Abstract
BACKGROUND AND OBJECTIVE:
Narrow-spectrum antibiotics are recommended as the first-line agent for children hospitalized with community-acquired pneumonia (CAP). There is little scientific evidence to support that this consensus-based recommendation is as effective as the more commonly used broad-spectrum antibiotics. The objective was to compare the effectiveness of empiric treatment with narrow-spectrum therapy versus broad-spectrum therapy for children hospitalized with uncomplicated CAP.
METHODS:
This multicenter retrospective cohort study using medical records included children aged 2 months to 18 years at 4 children's hospitals in 2010 with a discharge diagnosis of CAP. Patients receiving either narrow-spectrum or broad-spectrum therapy in the first 2 days of hospitalization were eligible. Patients were matched by using propensity scores that determined each patient’s likelihood of receiving empiric narrow or broad coverage. A multivariate logistic regression analysis evaluated the relationship between antibiotic and hospital length of stay (LOS), 7-day readmission, standardized daily costs, duration of fever, and duration of supplemental oxygen.
RESULTS:
Among 492 patients, 52% were empirically treated with a narrow-spectrum agent and 48% with a broad-spectrum agent. In the adjusted analysis, the narrow-spectrum group had a 10-hour shorter LOS (P = .04). There was no significant difference in duration of oxygen, duration of fever, or readmission. When modeled for LOS, there was no difference in average daily standardized cost (P = .62) or average daily standardized pharmacy cost (P = .26).
CONCLUSIONS:
Compared with broad-spectrum agents, narrow-spectrum antibiotic coverage is associated with similar outcomes. Our findings support national consensus recommendations for the use of narrow-spectrum antibiotics in children hospitalized with CAP.
Keywords: pneumonia, pediatric, antibiotics, comparative effectiveness, hospitalized
What’s Known on This Subject:
Broad-spectrum antibiotics are frequently used to empirically treat children hospitalized with community-acquired pneumonia despite recent national recommendations to use narrow-spectrum antibiotics.
What This Study Adds:
Narrow-spectrum antibiotics are similar to broad-spectrum antibiotics for the treatment of children hospitalized with community-acquired pneumonia in terms of clinical outcomes and resource utilization. This study provides scientific evidence to support national consensus guidelines.
Community-acquired pneumonia (CAP) is a common and serious cause of hospitalization in children, accounting for >150 000 hospitalizations each year in the United States.1 CAP ranks second in standardized cumulative cost for the most common inpatient pediatric diagnoses.2 Although Streptococcus pneumoniae remains the most likely bacterial cause of CAP, in the clinical setting it is uncommon to identify a specific pathogen. Uncertainty about the causative bacterium and its antimicrobial susceptibility pattern contributes to the use of empiric broad-spectrum antibiotics such as third-generation cephalosporins.3,4
In 2011, the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) published a guideline for the treatment of children with CAP.5 The guideline was created to reduce unwarranted variation in the treatment of children with CAP and to improve their clinical outcomes. The PIDS/IDSA guideline recommended the empiric use of narrow-spectrum coverage with ampicillin or penicillin G for children hospitalized with uncomplicated CAP. Although some studies suggest that penicillins are as effective as broad-spectrum antibiotics for empiric treatment of CAP due to S pneumoniae, few studies have directly compared the 2 regimens.4,6–8 Evidence showing the effectiveness of narrow-spectrum antibiotic therapy has the potential to improve adherence to the national guideline and minimize the development of bacterial resistance. The objective of this study was to compare the effectiveness of empiric therapy with narrow-spectrum antibiotics with empiric therapy with broad-spectrum antibiotics in children hospitalized with uncomplicated CAP.
Methods
Study Design, Data Source, and Study Population
This multicenter retrospective study was nested within a cohort of patients from a study to validate International Classification of Diseases, Ninth Revision, Clinical Modification, diagnostic codes for CAP.9 We used the Pediatric Health Information System (PHIS; Children’s Hospital Association, Overland Park, KS) to identify children discharged with a diagnosis of CAP from 4 freestanding children’s hospitals (Monroe Carell Jr Children’s Hospital at Vanderbilt, Nashville, TN; Children’s Mercy Hospitals and Clinics, Kansas City, MO; Seattle Children’s Hospital, Seattle, WA; and Cincinnati Children’s Hospital Medical Center, Cincinnati, OH). The institutional review board at each hospital approved the study.
Children who were 60 days to 18 years of age with a discharge diagnosis of pneumonia at a participating hospital between January 1, 2010, and December 31, 2010, were eligible for inclusion. A random subset of discharges was selected for medical record review to verify the diagnosis of CAP as previously described (n = 785).9 The medical records of children meeting the following definition of CAP were then reviewed to obtain data on presenting signs and symptoms, results of laboratory and radiologic studies, and hospital course: (1) provider diagnosis of pneumonia within the first 48 hours of hospitalization (mention of suspected CAP along with consistent management strategy), (2) fever within first 48 hours of admission or abnormal white blood cell (WBC) count, (3) evidence of respiratory illness (eg, cough or increased work of breathing), and (4) chest radiograph indicating pneumonia (eg, infiltrate or consolidation).
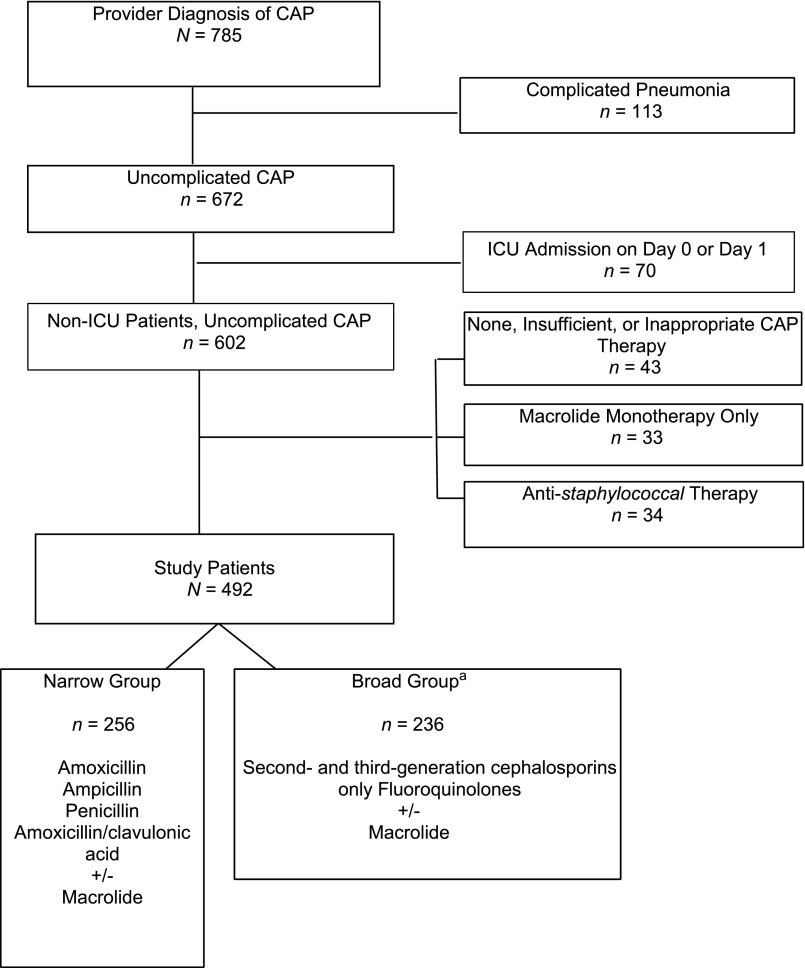
Children with chronic conditions predisposing to severe, recurrent, or health care–associated pneumonia were excluded with the use of a previously described classification scheme10 (Fig 1). We excluded children with complicated pneumonia (n = 113) and children who required intensive care within 2 calendar days of hospitalization (n = 70) because broad antimicrobial coverage is usually indicated in this population. Complicated pneumonia was defined as an imaging study indicating moderate to large pleural effusion, lung abscess or necrosis, or bronchopleural fistula or if there was a billing code for a pleural fluid drainage procedure.11 We also excluded patients who received staphylococcal coverage (n = 34), macrolide monotherapy (n = 33), or antibiotics not typically used to treat CAP (eg, carbapenem, nitrofurantoin, and gentamicin) (n = 5). Finally, we excluded children if they did not receive antibiotic therapy within the first 2 days of hospitalization or who received only 1 day of antibiotic therapy (n = 38).
FIGURE 1.
Consort diagram indicating identification of study population and reasons for exclusion. aFluoroquinolone exposure, n = 12; second- and third-generation cephalosporins only, n = 224.
Measured Outcomes
Main outcomes included hospital length of stay (LOS) measured in hours, readmission within 7 days of index hospitalization, durations of fever and supplemental oxygen use, and daily standardized pharmacy and overall cost. Duration of fever was defined as the time in hours from emergency department arrival to the time of last recorded fever. For patients receiving supplemental oxygen, the duration of supplemental oxygen was defined as the time in hours from emergency department arrival to the time oxygen was permanently discontinued. To use hospital costs as a marker of resource utilization, we used a previously described method to standardize the cost of individual items to remove the high interhospital variation in item costs.2 We modeled cost outcomes by mean LOS because room charges are a major driver of cost. Because more patients in the broad-spectrum group also received macrolide therapy, which could potentially drive pharmacy costs, we performed a sensitivity analysis that excluded patients in each group receiving macrolide therapy.
Measured Exposures
The main exposure of interest was initial antibiotic therapy, which included antibiotics administered before arrival to the hospital and antibiotics administered within the first 2 days of hospitalization. A minimum of 2 days of antibiotic therapy was required for study inclusion. We determined exposure to antibiotics before hospital arrival by medical record review and included antibiotics administered by any route. Antibiotics administered during the first 2 days of hospitalization were collected from the PHIS database. Prehospital antibiotics could account for no more than 1 day out of the 2 minimum days of therapy. All antibiotic exposure was classified as narrow or broad. We defined narrow-spectrum therapy as use of ampicillin, penicillin, or amoxicillin/clavulanic acid, with or without macrolide therapy. We defined broad-spectrum therapy as use of a second- or third-generation cephalosporin or fluoroquinolone, with or without macrolide therapy.
Covariates
Patient demographic characteristics were obtained from the PHIS database. A concomitant diagnosis of asthma was determined from the International Classification of Diseases, Ninth Revision, Clinical Modification, discharge codes for asthma or reactive airway disease. A concomitant diagnosis of bronchiolitis or viral infection was determined from either a discharge diagnosis code for bronchiolitis or viral lower respiratory infection or a positive test for a respiratory virus. Blood culture testing was determined from the PHIS database and culture results from chart review. Fever was defined as a temperature ≥38°C within first 48 hours of admission. Tachypnea was defined as a respiratory rate ≥90th percentile for age.12 An abnormal WBC count was defined as <5000 or >15 000 cells per mL.
Analysis
A propensity score was used to account for potential confounding by observed baseline covariates. We constructed propensity scores by using multivariable logistic regression to assess the likelihood of exposure to narrow-spectrum therapy because physicians may be less likely to prescribe narrow-spectrum antibiotics for patients who are more ill-appearing or who have persistent symptoms after taking outpatient antibiotics. Variables used to develop the propensity score included age, gender, race/ethnicity, payor, concurrent diagnosis of asthma or reactive airway disease, clinical characteristics upon presentation (eg, fever and tachypnea), exposure to previous antibiotic therapy, atypical antibiotic therapy, diagnosis of viral lower respiratory tract infection, admission to the ICU after the second day of hospitalization (n = 3), blood culture testing, positive blood culture result, abnormal WBC count, and baseline hospital-level cephalosporin use. Certain variables such as tachycardia, hypotension, and mental status change at the time of admission were not included because these events were not frequent enough in the 2 exposure groups to achieve convergence of the logistic regression for the propensity score. The model’s calculated C statistic was 0.70, indicating that the model provided a better estimate than expected by chance alone (ie, if the C statistic was equal to 0.5) but remained in a range indicating that there was overlapping propensity score distributions between the treatment groups.13 Narrow- and broad-spectrum recipients were matched on propensity score with the use of nearest-neighbor matching with a caliper set at one-quarter of the SD of the logit of the propensity scores.14
Because LOS, duration of fever, and duration of oxygen were not normally distributed, we log-transformed the data before modeling. We used linear mixed models with the hospital as a random effect and back-transformed the results onto the original scale. The analyses also accounted for the clustering of patients within hospital. All analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC), and P values <0.05 were considered statistically significant.
Results
A total of 492 patients with CAP were included in the study: 256 (52%) received a narrow-spectrum antibiotic and 236 (48%) received a broad-spectrum antibiotic (Table 1). Before matching, there were no differences between the 2 groups in age, gender, race, insurance type, asthma, reactive airway disease, or viral lower respiratory tract infection. However, patients in the narrow-spectrum antibiotic group were more likely to be 60 days to 2 years of age (44.1% vs 39.8%; P = .02) or have an abnormal WBC (41.0% vs 31.4%; P = .03). Patients in the broad-spectrum antibiotic group were more likely to receive antibiotics before hospital arrival (30.5% vs 18.4%; P = .002), receive macrolide antibiotics (25.8% vs 10.2%; P = .001), have a blood culture drawn (59.3% vs 44.2%; P = .001), or have a positive blood culture result (4.7% vs 1.2%; P = .02). There was significant variation in initial therapy choice across hospitals; the rate of narrow-spectrum use ranged from 18.6% to 88.3% (P < .001).
TABLE 1.
Patient Characteristics
| Total Population | Matched Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Population (N = 492) | Narrow-Spectrum (n = 256) | Broad-Spectrum (n = 236) | P | Cohort Population | Narrow-Spectrum | Broad-Spectrum | P | |
| Age | ||||||||
| 60 days to 2 years | 207 (42.1) | 113 (44.1) | 94 (39.8) | .02 | 184 (42.8) | 94 (43.7) | 90 (41.9) | .16 |
| 2–12 years | 275 (55.9) | 142 (55.5) | 133 (56.4) | 239 (55.6) | 120 (55.8) | 119 (55.3) | ||
| 12–18 years | 10 (2.0) | 1 (0.4) | 9 (3.8) | 7 (1.6) | 1 (0.5) | 6 (2.8) | ||
| Male | 268 (54.5) | 141 (55.1) | 127 (53.8) | .78 | 234 (54.4) | 117 (54.4) | 117 (54.4) | .95 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 269 (54.7) | 144 (56.3) | 125 (53.0) | .37 | 227 (52.8) | 117 (54.4) | 110 (51.2) | .81 |
| Non-Hispanic black | 80 (16.3) | 44 (17.2) | 36 (15.3) | 71 (16.5) | 36 (16.7) | 35 (16.3) | ||
| Hispanic | 53 (10.8) | 23 (9.0) | 30 (12.7) | 49 (11.4) | 23 (10.7) | 26 (12.1) | ||
| Asian | 15 (3.0) | 5 (2.0) | 10 (4.2) | 14 (3.3) | 5 (2.3) | 9 (4.2) | ||
| Other | 75 (15.2) | 40 (15.6) | 35 (14.8) | |||||
| Clinical characteristics | ||||||||
| Fever >38°C | 220 (44.7) | 107 (41.8) | 113 (47.9) | .18 | 194 (45.1) | 96 (44.7) | 98 (45.6) | .85 |
| Tachypnea | 108 (22.0) | 59 (23.0) | 49 (20.8) | .54 | 93 (21.6) | 47 (21.9) | 46 (21.4) | .91 |
| Tachycardiaa | 4 (0.8) | 1 (0.4) | 3 (1.3) | .28 | 3 (0.7) | 1 (0.5) | 2 (0.9) | .56 |
| Abnormal WBC | 179 (36.4) | 105 (41.0) | 74 (31.4) | .03 | 145 (33.7) | 74 (34.4) | 71 (33.0) | .76 |
| Hypotensiona | 4 (0.8) | 1 (0.4) | 3 (1.3) | .28 | 4 (0.9) | 1 (0.5) | 3 (1.4) | .32 |
| Altered mental statusa | 9 (1.8) | 6 (2.3) | 3 (1.3) | .38 | 9 (2.1) | 6 (2.8) | 3 (1.4) | .31 |
| Government insurance | 259 (52.6) | 132 (51.6) | 127 (53.8) | .62 | 235 (54.7) | 117 (54.4) | 118 (54.9) | .92 |
| Antibiotic therapy before admission | 119 (24.2) | 47 (18.4) | 72 (30.5) | .002 | 109 (25.3) | 47 (21.9) | 62 (28.8) | .10 |
| Macrolide empiric antibiotic therapy | 87 (17.7) | 26 (10.2) | 61 (25.8) | .001 | 70 (16.3) | 26 (12.1) | 44 (20.5) | .02 |
| Diagnosis of asthma or reactive airway diseaseb | 153 (31.1) | 89 (34.8) | 64 (27.1) | .07 | 125 (29.1) | 64 (29.8) | 61 (28.4) | .75 |
| Diagnosis of bronchiolitis viral infectionc | 130 (26.4) | 70 (27.3) | 60 (25.4) | .63 | 118 (27.4) | 62 (28.8) | 56 (26.0) | .52 |
| Blood culture obtained | 253 (51.4) | 113 (44.2) | 140 (59.3) | .001 | 234 (54.4) | 113 (52.6) | 121 (56.3) | .44 |
| Positive blood culture | 14 (2.8) | 3 (1.2) | 11 (4.7) | .02 | 11 (2.6) | 3 (1.4) | 8 (3.7) | .13 |
Data are presented as n (%).
Not frequent enough to include in the propensity score.
Includes International Classification of Diseases code 493.
Includes International Classification of Diseases, Ninth Revision, codes for viral pneumonia (480) or acute bronchiolitis (466.19) or a positive test for a viral pathogen.
In the unadjusted analysis, LOS was significantly shorter in the narrow-spectrum group (43 hours; 95% confidence interval [CI]: 28–62.5) compared with the broad-spectrum group (49 hours; 95% CI: 39–76) (Supplemental Table 3). The finding of a shorter LOS persisted in the adjusted analysis using propensity scores: the narrow-spectrum group had a 10-hour shorter LOS (P = .04). However, there was no significant difference in duration of oxygen, duration of fever, or readmission rate within 7 days (Table 2).
TABLE 2.
Adjusted Outcomes
| Narrow-Spectrum (n = 256) | Broad-Spectrum (n = 236) | P | |
|---|---|---|---|
| LOS, h | 43 (39–46) | 52.3 (48–57) | .04 |
| Duration of supplemental oxygen, h | 15.6 (12–20) | 21.8 (17–29) | .18 |
| Duration of fever, h | 6.5 (5–9) | 9.1 (7–12) | .23 |
| Standardized cost per day, $ | 2209 (2088–2338) | 2160 (2042–2286) | .62 |
| Standardized pharmacy cost per day, $ | 170 (153–188) | 188 (170–208) | .26 |
| Readmission within 7 daysa | Reference | 5.1 (0.3–83.6) | .25 |
Data are least-squares means (95% CI) unless otherwise indicated and were adjusted for age, gender, race, government insurance, concurrent diagnosis of asthma or reactive airway disease, previous antibiotic therapy, atypical antibiotic therapy, presence of effusion on chest radiograph, diagnosis of viral lower respiratory tract infection, admission to the ICU, blood culture utilization, presence of a positive blood culture, baseline hospital rates for cephalosporin use, tachypnea, fever, and abnormal WBC.
Data are adjusted odds ratios (95% CI) for broad-spectrum/penicillin therapy.
In the standardized cost analysis, the unadjusted cost was higher in the broad-spectrum group ($4704 vs $3933; P = .04). However, in the adjusted analysis modeled for LOS, there was no difference in average daily standardized cost (P = .62) or average daily standardized pharmacy cost (P = .26) (Table 2). In the subanalysis excluding patients who received macrolide therapy, there was still no difference in daily charges in the terms of average daily standardized cost (P = .48) or average daily standardized pharmacy cost (P = .32).
Discussion
We compared empiric narrow-spectrum therapy to broad-spectrum therapy for children hospitalized with uncomplicated CAP during the era of conjugate pneumococcal vaccination. In this multicenter study, we found that the narrow-spectrum therapy was not inferior to broad-spectrum antibiotics in all measured outcomes including LOS, duration of oxygen, duration of fever, daily standardized pharmacy and overall costs, or readmission rates within 7 days. The results of this study support the recently published PIDS/IDSA guideline, which recommends the empiric use of aminopenicillins in CAP of hospitalized pediatric patients.
In our study, only 33% of all patients with CAP received the recommended therapy with a narrow-spectrum penicillin or aminopenicillin. Broad-spectrum cephalosporins were the most commonly prescribed antibiotics, but they were used with substantial variability across the 4 participating hospitals. These findings are consistent with previous studies and indicate that physicians and some hospitals have yet to reliably change their practice. In 1 multicenter study of CAP, rates of ampicillin use at tertiary care children’s hospitals were as low as 5.5%.15 In another multicenter study, Ambroggio et al4 reported use of broad-spectrum therapy in up to 93% of children hospitalized for CAP across 33 children’s hospitals. Even among pediatric infectious disease physicians, there is considerable variation in empiric prescribing patterns, with only 21% recommending ampicillin or ampicillin/sulbactam alone for uncomplicated CAP.16 Adherence to recommended empiric antibiotics should be closely monitored as an indicator of quality and implementation efforts sought at local and national levels, especially with this new evidence to support recent PIDS/IDSA recommendations.
We found that narrow-spectrum penicillins were not inferior to broad-spectrum antibiotics across 4 freestanding children’s hospitals. Few studies have directly compared empiric antibiotics with patient outcomes and cost, especially across multiple settings. In 1 multicenter study, Ambroggio et al4 compared β-lactam monotherapy to β-lactam plus macrolide. In the subanalysis of patients receiving β-lactam monotherapy, 12% of the total cohort received an aminopenicillin with the remainder receiving a second- or third-generation cephalosporin. Readmission rates were not statistically different between the 2 groups. A single-center study by Newman et al6 also demonstrated similar outcomes between patients treated with aminopenicillins and ceftriaxone. Treatment failures were infrequent and not statistically different between the 2 groups.
Although the PIDS/IDSA CAP guideline noted that the cost of ampicillin and penicillin is less than that of other broad-spectrum agents, the utilization of hospital resources and overall costs of administration may be greater due to differences in dosing.5,17 However, when we analyzed standardized pharmacy charges, accounting for the impact of LOS, we found no significant difference between the 2 groups. This finding reveals that narrow-spectrum antibiotics are not only as clinically effective as broader coverage but, for the 4 hospitals in this study, they are also similar in cost.
There exist many reasons to preferentially use penicillins as first-line antibiotic therapy for CAP. First, penicillins provide appropriate coverage for the most prominent pathogen, S pneumoniae.5,18 Second, treatment of patients with non–central nervous system penicillin-resistant pneumococcal infections with penicillins has not been associated with treatment failures. These findings are consistent with in vitro data demonstrating bactericidal activity of penicillins at relatively low concentrations relative to the minimum inhibitory concentrations of pneumococcus.19,20 As a consequence, different breakpoints are used to determine pneumococcal susceptibility for infections outside of the central nervous system.21 Finally, the use of broad-spectrum antibiotics has been shown to increase the risk of developing subsequent infections with resistant organisms. In 2007, the IDSA noted that given the emergence of multiresistant organisms, appropriate use of antimicrobial agents has become a focus of patient safety and quality assurance.17
This study has several limitations. First, residual confounding may persist. The clinical impression and subsequent choice of empiric antibiotic therapy are influenced by many factors and we cannot be entirely certain that the impression was adequately accounted for by the propensity score. Nonetheless, outcomes of children receiving narrow-spectrum therapy were excellent and comparable to those in children receiving broad-spectrum antibiotics. Second, previous antibiotic exposure may influence empiric prescribing behavior. Although we included previous antibiotic exposure documented in the medical record, we cannot be entirely certain that it was consistently documented. Third, this study focused on the most common empiric antibiotic regimens for CAP. We did not evaluate patients receiving multiple antibiotics or antibiotics other than those included in our study (eg, targeted staphylococcal therapy). Finally, readmission was uncommon and our study does not have adequate power to detect small but potentially important differences in readmission rates.
Comparative effectiveness studies can be used to support consensus recommendations, especially when randomized controlled studies are costly or infeasible. Our study contributes to a growing body of evidence and consensus that broad-spectrum therapy is not needed in uncomplicated CAP and patients can be safely treated with narrow-spectrum antibiotics.
Supplementary Material
Glossary
- CAP
community-acquired pneumonia
- CI
confidence interval
- IDSA
Infectious Diseases Society of America
- LOS
length of stay
- PHIS
Pediatric Health Information System
- PIDS
Pediatric Infectious Diseases Society
- WBC
white blood cell
Footnotes
Dr Queen conceptualized and designed the study and drafted the initial manuscript; Dr Myers participated in data collection and analysis and drafted the initial manuscript; Dr Hall carried out the initial analyses and reviewed and revised the manuscript; Dr Shah conceptualized and designed the study and revised the manuscript; Dr Williams coordinated and supervised data collection and critically reviewed the manuscript; Drs Auger, Jerardi, and Statile participated in data collection and analysis and critically reviewed the manuscript; Dr Tieder conceptualized and designed the study, provided project oversight, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr Auger received funding from the Robert Wood Johnson Foundation Clinical Scholars Fellowship Program; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: No external funding.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126(2):204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keren R, Luan X, Localio R, et al. Pediatric Research in Inpatient Settings (PRIS) Network . Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164 [DOI] [PubMed] [Google Scholar]
- 3.Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambroggio L, Tabb LP, O’Meara T, Sheffler-Collins S, McGowan KL, Shah SS. Influence of antibiotic susceptibility patterns on empiric antibiotic prescribing for children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(4):331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–76 [DOI] [PMC free article] [PubMed]
- 6.Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3). Available at: www.pediatrics.org/cgi/content/full/129/3/e597 [DOI] [PubMed] [Google Scholar]
- 7.Dinur-Schejter Y, Cohen-Cymberknoh M, Tenenbaum A, et al. Antibiotic treatment of children with community-acquired pneumonia: comparison of penicillin or ampicillin versus cefuroxime. Pediatr Pulmonol. 2013;48(1):52–58 [DOI] [PubMed] [Google Scholar]
- 8.Vuori-Holopainen E, Peltola H, Kallio MJ, SE-TU Study Group . Narrow- versus broad-spectrum parenteral antimicrobials against common infections of childhood: a prospective and randomised comparison between penicillin and cefuroxime. Eur J Pediatr. 2000;159(12):878–884 [DOI] [PubMed] [Google Scholar]
- 9.Williams DJ, Shah SS, Myers AL, et al. Identifying pediatric community-acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9):851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/e99 [DOI] [PubMed] [Google Scholar]
- 11.Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonafide CP, Brady PW, Keren R, Conway PH, Marsolo K, Daymont C. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131(4). Available at: www.pediatrics.org/cgi/content/full/131/4/e1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13(12):841–853 [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum P, Rubin D. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38 [Google Scholar]
- 15.Weiss AK, Hall M, Lee GE, Kronman MP, Sheffler-Collins S, Shah SS. Adjunct corticosteroids in children hospitalized with community-acquired pneumonia. Pediatrics. 2011;127(2). Available at: www.pediatrics.org/cgi/content/full/127/2/e255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersh AL, Shapiro DJ, Newland JG, Polgreen PM, Beekmann SE, Shah SS. Variability in pediatric infectious disease consultants’ recommendations for management of community-acquired pneumonia. PLoS ONE. 2011;6(5):e20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177 [DOI] [PubMed]
- 18.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3). Available at: www.pediatrics.org/cgi/content/full/131/4/e964 [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Trallero E, Alkorta M, Giménez MJ, Vicente D, Aguilar L. Prediction of in-vivo efficacy by in-vitro early bactericidal activity with oral beta-lactams, in a dose-ranging immunocompetent mouse sepsis model, using strains of Streptococcus pneumoniae with decreasing susceptibilities to penicillin. J Chemother. 2001;13(2):118–125 [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Trallero E, Alkorta M, García-Arenzana JM, Iturzaeta A, Gomariz M. In-vitro, in-vivo and ex-vivo studies with oral beta-lactams against Streptococcus pneumoniae. J Antimicrob Chemother. 1998;41(6):629–634 [DOI] [PubMed] [Google Scholar]
- 21.Yu VL, Chiou CC, Feldman C, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003;37(2):230–237 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.