scoliosis is a contraindication to TrP-DN of both the thoracic multifidi and longissimus thoracis muscles, because of the vertebral rotation, which can alter boney landmarks and no longer ensures safety of needle insertion into the targetted tissue (Fig. 5).
Figure 5.
Scheme of the rotation of the thoracic vertebras in a patient with scoliosis.
Conclusion
The current paper summarises current evidence of the relevance of muscle pain in thoracic spine pain. Several neck, shoulder and spine muscles refer pain to the thoracic spine. TrP-DN can be safely applied to the thoracic spine musculature by an experienced clinician. There is a lack of research into this area since no study has investigated the effectiveness of TrP-DN application to the thoracic spine.
Disclaimer Statements
Contributors All authors have participated actively in the current paper.
Funding C Fernández-las-Peñas was partially fund supported by the ‘Grupo Excelencia Investigadora URJC-Banco Santander (N°30VCPIGI03): Investigación traslacional en el proceso de salud - enfermedad (ITPSE)’.
Conflicts of interest All authors of the manuscript declare no conflicts of interest.
Ethics approval None required.
References
- 1.Briggs AM, Bragge P, Smith AJ, Govil D, Straker LM. Prevalence and associated factors for thoracic spine pain in the adult working population: a literature review. J Occup Health. 2009;51:177–92. doi: 10.1539/joh.k8007. [DOI] [PubMed] [Google Scholar]
- 2.Briggs AM, Smith AJ, Straker LM, Bragge P. Thoracic spine pain in the general population: prevalence, incidence and associated factors in children, adolescents and adults: a systematic review. BMC Musculoskelet Disord. 2009;10:77. doi: 10.1186/1471-2474-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fouquet N, Bodin J, Descatha A, Petit A, Ramond A, Ha C et al. Prevalence of thoracic spine pain in a surveillance network. Occup Med. 2015;65(2):122–5. doi: 10.1093/occmed/kqu151. [DOI] [PubMed] [Google Scholar]
- 4.Dionne CE, Bourbonnais R, Frémont P, Rossignol M, Stock SR, Nouwen A et al. Determinants of “return to work in good health” among workers with back pain who consult in primary care settings: a 2-year prospective study. Eur Spine J. 2007;16:641–55. doi: 10.1007/s00586-006-0180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michael ALR, Newman J, Rao AS. The assessment of thoracic pain. Orthop Trauma. 2010;24:63–73. [Google Scholar]
- 6.Goodman CC, Sndyer TK. Differential diagnosis for physical therapists: screening for referral. 5th edn. St. Louis MO: Elsevier Saunders; 2013. [Google Scholar]
- 7.Mader R, Sarzi-Puttini P, Atzeni F, Olivieri I, Pappone N, Verlaan JJ et al. Extraspinal manifestations of diffuse idiopathic skeletal hyperostosis. Rheumatology. 2009;48:1478–81. doi: 10.1093/rheumatology/kep308. [DOI] [PubMed] [Google Scholar]
- 8.Moore KL. Clinically oriented anatomy. 3rd edn. Baltimore, MD: Williams & Wilkins; 1992. [Google Scholar]
- 9.Atluri S, Datta S, Falco FJ, Lee M. A systematic review of diagnostic utility and therapeutic effectiveness of thoracic facet joint interventions. Pain Phys. 2008;11:611–29. [PubMed] [Google Scholar]
- 10.Manchikanti L, Boswell MV, Singh V, Pampati V, Damron KS, Beyer CD. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15. doi: 10.1186/1471-2474-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manchikanti KN, Atluri S, Singh V, Geffert S, Sehgal N, Falco FJ. An update of evaluation and therapeutic thoracic facet interventions. Pain Phys. 2012;15:E463–81. [PubMed] [Google Scholar]
- 12.Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V, Fellows B. Comparative effectiveness of a one-year follow-up of thoracic medial branch blocks in management of chronic thoracic pain: a randomized, double-blind active controlled trial. Pain Phys. 2010;13:535–48. [PubMed] [Google Scholar]
- 13.Handwerker H, Arendt-Nielsen L. Pain model: translational relevance and applications. Seattle: IASP Press; 2013. [Google Scholar]
- 14.Simons DG, Travell JG, Simons LS. Myofascial pain and dysfunction: the trigger point manual (Vol 1). Upper half of the body. 2nd edn. Baltimore, MD: Williams & Wilkins; 1999. [Google Scholar]
- 15.Bron C, Dommerholt J, Stegenga B, Wensing M, Oostendorp RA. High prevalence of shoulder girdle muscles with myofascial trigger points in patients with shoulder pain. BMC Musculoskelet Disord. 2011;12:139. doi: 10.1186/1471-2474-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calandre EP, Hidalgo J, Garcia-Leiva J, Rico-Villademoros F. Trigger point evaluation in migraine patients: an indication of peripheral sensitization linked to migraine predisposition? Eur J Neurology. 2006;13:244–9. doi: 10.1111/j.1468-1331.2006.01181.x. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Carnero J, La Touche R, Ortega-Santiago R, Galan-del-Rio F, Pesquera J, Ge HY et al. Short-term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. J Orofac Pain. 2010;24:106–12. [PubMed] [Google Scholar]
- 18.Gonzalez-Perez L, Infante-Cossio P, Granados-Nuñez M, Urresti-Lopez FJ. Treatment of temporomandibular myofascial pain with deep dry needling. Med Oral Patol Oral Cir Bucal. 2012;17:781–5. doi: 10.4317/medoral.17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celik D, Yeldan I. The relationship between latent trigger points and muscle strength in healthy subjects: a double blind study. J Back Musculoskelet Rehabil. 2011;24:251–6. doi: 10.3233/BMR-2011-0302. [DOI] [PubMed] [Google Scholar]
- 20.Lucas KR, Rich PA, Polus BI. Muscle activation patterns in the scapular positioning muscles during loaded scapular plane elevation: the effects of latent myofascial trigger points. Clin Biomech. 2010;25:765–70. doi: 10.1016/j.clinbiomech.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Sikdar S, Shah JP, Gebreab T, Yen RH, Gilliams E, Danoff J et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90:1829–38. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turo D, Otto P, Shah JP, Heimur J, Gebreab T, Zaazhoa M et al. Ultrasonic characterization of the upper trapezius muscle in patients with neck pain. Ultrason Imaging. 2013;35:173–87. doi: 10.1177/0161734612472408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge HY, Arendt-Nielsen L, Madeleine P. Accelerated muscle fatigability of latent myofascial trigger points in humans. Pain Med. 2012;13:957–64. doi: 10.1111/j.1526-4637.2012.01416.x. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-de-Las-Peñas C, Galán-Del-Río F, Alonso-Blanco C, Jiménez-García R, Arendt-Nielsen L, Svensson P. Referred pain from muscle trigger points in the masticatory and neck-shoulder musculature in women with temporomandibular disorders. J Pain. 2010;11:1295–304. doi: 10.1016/j.jpain.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Giamberardino MA, Tafuri E, Savini A, Fabrizio A, Affaitati G, Lerza R et al. Contribution of myofascial trigger points to migraine symptoms. J Pain. 2007;8:869–78. doi: 10.1016/j.jpain.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RU, Sawyer T, Wise D, Morey A, Nathanson BH. Painful myofascial trigger points and pain sites in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2009;182:2753–8. doi: 10.1016/j.juro.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Ge HY, Wang Y, Fernández-de-las-Peñas C, Graven-Nielsen T, Danneskiold-Samsøe B, Arendt-Nielsen L. Reproduction of overall spontaneous pain pattern by manual stimulation of active myofascial trigger points in fibromyalgia patients. Arthritis Res Ther. 2011;13:R48. doi: 10.1186/ar3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-de-las-Penas C, Fernández J, Miangolarra JC. Musculoskeletal disorders in mechanical neck pain: myofascial trigger points versus cervical joint dysfunctions: a clinical study. J Musculoskeletal Pain. 2005;13:27–35. [Google Scholar]
- 29.Fernández-de-las-Penas C, Alonso-Blanco C, Alguacil-Diego IM, Miangolarra JC. Myofascial trigger points and posterior-anterior joint hypomobility in the mid-cervical spine in subjects presenting with mechanical neck pain: a pilot study. J Man Manip Ther. 2006;14:8894. [Google Scholar]
- 30.Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons' integrated hypothesis of trigger point formation. Curr Pain Headache Rep. 2004;8:468–75. doi: 10.1007/s11916-004-0069-x. [DOI] [PubMed] [Google Scholar]
- 31.Brückle W, Sückfull M, Fleckenstein W, Weiss C, Müller W. Gewebe-pO2-Messung in der verspannten Rückenmuskulatur (m. erector spinae) Zeitschrift fur Rheumatologie. 1990;49:208–16. [PubMed] [Google Scholar]
- 32.Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med. 2011;30:1331–40. doi: 10.7863/jum.2011.30.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh YL, Yang SA, Yang CC, Chou LW. Dry needling at myofascial trigger spots of rabbit skeletal muscles modulates the biochemicals associated with pain, inflammation, and hypoxia. Evid Based Complement Alternat Med. 2012;2012:342165. doi: 10.1155/2012/342165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerdle B, Ghafouri B, Ernberg M, Larsson B. Chronic musculoskeletal pain: review of mechanisms and biochemical biomarkers as assessed by the microdialysis technique. J Pain Res. 2014;7:313–26. doi: 10.2147/JPR.S59144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–84. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 36.Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89:16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Shah JP, Gilliams EA. Uncovering the biochemical milieu of myofascial trigger points in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther. 2008;12:371–84. doi: 10.1016/j.jbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Lewit K. The needle effect in the relief of myofascial pain. Pain. 1979;6:83–90. doi: 10.1016/0304-3959(79)90142-8. [DOI] [PubMed] [Google Scholar]
- 39.Bismil Q, Bismil M. Myofascial-entheseal dysfunction in chronic whiplash injury: an observational study. JRSM Short Rep. 2012;3:57. doi: 10.1258/shorts.2012.012052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langevin H, Bouffard NA, Fox JR, Palmer BM, Wu J, Iatridis JC et al. Fibroblast cytoskeletal remodeling contributes to connective tissue tension. J Cell Physiol. 2011;226:1166–75. doi: 10.1002/jcp.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langevin H, Churchill D, Cipolla MJ. Mechanical signaling through connective tissue: a mechanism for the therapeutic effect of acupuncture. FASEB J. 2001;15:2275–82. doi: 10.1096/fj.01-0015hyp. [DOI] [PubMed] [Google Scholar]
- 42.Dommerholt J. Fascia and dry needling. In: Dommerholt J, Fernández-de-las-Peñas C, editors. Trigger point dry needling: an evidence-based approach. Edinburgh: Elsevier; 2013. pp. 35–8. [Google Scholar]
- 43.Dunning J, Butts R, Mourad F, Young I, Flannagan S, Perreault T. Dry needling: a literature review with implications for clinical practice guidelines. Phys Ther Rev. 2014;19:252–65. doi: 10.1179/108331913X13844245102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haslam R. A comparison of acupuncture with advice and exercises on the symptomatic treatment of osteoarthritis of the hip: a randomised controlled trial. Acupunct Med. 2001;19:19–26. doi: 10.1136/aim.19.1.19. [DOI] [PubMed] [Google Scholar]
- 45.Inoue M, Kitakoji H, Ishizaki N, Tawa M, Yano T, Katsumi Y et al. Relief of low back pain immediately after acupuncture treatment: a randomised, placebo controlled trial. Acupunct Med. 2006;24:103–8. doi: 10.1136/aim.24.3.103. [DOI] [PubMed] [Google Scholar]
- 46.Melchart D, Thormaehlen J, Hager S, Liao J, Linde K, Weidenhammer W. Acupuncture versus placebo versus sumatriptan for early treatment of migraine attacks: a randomized controlled trial. J Intern Med. 2003;253:181–8. doi: 10.1046/j.1365-2796.2003.01081.x. [DOI] [PubMed] [Google Scholar]
- 47.APTA. Description of dry needling in clinical practice: an educational resource paper. Alexandria, VA, USA: APTA Public Policy, Practice, and Professional Affairs Unit; 2013. [Google Scholar]
- 48.Physiotherapy Acupuncture Association of New Zealand. Guidelines for safe acupuncture and dry needling practice. Physiotherapy Acupuncture Association of New Zealand; Wellington, New Zealand: 2014. [Google Scholar]
- 49.Jull GA. Management of cervical spine disorders: where to now? J Orthop Sports Phys Ther. 2012;40:A3–A7. [Google Scholar]
- 50.Bjorkedal E, Flaten MA. Expectations of increased and decreased pain explains the effect of conditioned pain modulation in females. J Pain Res. 2012;5:289–300. doi: 10.2147/JPR.S33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–24. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Rock JM, Rainey CE. Treatment of nonspecific thoracic spine pain with trigger point dry needling and intramuscular electrical stimulation: a case series. Int J Sports Phys Ther. 2014;9:699–711. [PMC free article] [PubMed] [Google Scholar]
- 53.Lucas KR, Polus BI, Rich PA. Latent myofascial trigger points: their effects on muscle activation and movement efficiency. J Bodyw Mov Ther. 2004;8:160–6. [Google Scholar]
- 54.Lucas KR. The impact of latent trigger points on regional muscle function. Curr Pain Headache Rep. 2008;12:344–9. doi: 10.1007/s11916-008-0058-6. [DOI] [PubMed] [Google Scholar]
- 55.Maher RM, Hayes DM, Shinohara M. Quantification of dry needling and posture effects on myofascial trigger points using ultrasound shear-wave elastography. Arch Phys Med Rehabil. 2013;94:2146–50. doi: 10.1016/j.apmr.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Llamas-Ramos R, Pecos-Martín D, Gallego-Izquierdo T, Llamas-Ramos I, Plaza-Manzano G, Ortega-Santiago R et al. Comparison of the short-term outcomes between trigger point dry needling and trigger point manual therapy for the management of chronic mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2014;44:852–61. doi: 10.2519/jospt.2014.5229. [DOI] [PubMed] [Google Scholar]
- 57.Mejuto-Vázquez MJ, Salom-Moreno J, Ortega-Santiago R, Truyols-Domínguez S, Fernández-de-las-Peñas C. Short-term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2014;44:252–60. doi: 10.2519/jospt.2014.5108. [DOI] [PubMed] [Google Scholar]
- 58.Osborne NJ, Gatt IT. Management of shoulder injuries using dry needling in elite volleyball players. Acupunct Med. 2010;28:42–5. doi: 10.1136/aim.2009.001560. [DOI] [PubMed] [Google Scholar]
- 59.Clewley D, Flynn TW, Koppenhaver S. Trigger point dry needling as an adjunct treatment for a patient with adhesive capsulitis of the shoulder. J Orthop Sports Phys Ther. 2014;44:92–101. doi: 10.2519/jospt.2014.4915. [DOI] [PubMed] [Google Scholar]
- 60.Kietrys DM, Palombaro KM, Azzaretto E, Hubler R, Schaller B, Schlussel JM et al. Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2013;43:620–34. doi: 10.2519/jospt.2013.4668. [DOI] [PubMed] [Google Scholar]
- 61.Ong J, Claydon LS. The effect of dry needling for myofascial trigger points in the neck and shoulders: a systematic review and meta-analysis. J Bodyw Mov Ther. 2014;18:390–8. doi: 10.1016/j.jbmt.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Dommerholt J. Dry needling: peripheral and central considerations. J Man Manip Ther. 2011;19:223–7. doi: 10.1179/106698111X13129729552065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim PS, Hsu W. Discitis in an adult following acupuncture treatment: a case report. J Can Chiropr Assoc. 2004;48:132–6. [PMC free article] [PubMed] [Google Scholar]
- 64.Cummings M, Ross-Marrs R, Gerwin R. Pneumothorax complication of deep dry needling demonstration. Acupunct Med. 2014;32:517–9. doi: 10.1136/acupmed-2014-010659. [DOI] [PubMed] [Google Scholar]
- 65.Peuker E, Grönemeyer D. Rare but serious complications of acupuncture: traumatic lesions. Acupunct Med. 2001;19:103–8. doi: 10.1136/aim.19.2.103. [DOI] [PubMed] [Google Scholar]
- 66.McDowell JM, Johnson GM, Hale L. Adverse reactions to acupuncture: policy recommendations based on practitioner opinion in New Zealand. New Zealand J Physiother. 2013;41:94–101. [Google Scholar]
- 67.Brady S, McEvoy J, Dommerholt J, Doody C. Adverse events following trigger point dry needling: a prospective survey of chartered physiotherapist. J Man Manip Ther. 2014;22:134–40. doi: 10.1179/2042618613Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCutcheon L, Yelland M. Iatrogenic pneumothorax: safety concerns when using acupuncture or dry needling in the thoracic region. Phys Ther Rev. 2011;16:126–32. [Google Scholar]
- 69.Dommerholt J, Fernandez-de-las-Peñas C. Trigger point dry needling: an evidence and clinical-based approach. 1st edn. London: Churchill Livingstone: Elsevier; 2013. [Google Scholar]