Abstract
The immunochemical faecal occult blood test (iFOBT) is a simple, non-invasive colorectal cancer (CRC) screening method for reducing CRC-related mortality. However, the sensitivity of iFOBT is imperfect and certain colonic neoplasms that require removal may be missed. The aim of this study was to investigate the incidence and characteristics of CRC in asymptomatic, iFOBT-negative patients who underwent opportunistic screening. A total of 919 subclinical patients (276 iFOBT-positive and 643 iFOBT-negative) in the health screening program of our hospital underwent total colonoscopy (TCS) within 2 years after iFOBT. The patients were divided into an iFOBT-positive and an iFOBT-negative group and the TCS findings were compared between the two groups. Although the incidence of advanced neoplasia (CRC, high-grade dysplasia, adenoma sized ≥10 mm and tubulovillous adenoma) was significantly higher in the iFOBT-positive group, these lesions were also found in 6.3% of iFOBT-negative patients. The lesions tended to be proximally located and non-protruding. In conclusion, screening with iFOBT remains clinically significant. However, colonoscopy is indispensable for reducing the incidence and mortality of CRC.
Keywords: colorectal cancer screening, immunochemical faecal occult blood test, colonoscopy, advanced neoplasia, advanced adenoma, asymptomatic patient, opportunistic screening
Introduction
The incidence and mortality of colorectal cancer (CRC) have been increasing in Japan (1). Therefore, screening is crucial for the early detection of CRC. The faecal occult blood test (FOBT) is a simple, low-cost, non-invasive screening method. Although annual or biennial guaiac-based FOBT screening reduces the incidence of CRC by 17–20% (2) and CRC mortality by 16–33% (3–5), this screening method has been criticised due to its poor sensitivity (6,7). Immunochemical FOBT (iFOBT) exhibits improved sensitivity and specificity and involves no dietary restrictions, resulting in fewer abnormalities due to interfering substances (8). Therefore, iFOBT has been recommended as a population-based CRC screening test in Japan since 1992 (9). Colonoscopy (CS) is the most accurate test for detecting early cancer and for detecting and removing advanced adenomas (10–17). However, due to its potential limitations, low availability of qualified endoscopists and high cost, CS is considered an opportunistic screening or detailed examination method for patients with positive FOBT results in population-based screening. Therefore, the characteristics of FOBT-negative colorectal tumours may not be evident, as patients do not generally undergo CS when their FOBT results are negative.
The aim of this study was to elucidate the characteristics of iFOBT-negative colorectal tumours in asymptomatic patients who underwent opportunistic screening in our hospital.
Materials and methods
Patients
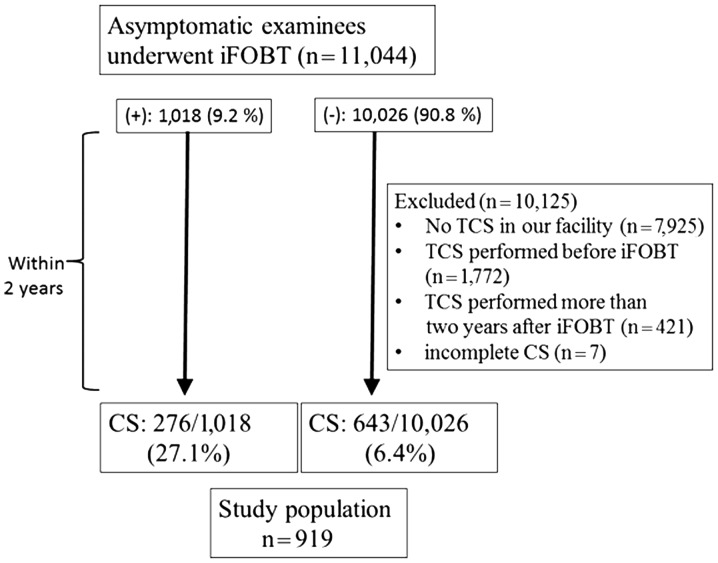
Between December, 2001 and August, 2012, iFOBT was performed in 11,044 subclinical patients in the health screening program of Showa University Northern Yokohama Hospital (Yokohama, Japan). The study protocol from iFOBT to CS is outlined in Fig. 1. Total colonoscopy (TCS) involved CS from the caecum to the rectum. A total of 7,801 patients did not undergo TCS or underwent TCS in other facilities, 2,317 underwent TCS prior to iFOBT, 421 underwent TCS >2 years after iFOBT and 7 underwent incomplete CS. For these reasons, 10,125 patients were excluded from this study. A total of 919 patients (27.1% iFOBT-positive and 6.4% iFOBT-negative) underwent TCS in our facility within 2 years after iFOBT, regardless of the test results. All the eligible patients were asymptomatic. The patients were divided into iFOBT-positive and -negative groups and the characteristics of TCS were compared between the two groups within 2 years after iFOBT.
Figure 1.
Study flow chart. iFOBT, immunochemical faecal occult blood test; CS colonoscopy; TCS, total CS.
This study's protocol was approved by the Clinical Research Ethics Committee of Showa University Northern Yokohama Hospital. The study was performed in accordance with the principles of the Declaration of Helsinki. This study is registered in the University Hospital Medical Network Clinical Trials Registry (UMIN000012116). We used individual and endoscopic data from the database of Showa University Northern Yokohama Hospital and written informed consent for TCS was obtained from all the examinees prior to conducting the original procedures.
iFOBT
We performed 1-day iFOBT. The patients were asked to prepare a faecal sample from a specimen using an iFOBT kit. The OC-Hemodia was used between December, 2001 and March, 2008 and the OC-Hemocatch S between April, 2008 and August, 2012 (both from Eiken Chemical Co., Ltd., Tokyo, Japan). The faecal sample was delivered to the hospital within 3 days and tested immediately.
CS examination and pathological findings
All the patients underwent bowel preparation with 2–3 l polyethylene glycol solution prior to CS. Diazepam and butylscopolamine were intravenously administered for sedation and prevention of peristalsis. All the detected lesions were endoscopically examined at ~80- to 100-fold magnification (CF-240ZI, CF-H260AZI, or PCF-240ZI; Olympus, Tokyo, Japan). Following conventional examination, the shape of each lesion was classified according to the Paris classification system (18). For colour staining, 0.2% indigo carmine dye and 0.05% crystal violet were applied directly through the endoscope channel and the pit pattern was determined according to the Kudo's pit pattern classification with the magnifying view (19–22). The Kudo's classification system involves morphological analysis of the colorectal crypts for diagnosis. The pattern is classified as one of five typesas follows: type I, round pits; type II, stellar pits; type III, tubular or small, roundish pits; type IV, branch-like or gyrus-like pits; and type V, irregular or non-structural pits (19,21–23). Lesions with type I or II patterns are defined as non-neoplastic. Type III, IV, or VI low-grade patterns are defined as adenoma (including high-grade dysplasia) or slightly invasive cancer that may be completely resected with endoscopy. Type VI high-grade and type VN patterns are defined as massively invasive cancer. The degree of submucosal invasion was classified into two groups: Slightly invasive submucosal cancer (SMs; invasion depth <1,000 µm) and massively invasive submucosal cancer (SMm; invasion depth ≥1,000 µm) (23,24). SMs does not metastasise as readily as adenomas, making it a good indication for endoscopic resection, whereas SMm exhibits nodal metastasis (~10%), thus requiring surgical resection. All the observed lesions with data on the pit pattern findings, location, shape and diameter were documented in the electronic medical charts. The CS findings were classified according to the most advanced histological lesion found and the results were expressed in terms of number of patients and number of polyps. When neither polyps (adenomatous, hyperplastic, juvenile or inflammatory) nor cancer was detected, the CS findings were classified as normal. If possible, all the observed neoplastic lesions were removed endoscopically or surgically and other lesions were biopsied if necessary. If histopathological evaluation was not possible (e.g., the specimen could not be collected, the patient was on oral anticoagulants, or numerous lesions were present), the pit pattern diagnosis was substituted for the pathological diagnosis. When the location of the lesions was analysed, the distal colon was defined as the rectum plus the sigmoid and descending colon, whereas the proximal colon was defined as the transverse and ascending colon plus the caecum.
The pathological findings were evaluated by experienced pathologists in our facility. Patients with intramucosal carcinoma or carcinoma in situ were considered to have high-grade dysplasia. CRC was defined as invasion of the malignant cells beyond the muscularis mucosae. Advanced neoplasia (AN), which was considered to require intensive therapy, was defined as a CRC or advanced adenoma (adenoma ≥10 mm in size, ≥20% villous component, or high-grade dysplasia). Non-AN was defined as an adenoma of <10 mm, without a villous component. Neoplasia was defined as CRC, advanced adenoma, or non-AN.
Outcome measures and statistical analysis
SPSS for Windows version 20.0 statistical software (IBM Corp., Chicago, IL, USA) was used for data analysis. For descriptive findings, quantitative data are presented as means and standard deviations (SDs) and categorical variables are presented as percentages. Differences in demographic characteristics between participants with positive and negative faecal test results were determined using the Student's t-test, χ2 test, or Fisher's exact test. A two-tailed P-value of <0.05 indicated statistical significance.
Results
Patient characteristics
Of the 11,044 patients, 926 underwent CS within 2 years after iFOBT. The remaining 919 patients (564 men and 355 women) were included in the study. Of the 919 patients, 721 underwent TCS for the first time in our facility; the remaining patients had a history of previous TCS in our facility.
The demographic characteristics of the included patients are summarised in Table I. The mean age of the patients was 58.0 years (SD, 11.7 years). The average inspection interval between iFOBT and CS was 365.2 days (SD, 214.71 days). Of the 919 patients, 276 were included in the iFOBT-positive and 643 in the iFOBT-negative group. No significant differences in age were present between the two groups. However, the male-to-female ratio was significantly higher in the iFOBT-negative compared with that in the iFOBT-positive group (P<0.05). Additionally, the inspection interval between iFOBT and CS was significantly longer in the iFOBT-negative group (P<0.001). Neoplastic lesions were observed in 318 of the 643 iFOBT-negative patients (49.3%) and in 213 of the 276 iFOBT-positive patients (77.2%). The average tumour size (including adenomas <10 mm) was significantly smaller in the iFOBT-negative compared with that in the iFOBT-positive group (4.36±3.96 vs. 5.81±6.54 mm, respectively; P<0.001) (Table II). AN was observed in 40 of the 643 iFOBT-negative patients (6.2%) and in 52 of the 276 iFOBT-positive patients (18.8%). CRC was observed in 1 of the 643 iFOBT-negative patients (0.16%) (Fig. 2) and in 10 of the 276 iFOBT-positive patients (3.62%). The detection rates of neoplasia, AN and CRC were significantly lower in the iFOBT-negative compared with that in the iFOBT-positive group (P<0.001). The number of non-AN lesions was 470 in the iFOBT-negative group and 338 in the iFOBT-positive group. The ratio of non-AN lesions was significantly higher in the iFOBT-negative compared with the iFOBT-positive group (91.6 vs. 82.4%, respectively; P<0.001).
Table I.
Demographic characteristics of patients according to iFOBT results.
| Variables | iFOBT-negative (n=643) | iFOBT-positive (n=276) | P-value |
|---|---|---|---|
| Gender, male/female | 409/234 | 155/121 | <0.05 |
| Age, years (mean ± SD) | 57.7±11.2 | 58.5±12.7 | 0.3431 |
| iFOBT to CS interval, days (mean ± SD) | 379±200 | 131±131 | <0.001 |
| Neoplasia | |||
| Cases/total (%) | 318/643 (49.3) | 213/276 (77.2) | <0.001 |
| Total lesions | 513 | 410 | |
| Non-advanced neoplasia | |||
| Cases/total (%) | 260/643 (40.4) | 181/276 (65.6) | <0.001 |
| Total lesions | 470 | 338 | |
| Advanced neoplasia | |||
| Cases/total (%) | 40/643 (6.2) | 52/276 (18.8) | <0.001 |
| Total lesions | 43 | 72 | |
| Advanced adenoma | |||
| Cases/total (%) | 39/643 (6.1) | 43/276 | <0.001 |
| Total lesions | 42 | 61 (15.6) | |
| Colorectal cancer | |||
| Cases/total (%) | 1/643 (0.16) | 10/276 (3.62) | <0.001 |
| Total lesions | 1 | 11 |
iFOBT, immunochemical faecal occult blood test; CS, colonoscopy; SD, standard deviation.
Table I.
Comparison of neoplasm characteristics between the two groups.
| Variables | iFOBT-negative (513 lesions) | iFOBT-positive (410 lesions) | P-value |
|---|---|---|---|
| Non-advanced neoplasia | 470 | 338 | |
| Average size, mm (mean ± SD) | 3.68±1.44 | 3.89±1.58 | 0.05 |
| Location, proximal/total (%) | 260/470 (55.3) | 191/338 (56.5) | 0.737 |
| Shapea, protruding/total (%) | 198/470 (42.1) | 190/338 (56.2) | <0.001 |
| Advanced neoplasia | 43 | 72 | |
| Average size, mm (mean ± SD) | 11.8±10.3 | 14.8±11.6 | 0.080 |
| Location, proximal/total (%) | 20/43 (46.5) | 28/72 (38.9) | 0.423 |
| Shapea, protruding/total (%) | 20/42a (47.6) | 43/68a (63.2) | 0.108 |
| Advanced adenoma | 42 | 61 | |
| Average size, mm (mean ± SD) | 10.5±6.24 | 12.3±7.39 | 0.197 |
| Location, proximal/total (%) | 19/42 (45.2) | 25/61 (41.0) | 0.668 |
| Shape, protruding/total (%) | 20/42 (47.6) | 41/61 (68.3) | <0.05 |
| Colorectal cancer | 1 | 11 | |
| Average size (mm) | 65.0 | 28.6±19.5 | |
| Location, proximal/total (%) | 1/1 (100) | 3/11 (27.3) | 0.333 |
| Shapea, protruding/total (%) | (advanced cancer) | 2/7a (18.2) | |
| Combined (total neoplasia) | 513 | 410 | |
| Average size, mm (mean ± SD) | 4.36±3.96 | 5.8±6.54 | <0.001 |
| Location, proximal/total (%) | 280/513 (54.6) | 219/410 (53.4) | 0.724 |
| Shapea, protruding/total (%) | 218/512a (42.6) | 233/406a (57.4) | <0.001 |
| Rate of non-advanced neoplasia | 470/513 (91.6) | 338/410 (82.4) | <0.001 |
| Rate of advanced neoplasia | 43/513 (8.4) | 72/410 (17.6) | <0.001 |
| Rate of advanced adenoma | 42/513 (8.2) | 61/410 (14.9) | <0.01 |
| Rate of colorectal cancer | 1/513 (0.19) | 11/410 (2.7) | <0.01 |
Cases of advanced colorectal cancer were excluded. iFOBT, immunochemical faecal occult blood test; standard deviation.
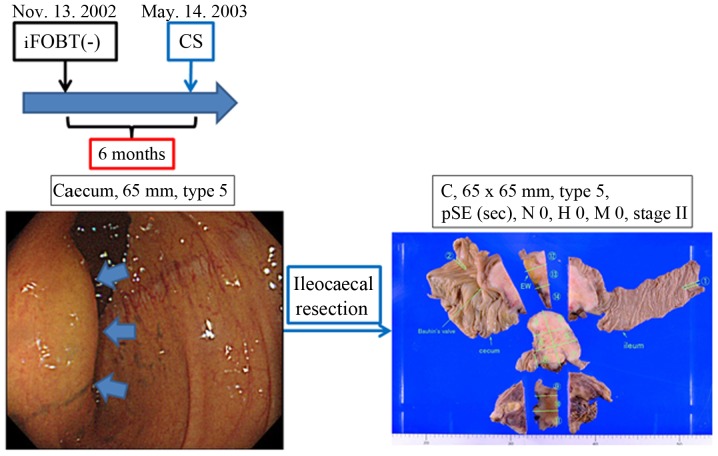
Figure 2.
A case of iFOBT-negative CRC in the cecum. A 65-mm CRC was discovered in the cecum of a 56-year-old woman with TCS, despite the fact that her iFOBT had been negative 6 months prior. iFOBT, immunochemical faecal occult blood test; CRC, colorectal cancer; CS, colonoscopy; TCS, total colonoscopy; pSE, CRC infiltrated the serosa pathologically.
Neoplasm characteristics
The comparison of neoplasm characteristics between the two groups is summarised in Table II. The number of AN lesions was 43 in the iFOBT-negative group (CRC, n=1; high-grade dysplasia, n=6; adenoma ≥10 mm, n=21; and tubulovillous adenoma, n=15) and 72 in the iFOBT-positive group (CRC, n=11; high-grade dysplasia, n=19; adenoma ≥10 mm, n=31; and tubulovillous adenoma, n=11). The ratios of CRC and AN were significantly lower in the iFOBT-negative compared with those in the iFOBT-positive group (0.19 vs. 2.7% and 8.4 vs. 17.6%, respectively; P<0.001). With respect to location, the rate of proximal-sided neoplasia (neoplasia or AN or CRC) tended to be higher in the iFOBT-negative compared with that in the iFOBT-positive group; however, there were no significant differences between the two groups. With respect to shape (excluding advanced CRC), the ratio of protruding neoplasia was significantly lower in the iFOBT-negative compared with that in the iFOBT-positive group.
Discussion
The effect of FOBT screening on the reduction of mortality due to CRC has been established (3–5). However, due to the imperfect sensitivity of FOBT, a certain risk of missing advanced lesions is always present (25,26). Although a number of previous studies have reported the performance of iFOBT, CS was not performed in iFOBT-negative patients (25,26). Additionally, certain studies on iFOBT invited asymptomatic patients with negative iFOBT results to undergo CS to validate the test results (27–31). To date, very few studies have investigated the characteristics of colorectal tumours in iFOBT-negative patients. As we sought to characterise colon tumours using our original diagnostic standard with magnifying endoscope technology (19–21), we were specifically interested in the characteristics of neoplastic lesions in iFOBT-negative patients and have herein attempted to elucidate the incidence, location and shape of these lesions. The aim of the present study was to determine the characteristics of colonic neoplasms that tend to be missed by iFOBT screening. The knowledge obtained herein may be useful in cancer screening using CS, particularly for patients without iFOBT results.
As expected, the incidence and average size of neoplasia, non-AN, AN and CRC were lower in iFOBT-negative compared with that in iFOBT-positive patients. Unfortunately, we identified no characteristic findings that were significantly specific to iFOBT-negative patients. However, there were certain potentially informative findings that provided clues to determining the weak points of iFOBT. First, although there were no differences in tumour location between the two groups, AN in iFOBT-negative patients tended to be located in the proximal colon more often compared with AN in iFOBT-positive patients. Additionally, CRC in iFOBT-negative patients, which only involved one lesion in this study, was also found in the proximal colon; this case of an iFOBT-negative CRC, which infiltrated the serosa, is shown in Fig. 2. These findings suggest that patients with tumours in the proximal colon may have negative iFOBT results, even when the tumours have malignant potential. Second, the rate of protruding non-AN (small lesions) was significantly lower in iFOBT-negative compared with iFOBT-positive patients. This tendency was not significantly evident for AN, suggesting that lesion size more significantly affected the sensitivity of iFOBT rather than lesion shape. However, the rate of protruding total neoplasia (non-AN and AN) was significantly lower in iFOBT-negative compared with that in iFOBT-positive patients. These results suggest that proximal and/or non-protruding (particularly small) tumours may be iFOBT-negative.
Sessile serrated adenoma/polyps (SSA/P), which were recently recognised as precancerous lesions of CRC, also tend to be proximally located and of the flat-elevated type (i.e., 0-IIa or 0-IIb in the Paris classification) (32,33). This characteristic feature corresponds with their tendency to be missed by iFOBT in the present study. In fact, we observed three cases of histologically proven SSA/P (average diameter ± SD of 16.0±4.62 mm), but all three were iFOBT-negative. This finding indicates that SSA/P may be missed during CRC surveillance using iFOBT.
The iFOBT was confirmed as an excellent method of CRC screening, as the detection rate of CRC was significantly higher in iFOBT-positive compared with that in iFOBT-negative patients (3.62 vs. 0.16%, respectively). Moreover, the detection rate of AN was lower in iFOBT-negative compared with that in iFOBT-positive patients (8.4 vs. 17.6%, respectively). However, it should be emphasised that neoplasia was found in almost half (49.3%) of the iFOBT-negative patients, suggesting that iFOBT screening is insufficient for targeting neoplasia, irrespective of the malignant potential of the neoplasia. In particular, 6.3% of the iFOBT-negative patients had AN, including 1 patient with CRC who required therapeutic intervention. We consider that this number is not insignificant and requires clinical attention. Park et al (26) also reported that the sensitivity for AN was markedly lower compared with that for CRC. As advanced adenoma is considered to be a precancerous lesion (31), endoscopic treatment for these lesions may reduce the incidence and mortality of CRC. Therefore, regularly conducting iFOBT alone for cancer screening is insufficient for detecting all CRC lesions; it is necessary to occasionally complement iFOBT with CS to compensate for the inaccuracy of iFOBT.
Our study had several limitations. First, we used the 1-day iFOBT, despite an earlier study recommending 2-day iFOBT, which is more cost-effective compared with the 1-day iFOBT (27). However, the compliance associated with the 2-day method is lower, as it involves more complicated procedures. The 1-day method was used in our study to allow for simpler data analysis. In fact, CRC screening programs vary among countries. For example, Australia uses the annual 2-day iFOBT, most European countries use the annual 1-day iFOBT and Italy uses the biennial 1-day iFOBT (27–30,34,35). The second limitation is that this study focused on patients undergoing CS within 2 years after iFOBT. The interval of 2 years may be relatively long, since, with the exception of Italy, CRC screening in several countries is performed annualy. The third limitation is that the population of the present study did not comprise the participants of a population-based screening program, but rather the participants of an opportunistic screening program. According to a 2009 national survey by the Japanese Society of Gastrointestinal Cancer Screening, the CRC detection rate was 0.051% (1,617/3,195,750) in opportunistic screening and 0.21% (5,309/2,508,0197) in population-based screening (http://www.jsgcs.or.jp/files/uploads/iinkai_h21.pdf). Moreover, the patients in the population-based screening program tended to be older compared with those in the opportunistic screening program. The survey also reported that the adenoma and CRC detection rates increased with age. We therefore expect the CRC and adenoma detection rates to be higher in population-based screening compared with those in the present study. The fourth limitation is that this was a retrospective study that may contain selection bias, compromising the ability to generalize the study results. Several patients were excluded from this study (n=10,125). However, the majority of the excluded patients were iFOBT-negative patients who did not undergo TCS in the population-based screening. In fact, 9,383 of the 10,125 excluded patients were iFOBT-negative. Therefore, a prospective follow-up study in which iFOBT-negative patients undergo TCS is desired.
Despite these limitations, our study demonstrated the clinical significance of CS during CRC screening. Nishihara et al (17) also reported that the multivariate hazard ratio for death from CRC was 0.32 (95% CI: 0.24–0.45) after screening CS. CS may detect small, non-protruding and proximally located colorectal tumours in iFOBT-negative patients. In particular, as certain precancerous lesions that are curable with endoscopic therapy are not detectable by iFOBT, screening using CS is crucial for reducing the mortality and incidence of CRC.
Acknowledgements
We would like to express our appreciation to Daisuke Watanabe (Kobe University), Nobunao Ikehara (Ikehara Clinic) and Yoko Tanaka (Showa University Northern Yokohama Hospital) for their instructive advice regarding this article.
References
- 1.Wan DS. Epidemiologic trend of and strategies for colorectal cancer. Ai Zheng. 2009;28:897–902. doi: 10.5732/cjc.008.10833. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 2.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, Snover DC, Schuman LM. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 3.Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 6.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. (Colorectal Cancer Study Group). Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 7.Rozen P, Levi Z, Hazazi R, Waked A, Vilkin A, Maoz E, Birkenfeld S, Niv Y. Quantitative colonoscopic evaluation of relative efficiencies of an immunochemical faecal occult blood test and a sensitive guaiac test for detecting significant colorectal neoplasms. Aliment Pharmacol Ther. 2009;29:450–457. doi: 10.1111/j.1365-2036.2008.03898.x. [DOI] [PubMed] [Google Scholar]
- 8.Saito H, Soma Y, Nakajima M, Koeda J, Kawaguchi H, Kakizaki R, Chiba R, Aisawa T, Munakata A. A case-control study evaluating occult blood screening for colorectal cancer with hemoccult test and an immunochemical hemagglutination test. Oncol Rep. 2000;7:815–819. doi: 10.3892/or.7.4.815. [DOI] [PubMed] [Google Scholar]
- 9.Saito H. Screening for colorectal cancer: Current status in Japan. Dis Colon Rectum. 2000;43(Suppl):S78–S84. doi: 10.1007/BF02237230. [DOI] [PubMed] [Google Scholar]
- 10.Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218–1224. doi: 10.1056/NEJMoa0803597. [DOI] [PubMed] [Google Scholar]
- 11.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 12.Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770–775. 711. doi: 10.1016/j.cgh.2008.12.030. quiz. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H, Haug U, Arndt V, Stegmaier C, Altenhofen L, Hoffmeister M. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology. 2010;138:870–876. doi: 10.1053/j.gastro.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 14.Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Long-term risk of colorectal cancer after negative colonoscopy. J Clin Oncol. 2011;29:3761–3767. doi: 10.1200/JCO.2011.35.9307. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 16.Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, Andreu M, Carballo F, Morillas JD, Hernández C, et al. COLONPREV Study InvestigatorsColonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 17.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Participants in the Paris Workshop, corp-author. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(Suppl):S3–S43. doi: 10.1016/S0016-5107(03)02159-X. [DOI] [PubMed] [Google Scholar]
- 19.Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo S, Rubio CA, Teixeira CR, Kashida H, Kogure E. Pit pattern in colorectal neoplasia: Endoscopic magnifying view. Endoscopy. 2001;33:367–373. doi: 10.1055/s-2004-826104. [DOI] [PubMed] [Google Scholar]
- 21.Kashida H, Kudo SE. Early colorectal cancer: Concept, diagnosis, and management. Int J Clin Oncol. 2006;11:1–8. doi: 10.1007/s10147-005-0550-5. [DOI] [PubMed] [Google Scholar]
- 22.Huang Q, Fukami N, Kashida H, Takeuchi T, Kogure E, Kurahashi T, Stahl E, Kudo Y, Kimata H, Kudo SE. Interobserver and intra-observer consistency in the endoscopic assessment of colonic pit patterns. Gastrointest Endosc. 2004;60:520–526. doi: 10.1016/S0016-5107(04)01880-2. [DOI] [PubMed] [Google Scholar]
- 23.Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/S0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 24.Tsuruta O, Toyonaga A, Ikeda H, Tanikawa K, Morimatsu M. Clinicopathological study of superficial-type invasive carcinoma of the colorectum. Int J Oncol. 1997;10:1003–1008. doi: 10.3892/ijo.10.5.1003. [DOI] [PubMed] [Google Scholar]
- 25.van Rossum LG, van Rijn AF, van Oijen MG, Fockens P, Laheij RJ, Verbeek AL, Jansen JB, Dekker E. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125:746–750. doi: 10.1002/ijc.24458. [DOI] [PubMed] [Google Scholar]
- 26.Park DI, Ryu S, Kim Y-H, Lee SH, Lee CK, Eun CS, Han DS. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol. 2010;105:2017–2025. doi: 10.1038/ajg.2010.179. [DOI] [PubMed] [Google Scholar]
- 27.Nakama H, Zhang B, Fattah AS. A cost-effective analysis of the optimum number of stool specimens collected for immunochemical occult blood screening for colorectal cancer. Eur J Cancer. 2000;36:647–650. doi: 10.1016/S0959-8049(00)00020-4. [DOI] [PubMed] [Google Scholar]
- 28.Bampton PA, Sandford JJ, Cole SR, Smith A, Morcom J, Cadd B, Young GP. Interval faecal occult blood testing in a colonoscopy based screening programme detects additional pathology. Gut. 2005;54:803–806. doi: 10.1136/gut.2004.043786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer. 2006;107:2152–2159. doi: 10.1002/cncr.22230. [DOI] [PubMed] [Google Scholar]
- 30.van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH, Verbeek AL, Jansen JB, Dekker E. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135:82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. (United States Multi-Society Task Force on Colorectal Cancer). Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Jass JR. Hyperplastic-like polyps as precursors of microsatellite-unstable colorectal cancer. Am J Clin Pathol. 2003;119:773–775. doi: 10.1309/UYN70N9W2DVN9ART. [DOI] [PubMed] [Google Scholar]
- 33.Lambert R, Kudo SE, Vieth M, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, et al. Pragmatic classification of superficial neoplastic colorectal lesions. Gastrointest Endosc. 2009;70:1182–1199. doi: 10.1016/j.gie.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Castiglione G, Grazzini G, Miccinesi G, Rubeca T, Sani C, Turco P, Zappa M. Basic variables at different positivity thresholds of a quantitative immunochemical test for faecal occult blood. J Med Screen. 2002;9:99–103. doi: 10.1136/jms.9.3.99. [DOI] [PubMed] [Google Scholar]
- 35.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Sakaguchi K, Shiratori Y. Sensitivity of immunochemical fecal occult blood test to small colorectal adenomas. Am J Gastroenterol. 2007;102:2259–2264. doi: 10.1111/j.1572-0241.2007.01404.x. [DOI] [PubMed] [Google Scholar]