Abstract
Breast cancer is the most frequently diagnosed cancer in women worldwide and the second cause of cancer-related mortality. A total of 20–30% of patients with early-stage breast cancer develop recurrence within the first 5 years following diagnosis. Trastuzumab significantly improves overall survival and disease-free survival (DFS) in women with human epidermal growth factor receptor 2 (HER2)-positive early and locally advanced breast cancer. This study aimed to determine the factors that affect DFS following adjuvant transtuzumab therapy. A total of 62 patients treated with trastuzumab for early and locally advanced breast cancer were included in our study. Data, including pathology, treatment and treatment outcome, rate of recurrence and laboratory tests, were retrospectively collected. There was no significant association between DFS and age, menopausal status, disease stage and hormone receptor status. The median follow-up was 48.4 months. The median DFS of patients treated with adjuvant trastuzumab was 64.1 months. In addition, the median DFS was 44.3 vs. 66.8 months in patients with platelet-lymphocyte ratio (PLR) ≤200 vs. >200, respectively (log-rank test; P=0.001), and 70 vs. 45 months in patients with eosinophil count ≤70 vs. >70×103/mm3 (log-rank test; P=0.001). Our data revealed the prognostic relevance of a decrease in the peripheral blood eosinophil count and PLR value following trastuzumab therapy in breast cancer. PLR and eosinophil count measurements are cost-effective, readily available worldwide, non-invasive and safe. Combined with other markers, such as patient age, tumor stage and tumor histology, may be effectively used for patients with breast cancer.
Keywords: breast cancer, trastuzumab, platelet-lymphocyte ratio, eosinophil count
Introduction
Breast cancer is the most frequently diagnosed cancer in women worldwide and the second leading cause of cancer-related mortality, accounting for 23% of the total new cases of cancer (1.38 million) and 14% of the total cancer deaths (458,400) (1).
Breast cancer recurrence usually occurs within the first 5 years following diagnosis, with the majority of these recurrences being hormone receptor-negative or human epidermal growth factor receptor 2 (HER2)-positive. In certain cases, relapse may occur after 5 years, which is more common in cases of hormone receptor-positive cancer with indolent disease and HER2-negative cancer. A retrospective study evaluating 2,838 cases reported that the 5-year recurrence risk for patients with stage I, II and III breast cancer receiving adjuvant therapy was 7, 11 and 13%, respectively (2,3).
T cells are known to play a critical role in tumor immune surveillance. Although the role of immune response in breast cancer has yet to be fully elucidated, certain studies reported that chemotherapy contributes to overall treatment response by stimulating the immune response. Antibody-dependent cellular cytotoxicity plays an important role in the mechanism of action of trastuzumab and peritumoral lymphocyte infiltration has been found to be associated with improved response and survival rates in antineoplastic and trastuzumab therapy (4–7).
Platelets play a balancing role in health and disease and are the origin of active metabolites and proteins. Platelets release growth factors, such as platelet-derived growth factor, platelet factor 4, transforming growth factor β and vascular endothelial growth factor, which may stimulate tumor growth and angiogenesis. The association of poor prognosis and the increase in white blood cells, platelets, or their ratio may be explained through an inflammatory process evoked by cancer cells (8,9).
The aim of this study was to retrospectively examine the survival data of breast cancer patients who received adjuvant trastuzumab and determine the prognostic value of different peripheral blood parameters in association with disease-free survival (DFS).
Patients and methods
Patients
A total of 62 patients treated at the Akdeniz University Hospital between January, 2008 and August, 2010, who received adjuvant trastuzumab for early and locally advanced breast cancer, were retrospectively reviewed.
The study was approved by the Akdeniz University Clinical Research Ethics Committee.
Statistical analysis
Statistical analyses were performed using SPSS software, version 20.0 (IBM Corp., Armonk, NY, USA). DFS was defined as the time period between initial diagnosis and detection of the first tumor recurrence based on radiological criteria. Survival was analyzed by the Kaplan-Meier method and the univariate Cox regression analysis. The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk's test) to determine whether they are normally distributed. A descriptive analysis was presented using means and standard deviations for normally distributed variables [platelet-lymphocyte ratio (PLR) measurements]. Variables with a P-value of <0.10 in the univariate analysis were also evaluated by multivariate analysis. A P-value of <0.05 was considered to indicate statistically significant differences.
PLR
Prior to treatment, PLR was calculated as the platelet count divided by the lymphocyte count. Using a number of different cut-off points, a PLR of 200 was found to represent the optimum stratification point at which the survival difference between two groups was maximized.
Results
Patient, disease and treatment characteristics
In this study, we evaluated the data of 62 patients diagnosed with breast cancer. The study group consisted of all patients diagnosed with breast cancer between January, 2008 and August, 2010. The median follow-up period was 48.4 months. The median age of the patients was 52 years (range, 24–73 years).
As regards menopausal status, 26 (41.9%) of the patients were premenopausal and 36 postmenopausal (58.1%). A total of 75.8% of the patients underwent modified radical mastectomy. The majority of the tumors were invasive ductal carcinomas (77.4%). At the time of diagnosis, 11.1% of the patients had stage I, 53.2% had stage II and 33.9% had stage III disease. Adjuvant chemotherapy included anthracylines and taxanes in 96.8% of the cases (Table I).
Table I.
Baseline characteristics of breast cancer patients.
| Characteristics | Patient no. (%) (n=62) |
|---|---|
| Age, years | |
| Median | 52 |
| Range | 24–73 |
| Stage | |
| I | 7 (11.3) |
| II | 33 (53.2) |
| III | 21 (33.9) |
| Missing | 1 (1.6) |
| Menopausal status | |
| Premenopausal | 26 (41.9) |
| Postmenopausal | 36 (58.1) |
| Tumor type | |
| Ductal invasive | 48 (77.4) |
| Lobular invasive | 14 (22.6) |
| Tumor grade | |
| I | 6 (9.7) |
| II | 25 (40.3) |
| III | 30 (48.4) |
| Missing | 1 (1.6) |
| Hormone receptor status | |
| Positive | 35 (56.5) |
| Negative | 27 (43.5) |
| Chemotherapy regimens | |
| FACa-docetaxel | 46 (74.2) |
| FECb-docetaxel | 9 (14.5) |
| TACc | 6 (9.7) |
| CMFd | 1 (1.6) |
| Operation | |
| Breast conserving surgery | 15 (24.2) |
| Modifiied radical mastectomy | 47 (75.8) |
| Baseline eosinophil count, ×103/mm3 | |
| Median | 70 |
| Range | 10–680 |
| Baseline PLR | |
| 200≥ | 36 (58.1) |
| 200< | 26 (41.9) |
Fluorouracil, doxorubicin, cyclophosphamide.
Fluorouracil, epirubicine, cyclophosphamide.
Docetaxel, doxorubicin, cyclophosphamide.
Fluorouracil, methotrexate, cyclophosphamide.
PLR, platelet-lymphocyte ratio.
A total of 22 patients (35.5%) developed metastasis after diagnosis, with 18 (29%) of the patients developing distant and 4 (6.5%) local metastasis, whereas 3 (4.8%) patients developed brain metastasis (data not shown).
Survival analysis
The median follow-up period was 48.4 months and the patients had a median DFS of 64.1 months.
There were no significant associations between DFS and age, menopausal status, stage or hormone receptor status. The univariate analysis revealed that DFS was significantly affected by tumor grade [P=0.086 (95% CI for HR: 0.96–1.62)], PLR [P=0.021 (95% CI for HR: 1.33–4.16)] and eosinophil count [P=0.029 (95% CI for HR: 1.03–1.91)]. However, eosinophil count [P= 0.017 (95% CI for HR: 1.23–8.69)] retained significance with multivariate analysis.
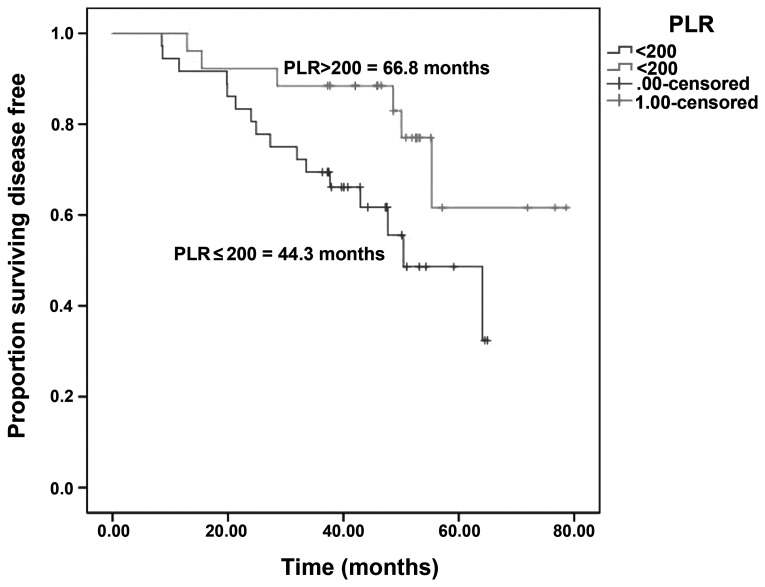
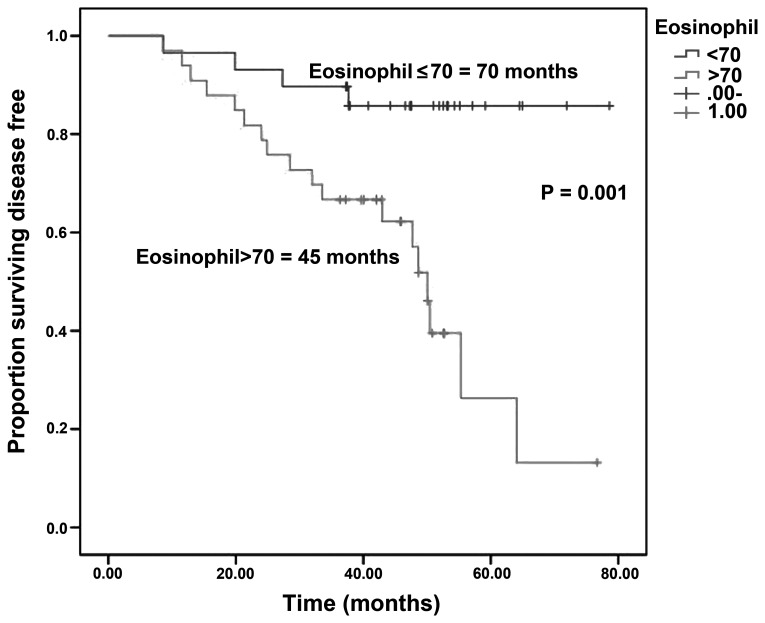
The median DFS was 44.3 vs. 66.8 months in patients with PLR ≤200 vs. >200 (log-rank test; P=0.001) (Fig. 1) and 70 vs. 45 months in patients with eosinophil count ≤70 vs. >70×103/mm3 (log-rank test; P=0.001) (Fig. 2).
Figure 1.
Disease-free survival in patients with platelet-lymphocyte ratio (PLR)≤200 vs. >200.
Figure 2.
Disease-free survival in patients with an eosinophil count of ≤70 vs. >70×103/mm3.
Discussion
To the best of our knowledge, this study is the first to demonstrate the significance of PLR and eosinophil count in breast cancer patients receiving adjuvant trastuzumab therapy.
Blood markers such as PLR and eosinophil count are simple, rapidly available laboratory markers. A previous study, including >25,000 patients, demonstrated the significance of inflammatory markers in the prediction of the outcome of various types of cancer (10). Feng et al found that PLR is associated with tumor progression and may be considered as an independent marker of poor prognosis in patients who undergo esophagectomy for esophageal squamous cell carcinoma without neoadjuvant or adjuvant treatment (11). Certain studies demonstrated that the presence of pretreatment lymphopenia is associated with poor cancer survival or tumor response and it has not only prognostic, but also predictive potential (12–14). In a study conducted by the Radiation Therapy Oncology Group, lymphopenia and hormone receptor negativity were found to be independent prognostic factors indicating poor survival in breast cancer patients with brain metastases (15). A more recent study conducted by the same group concluded that lymphocyte count and LDH levels may predict overall survival (16). Peritumoral lymphocyte infiltration in trastuzumab therapy is known to enhance treatment response and survival (7). Combination chemotherapies were shown to reduce peripheral lymphocyte count in cancer patients. These findings suggest that treatment-induced lymphopenia may be associated with an increased tumor response (17). In our study, we demonstrated that patients with PLR >200 and eosinophil count <70×103/mm3 exhibited better DFS rates.
In early-stage breast cancer, the recurrence rate varies between 20 and 30%. Trastuzumab therapy is known to significantly reduce recurrence and mortality, with a 50% reduced risk of breast cancer recurrence and 30% improved survival rate. In patients with HER2-overexpressing early-stage breast cancer treated with trastuzumab-based therapy, the most common location for disease progression is the isolated central nervous system (18–20). This has been associated with the inability of trastuzumab to penetrate the blood-brain barrier or the brain-metastatic breast tumor cells losing the expression of HER2; it may also be explained by the overall effectiveness of trastuzumab in disease control, except in the central nervous system (21). In our study, 35.3% of our patients receiving adjuvant trastuzumab therapy developed recurrence. Of these patients, 3 (6.3%) developed isolated brain metastases. The respective percentage was 2.56% in the study conducted by Olson et al (21).
When recurrence of breast cancer is diagnosed, the initial evaluation should include hormone receptor status, DFS, age and menopausal status. Previous research in this field has demonstrated that estrogen and progesterone receptor status are independent predictors of survival after the first recurrence (22). While Clark et al (23) and Insa et al (24) found that these were independent predictors of patient survival following relapse, Koenders et al (25) found no such association. In our study, we observed no correlations between hormone receptor status, menopausal status and disease-free survival.
In conclusion, PLR and eosinophil count are cost-effective, readily available worldwide, non-invasive and safe and, when combined with other markers, such as patient age, tumor stage and tumor histology, may be effectively used for patients with breast cancer.
References
- 1.Jemal A, Bray F. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179–1183. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vicente Conesa MA, Garcia-Martinez E, Billalabeitia EG, Benito AC, Garcia TG, Garcia VV, Ayala de la Peña F. Predictive value of peripheral blood lymphocyte count in breast cancer patients treated with primary chemotherapy. The Breast. 2012;21:468–474. doi: 10.1016/j.breast.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Mackall CL, Fleisher TA, Brown MR, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. 1994;84:2221–2228. [PubMed] [Google Scholar]
- 6.Smith I, Procter M, Gelber RD, et al. HERA study team: 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 7.Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperativetrastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105:13–33. doi: 10.1160/THS10-11-0720. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 10.Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome study. Eur J Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Feng JF, Huang Y, Liu JS. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2013;6:1605–1612. doi: 10.2147/OTT.S52501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papatestas AE, Lesnick GJ, Genkins G, Aufses AH., Jr The prognostic significance of peripheral lymphocyte counts in patients with breast carcinoma. Cancer. 1976;37:164–168. doi: 10.1002/1097-0142(197601)37:1<164::AID-CNCR2820370123>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Pattison CW, Woods KL, Morrison JM. Lymphocytopenia as an independent predictor of early recurrence in breast cancer. Br J Cancer. 1987;55:75–76. doi: 10.1038/bjc.1987.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray-Coquard I, Cropet C, Van GM, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Scodan R, Massard C, Mouret-Fourme E, et al. Brain metastases from breast carcinoma: validation of the Radiation Therapy Oncology Group recursive partitioning analysis classification and proposition of a new prognostic score. Int J Radiat Oncol Biol Phys. 2007;69:839–845. doi: 10.1016/j.ijrobp.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Le Scodan R, Massard C, Jouanneau L, Coussy F, Gutierrez M, Kirova Y, Lerebours F, Labib A, Mouret-Fourme E. Brain metastases from breast cancer: proposition of new prognostic score including molecular subtypes and treatment. J Neurooncol. 2012;106:169–176. doi: 10.1007/s11060-011-0654-x. [DOI] [PubMed] [Google Scholar]
- 17.Delyon J, Mateus C, Lefeuvre D, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24:1697–1703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 18.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), corp-author Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 21.Olson EM, Abdel Rausoul M, Maly J, Wu CS, Lin NU, Shapiro CL. Insidence and risk of central nervous system metastases as site of first recurrence in patients in with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24:1526–1533. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji W, Teramukai S, Ueno M, Toi M, Inamoto T. Prognostic factors for survival after first recurrence in breast cancer: a retrospective analysis of 252 recurrent cases at a single institution. Breast Cancer. 2014;21:86–95. doi: 10.1007/s12282-012-0358-x. [DOI] [PubMed] [Google Scholar]
- 23.Clark GM, Sledge GW, Jr, Osborne CK, McGuire WL. Survival from first recurrence: relative importance of prognostic factors in 1,015 breast cancer patients. J Clin Oncol. 1987;5:55–61. doi: 10.1200/JCO.1987.5.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Insa A, Lluch A, Prosper F, Marugan I, Martinez-Agullo A, Garcia-Conde J. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67–78. doi: 10.1023/A:1006285726561. [DOI] [PubMed] [Google Scholar]
- 25.Koenders PG, Beex LV, Kloppenborg PW, Smals AG, Benraad TJ. Human breast cancer: survival from first metastasis. Breast Cancer Study Group. Breast Cancer Res Treat. 1992;21:173–180. doi: 10.1007/BF01975000. [DOI] [PubMed] [Google Scholar]