Abstract
The effect of thyroxine (T4) on natural killer (NK) activity of peripheral blood lymphocytes (PBL) was investigated, using a 4-hr 51Cr release assay, in 18 patients with previously untreated Graves’ disease and in 18 controls. NK activity in patients with Graves disease was not significantly different from that in the controls. Normal T4 (NT) and high T4 (HT) medium, free T4 concentrations in which were 1.01 and 16.3 ng/dl, respectively, were used to evaluate the effect of T4 on NK activity. In the controls, NK activity increased in the NT or HT medium compared with that in the control medium at effector to target cell (E : T) ratios of 25 : 1 and 50 : 1. NK activity in the Graves’ disease patients, however, did not increase when either the NT or HT medium was used at E : T ratios of 25 : 1 and 50 : 1. These results suggest that patients with Graves’ disease have a similar NK activity to the controls but have a defect in the peripheral blood lymphocytes to increase NK activity in response to T4.
Keywords: Natural killer activity, Thyroxine, Graves’ disease
INTRODUCTION
Natural killer (NK) activity has been functionally characterized as spontaneous cytotoxicity of lymphocytes to a variety of cells in vitro in the absence of antibody and previous sensitization1) and reported to be influenced by numerous cytokines and hormones2–4). In some autoimmune diseases, NK activity has been reported to be depressed, although the exact role of the depressed NK activity in development and progression of the disease is not clearly defined5–7).
Previous reports have shown that NK activity decreased in patients with Graves’ disease, one of the organ-specific autoimmune diseases, and in patients with long-term treatment with thyroid hormones8,9), while conflicting results have been reported10). However, there has been no report regarding the modulatory effects of thyroid hormones on NK activity in patients with Graves’ disease.
This study was conducted to investigate NK activity in Graves’ disease patients and to evaluate the effect of thyroxine (T4) on NK activity of peripheral blood lymphocytes (PBL) in patients with Graves’ disease and in controls.
PATIENTS AND METHODS
1. Patients
Eighteen patients (4 male and 14 female) with previously untreated Graves’ disease and 18 controls (14 male and 4 female) were studied. The median ages of the patients and the controls were 35 years (range: 24 – 53) and 38.5 years (range: 25 – 58), respectively. Graves’ disease was diagnosed on the basis of symptoms and signs of thyrotoxicosis and abnormal thyroid function tests including triiodothyronine (T3), T4, free T4, thyroid stimulating hormone (TSH), and TSH-binding inhibitory immunoglobulin.
2. Preparation of PBL and Target Cells
Mononuclear cells were isolated by centrifugation of heparinized venous blood diluted with the same volume of Hank’s balanced salt solution (HBSS) (Gibco Laboratories, Grand Island, NY, USA) on a Ficoll-Paque (Sigma, St. Louis, MO, USA) density gradient. The collected cells were washed twice with HBSS and suspended in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 U/ml of penicillin and 100 μg/ml of streptomycin (Sigma) (RPMI-FBS). After incubation on a 35-mm petri dish (Green Cross, Seoul, Korea) in a humidified atmosphere of 5% CO2 at 37°C for 50 min, the nonadherent cells were colleted by repeated washing with RPMI-FBS and resuspended in PRMI-FBS at concentrations of 2.5 × 106/ml and 5 × 106/ml. More than 95% of these nonadherent cells were lymphocytes. As target cells, K562, human chronic myelogenous leukemia11) cells were labeled by incubation of 1 × 106 cells in 0.1 ml of RPMI-FBS with 20 μCi of Na2 51CrO4 (Dupont, Boston, MA, USA) at 37°C for 50 min. After washing twice with HBSS and once with RPMI-FBS, the cells were suspended in RPMI-FBS at a concentration of 1 × 105/ml.
3. Measurement of NK Activity
NK activity of PBL was determined by a 4-hr 51Cr release assay according to the method previously described12). In brief, 0.1 ml of 51Cr-labeled target cell suspension was mixed with 0.1 ml of effector cell suspension (2.5 × 106/ml and 5 × 106/ml at effector cell to target cell (E : T) ratios of 25 : 1 and 50 : 1, respectively, in each experiment) in each well of a 96-well microtest plate (Flow, McLean, VA, USA). The plates were incubated for 4 hr at 37°C in a humidified atmosphere of 5% CO2 and then centrifuged at 400 g for 10 min. Then, 0.1 ml of the supernatant was collected from each well, and its radioactivity was measured with a gamma-counter (Beckman Instruments Inc., USA). Each experiment was performed in triplicate. Spontaneous and maximum release from the target cells were determined from supernatants in the cultures of the target cells alone and target cells with Triton X-100 (S.P.C GR Reagent, Japan), respectively. The calculation of NK activity was done using the following formula:
4. Effect of T4 on NK Activity
T4 stock solution was prepared by dilution of T4 (Sigma, USA) in 0.05 N NaOH with deionized water to concentrations of 1 mg/dl and 10 mg/dl for normal T4 (NT) medium and high T4 (HT) medium, respectively. NT and HT media were made by adding 40 μl of 1 mg/dl and 10 mg/dl T4 stock solutions to 10 ml of RPMI-FBS, respectively. The control medium was made by adding 40 μl of deionized water to the same volume of RPMI-FBS.
The concentrations of free T4 were measured with a radioimmunoassay kit (Diagnostic Products Corporation, Los Angeles, CA, USA), showing 0. 14 ± 0.03, 1.01 ± 0.17, and 16.3 ± 9.2 ng/dl in the control medium, NT medium, and HT medium, respectively. The normal value of free T4 in the Korea Cancer Center Hospital was 0.8–2.3 ng/dl. The intra-assay coefficient of variation was 7.6% at a concentration of 1.0 ng/dl.
5. Statistical Analysis
Unpaired t-test was used to test the difference in NK activities between Graves’ disease patients and the normal controls. Paired t-test was employed to compare NK activity measured in the control medium with that in the NK medium or HT medium. A p value below 0.05 was regarded as statistically significant.
RESULTS
1. NK Activity in Graves’ Disease
NK activity of PBL was measured in 18 patients with Graves’ disease and 18 controls. NK activity in Graves’ disease measured in the control medium was 39.8 ± 23.3% and 54.7 ± 20.3%, at E : T ratios of 25 : 1 and 50 : 1, respectively, and was not significantly different from that in the normal controls (Table 1).
Table 1.
NK Activity in 18 Patients with Graves’ Disease and in 18 Controls
| E : T ratio | Graves’ disease | Controls | ||
|---|---|---|---|---|
|
| ||||
| 25 : 1 | 50: 1 | 25: 1 | 50 : 1 | |
| Control medium | 39.8 ± 23.3a) | 54.7 ± 20.3 | 46.0 ± 18.2 | 59.7 ± 15.6 |
| NT medium | 41.2 ± 23.3 | 58.2 ±21.5 | 54.0 ± 20.0 | 69.5 ± 17.7 |
| HT medium | 42.5 ± 25.3 | 57.9 ± 22.5 | 52.4 ±21.1 | 68.3 ± 19.7 |
NT : normal T4 : HT : high T4
mean ± SD of NK activity
2. Effect of T4 on NK Activity
In the 18 controls, NK activity measured in the NT medium increased compared with that in the control medium when tested with paired t-test at E : T ratios of 25 : 1 and 50 : 1 (p<0.05) (Table 1) (Fig. 1). NK activity of the controls measured in the HT medium also increased compared with that in the control medium when tested with paired t-test at E : T ratios of 25 : 1 and 50 : 1 (p <0.01). NK activity in the HT medium, however, was not significantly different from that measured in the NT medium at E : T ratios of 25 : 1 and 50 : 1. In patients with Graves’ disease, NK activity measured in either the NT or HT medium was not significantly different from that in the control medium at E : T ratios of 25 :1 and 50 : 1 (Fig. 2).
Fig. 1.
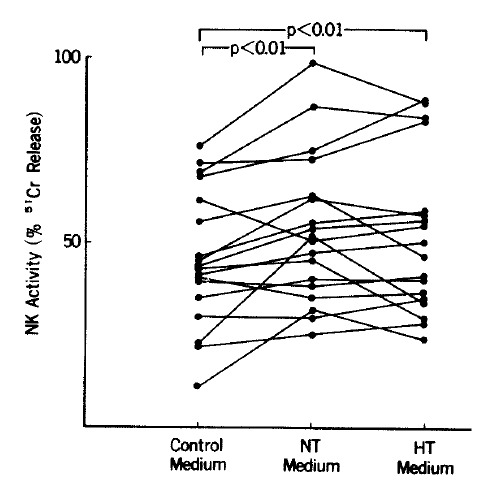
NK activity in controls at an E : T ratio of 25 : 1. NK activity increased in NT and HT media compared with that in control medium. NT: normal T4; HT: high T4.
Fig. 2.
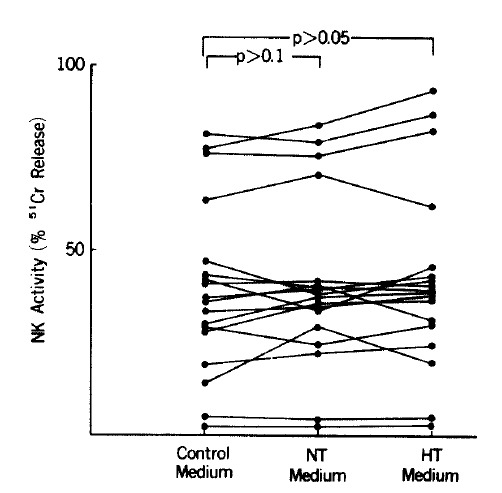
NK activity in patients with Graves’ disease at an E : T ratio of 25 : 1. NK activity measured in NT and HT media was not different from that in control medium. NT: normal T4; HT: high T4.
DISCUSSION
In this study, NK activity of PBL obtained from patients with Graves’ disease was slightly lower than that of the controls, but the difference was not significant. This result is consistent with the report by Pedersen et al.7), in which they observed a small and insignificant difference in NK activity of PBL between controls and patients with Graves’ disease. On the other hand, Wang et al.4). and Papic et al.8) reported that NK activity decreased in association with hyperthyroxinemia regardless of the etiology. The reason for this discrepancy is unclear. In a clinical aspect, the absence of a significant difference in NK activity between Graves’ disease and the controls seems to be more compatible with the absence of a conclusive report indicating an increased incidence of infection or malignancy in patients with Graves’ disease.
In the study populations of this study, age was matched but sex was not matched between Graves’ disease and the controls. It is preferable for the study populations to be matched for age and sex. However, it does not seem to be a significant problem in this study, because several previous studies have shown that NK activity is not significantly different betweeen the sexes13–15).
In an attempt to investigate the influence of T4 on NK activity, we measured free T4 concentration in the control medium and added T4 to make an NT medium with free T4 concentration simulating that in the plasma of the normal subjects. For the HT medium, a ten-fold amount of T4 used for the NT medium was added to control medium. To rule out direct cytotoxic effect of T4 and the the solvent used, spontaneous releases from the control medium, NT medium, and HT medium were measured. They were not significantly different. We observed an increase in NK activity by the addition of T4 to the control medium of T4 up to a free T4 concentration of 16.3 ng/dl. The exact mechanism of this phenomenon is not clear, although some defect of PBL in Graves’ disease patients may occur from prolonged exposure to the high concentration of thyroid hormones. In contrast to these results, Provinciali et al.18) reported that T4 alone did not modify NK activity but increased an interferon-induced NK activity. However, their study differs from ours on three points: first, they employed only one T4 concentration and did not measure the concentration of free T4, which is considered to exert the main biologic activity; second, they measured the NK activity of splenocytes pretreated with T4; and finally, they measured NK activity in a mouse system, not in a human system.
These results demonstrating that NK activity increases by the addition of T4 to a medium in the controls but not in patients with Graves’ disease suggest that NK activity in Graves’ disease is not different from that in the controls, however, PBL in Graves’ disease may have some defect to increase NK activity in response to T4.
Acknowledgments
We wish to thank Miss Young-Sun Kim for her excellent technical help.
REFERENCES
- 1.Trinchieri G, Perussia B. Human natural killer cells: biologic and pathologic aspects. Laborat Invest. 1984;50:489. [PubMed] [Google Scholar]
- 2.Ortaldo JR, Herberman RB. Heterogeneity of natural killer cells. Ann Rev Immunol. 1984;2:359. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- 3.Hochman PS, Cudkowicz G. Suppression of natural cytotoxicity by spleen cells of hydrocortisone-treated mice. J Immunol. 1979;123:968. [PubMed] [Google Scholar]
- 4.Seaman WE, Merigan TC, Talal N. Natural killing in estrogen-treated mice responds poorly to poly I : C despite normal stimulation of circulating interferon. J Immunol. 1979;123:2903. [PubMed] [Google Scholar]
- 5.Mackay P, Jacobson J, Rabinovitch A. Spontaneous diabetes mellitus in the Bio-Breeding/Worcester rat-evidence in vitro for natural killer cell lysis of islet cells. J Clin Invest. 1986;77:916. doi: 10.1172/JCI112390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nerup J, Mandrup-Poulsen T, Molvig J, Helgvist S, Wogensen L, Egeberg J. Mechanisms of pancreatic β-cell destruction in type I diabetes. Diabetes Care. 1988;11(Suppl 1):16. [PubMed] [Google Scholar]
- 7.Pederson BK, Perrild H, Feldt-Rasmussen U, Christensen T, Klarlund K, Hansen JM. Suppressed natural killer cell activity in patients with euthyroid Graves’ opthalmopathy. Autoimmunity. 1989;2:291. doi: 10.3109/08916938908997155. [DOI] [PubMed] [Google Scholar]
- 8.Papic M, Stein-Streilein J, Zakarija M, McKenzie JM, Guffee J, Fletcher MA. Suppression of peripheral blood natural killer cell activity by excess thyroid hormone. J Clin Invest. 1987;79:404. doi: 10.1172/JCI112826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P-W, Luo S-F, Huang B-Y, Lin J-D, Huang M-J. Depressed natural killer activity in Graves’ disease and during antithyroid medication. Clin Endocrinol. 1988;28:205. doi: 10.1111/j.1365-2265.1988.tb03657.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharma SD, Tsai V, Proffitt MR. Enhancement of mouse natural killer activity by thyroxine. Cell Immunol. 1982;73:83. doi: 10.1016/0008-8749(82)90437-3. [DOI] [PubMed] [Google Scholar]
- 11.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321. [PubMed] [Google Scholar]
- 12.Hong W-S, Kim C-M, Lee J-O, Kang T-W, Yun T-K, Kim C-Y. Natural killer and lymphokine-activated killer activities in stomach cancer patients with special emphasis on the effect of 5-fluouracil, adriamycin and mitomycin-C chemotherapy. Jpn J Clon Oncol. 1990;20:87. [PubMed] [Google Scholar]
- 13.Yun Taik-Koo, Yun Yeon-Sook, Jo Sung-Kee, Moon Hae-Sun. Natural killer activity of human peripheral blood lymphocytes. J Korean Cancer Res Ass. 1987;19:14. [Google Scholar]
- 14.Kim J-P, Park J-G. Natural killer activity of PBLs from normal volunteers and cancer patients. Korean J Immunol. 1984;6(1) [Google Scholar]
- 15.Fujita J, Saijo N, Sasaki Y, et al. Natural killer (NK) and lymphokine-activated killer (LAK) cell activity in healthy volunteers with special emphasis on the familial incidence of cancer. Jpn J Clin Oncol. 1985;15:589. [PubMed] [Google Scholar]
- 16.Provinciali M, Muzzioli M, Fabris N. Thyroxine-dependent modulation of natural killer activity. J Exp Pathol. 1987;3:617. [PubMed] [Google Scholar]


