Abstract
OBJECTIVE:
To develop and evaluate a system for reliable and efficient individualized risk-based monitoring of cholesterol and 11 other tests after kidney transplantation in children.
METHODS:
We identified system components that drive reliable individualized monitoring and used quality improvement methods to develop and implement interventions, including (1) monitoring schedules individualized by dyslipidemia risk assigned to each patient, (2) automated previsit decision support from our electronic medical record, (3) standardized work flow and responsibility, and (4) automated forwarding of results to providers. We measured the proportion of patients due for cholesterol testing who had it performed within 1 week of their clinic visit and the proportion of patients in our population who achieved low-density lipoprotein (LDL) cholesterol control at baseline and for 2 years after improved monitoring.
RESULTS:
The proportion of visits in which cholesterol monitoring was completed when indicated improved from 80% to 98% within 8 months and was sustained for more than 1 year. The number of patients with controlled LDL (<130 mg/dL, 3.3 mmol/L) improved from 44 (71%) of 62 at the start of our project to 58 (94%) of 62 (P = .002) at an average follow-up of 24 months.
CONCLUSIONS:
Using quality improvement and health information technology, we achieved sustained, reliable and efficient personalized monitoring of cholesterol and 11 other tests. This approach enabled substantial improvement in LDL cholesterol control. Structured methods of system redesign that leverage information technology systems hold promise for rapidly achieving reliable individualized care in other settings.
Keywords: cholesterol, dyslipidemia, kidney transplant, low-density lipoprotein cholesterol, reliable systems, quality improvement, cardiovascular disease, preventive cardiology
Cardiovascular disease (CVD) is the leading cause of mortality in adults who undergo kidney transplantation (KT) during childhood, with the risk of cardiac death up to 50-fold greater than in the general population.1 Accordingly, guidelines place pediatric KT recipients (KTRs) in the highest CVD risk category.2–4 Among other CVD risk factors, pediatric KTRs have a high prevalence of dyslipidemia,5 which is a strong predictor of atherosclerosis in children6 and may compound the CVD risk conferred by coronary artery media calcifications in children receiving dialysis before KT.7,8 Dyslipidemia in KTRs is multifactorial, including traditional risk factors (eg, genetic predisposition, obesity, high-fat diet) and those specific to kidney disease and transplantation (proteinuria, and antihypertensive and immunosuppressive medications, especially sirolimus9). Thus, guidelines recommend regular cholesterol monitoring and control through therapeutic lifestyle change (TLC)2–4,10–12 for all pediatric KTRs and through pharmacotherapy with 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (statins) for those >10 years of age with low-density lipoprotein cholesterol (LDL) persistently >130 mg/dL (3.3 mmol/L) despite a trial of TLC.3,4,11
Dyslipidemia guidelines for pediatric KTRs have not been reliably implemented into clinical practice. At 8 Midwest Pediatric Nephrology Consortium centers, only 47% of adolescents with kidney disease or transplant had a cholesterol documented in their chart and, of those with high cholesterol, 44% were on medical therapy.13 Thus, the high cardiovascular risk for these patients in adulthood may be, in part, related to unreliable systems for preventive CVD care as children.
Evidence suggests that using structured quality improvement (QI) methods to redesign systems for reliable chronic care delivery can improve clinical outcomes.14,15 Moreover, removing unwanted variation in clinical practice may allow clinicians to better understand patient-specific variation.16
In 2008, the KT program at our center started a QI initiative to use a new electronic medical record (EMR) to improve our system of monitoring cholesterol and 11 other tests according to individualized schedules. We hypothesized that reliable cholesterol monitoring, individualized by dyslipidemia risk, would lead to improved treatment and control of dyslipidemia in our population.
Methods
Study Design
We used an interrupted time series design to assess the impact of structured QI methods and iterative plan-do-study-act cycles to test, refine, and reliably implement interventions to improve our system of cholesterol monitoring.17–19 This study was approved by our center’s institutional review board.
Setting
The KT program at our center performs 15 to 25 transplants yearly and follows 70 to 100 KTRs at any given time. Our multidisciplinary team includes nurse coordinators, clinic nurses, physicians, a renal dietician, a social worker, and a psychologist. Immunosuppression includes induction with basiliximab and methylprednisolone. Maintenance immunosuppression consists of prednisone, mycophenolate mofetil and tacrolimus. We dose prednisone initially at 1.5 to 2.0 mg/kg/d and wean over 6 months to a dosage of 0.1 mg/kg every 24 to 48 hours. We offer conversion from tacrolimus to sirolimus at 6 months after transplantation to standard- or low-immunologic-risk patients. Because of dyslipidemia risk, other comorbidities, and drug side effects, our practice before October 2008 was to monitor 23 different tests according to a complex algorithm individualized by time since transplant, current medications (eg, statins, sirolimus), and infection risk (Table 1). KTRs were seen by 1 of 9 pediatric nephrologists at 6 outpatient locations. The providers were notified of testing algorithms by e-mail dissemination of a 1-page spreadsheet listing all 23 tests and how often each should be monitored. Clinic documentation was performed in paper charts, whereas internal laboratory results were reported in an EMR. In October 2008, our division implemented a new, comprehensive EMR and we redesigned our process to be more efficient and reliable.
TABLE 1.
Posttransplant Monitoring (September 2008)
| Tests Monitored at Every Visit | |
|---|---|
| Urinalysis | |
| Complete blood count | |
| Renal profile | |
| Calcium, magnesium, phosphorus | |
| Glucose | |
| Medication levels (tacrolimus, sirolimus, cyclosporine) | |
| Tests Not Monitored at Every Visit | |
| Test | Frequency |
| Liver profile | (Not on statin) at 6 mo, 1 y, 2 y, and every 2 y |
| (On a statin) before statin, monthly for 3 mo, then at 6 mo after statin and every 6 mo thereafter | |
| Creatine kinase (only if on statin) | See liver profile “on a statin” |
| Uric acid | At 6 mo, 1 y, 2 y, and every 2 y |
| Fasting lipid profile | (Not on sirolimus) At 6 mo, 1 y, 2 y, and every 2 y |
| (On sirolimus) every 6 mo | |
| Nuclear glomerular filtration rate | At 6 mo, 1 y, 2 y, and every 2 y |
| Cystatin C | At 6 mo, 1 y, 2 y, and every 2 y |
| Urine BK virus | At 1 mo, at 6 mo, at 1 y, and yearly |
| Testosterone, LH, FSH (only if on sirolimus) | At time of sirolimus initiation, every 6 mo while on sirolimus |
| Echocardiogram | At 1 y, 2 y, and every 2 y |
| Transplant ultrasound | At 3 mo, 1 y, 2 y, and every 2 y |
| EBV IgG & IgM, EBV quantitative PCR (only for EBV IgG-negative patients) | At 1 mo, quarterly during the first 2 y, and yearly until converted |
EBV, Epstein-Barr virus; FSH, follicle stimulating hormone; Ig, immunoglobulin; LH, luteinizing hormone; PCR, polymerase chain reaction.
Planning the Intervention
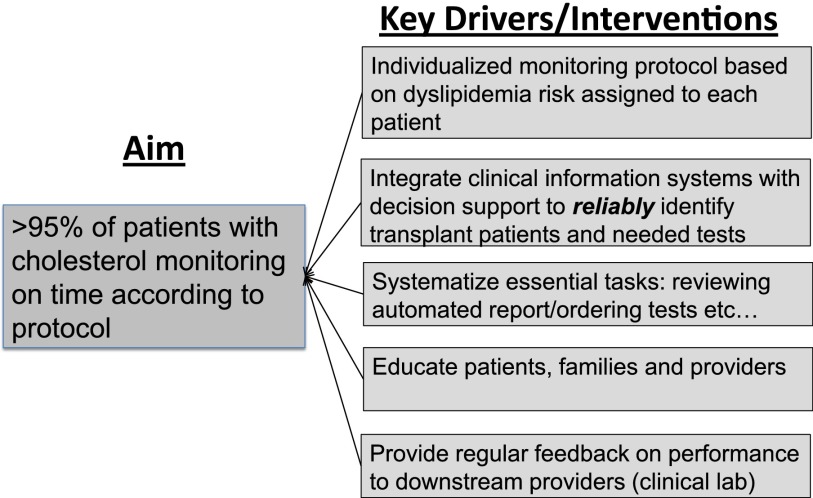
A pediatric nephrology fellow (D.K.H.) and KT nurse coordinator (J.R.) co-led a multidisciplinary team composed of an EMR specialist, a computer analyst, the medical director of KT, and a QI consultant. The team first identified a set of system factors, termed key drivers, that we thought to be necessary for reliable individualized monitoring (Fig 1). These key drivers were informed by the Chronic Care Model20,21 and incorporated principles of reliability science.22 Our aim was to develop a system that would achieve cholesterol monitoring in at least 95% of patients due for a cholesterol test at the time of their clinic visit. We chose this goal based on our perception of patients’ expectations and our belief that we could achieve this level of reliability22 through an intentionally redesigned process using human factors engineering principles.
FIGURE 1.
Key driver diagram for improving cholesterol monitoring in pediatric kidney transplant recipients.
We reviewed our current process to ensure proper monitoring and found that it relied on the specialized knowledge and diligence of our most experienced transplant nurse coordinator. Her process consisted of reviewing clinic rosters for the coming week to identify scheduled KTRs and reviewing medical records (both electronic and paper) to determine whether testing was indicated. Her primary intervention was to place “sticky notes” in patient charts reminding physicians to order indicated tests. Once a test was ordered, she would contact the patient/family and provide necessary instructions. This process was time-consuming and unreliable because it did not happen if she was absent.
Improvement Activities
We, first, simplified our laboratory monitoring schedules. The pediatric nephrology fellow (D.K.H.) and medical director of KT (J.G.) reviewed all laboratory tests and identified those that were monitored infrequently, rather than at every medical encounter (Table 1). We selected 12 tests, including fasting lipid profile, for personalized monitoring and developed 18 discrete individualized schedules based on evidence and published guidelines (Table 2).11 Cholesterol monitoring frequency was determined by dyslipidemia risk (Table 3). The highest-risk patients with dyslipidemia (on a statin <6 months) were monitored quarterly. Patients with moderate risk (<1 year posttransplant, on sirolimus, or treated long term with a statin) were monitored semiannually. Lowest-risk patients (>1 year posttransplant and not on sirolimus or a statin) were monitored yearly.
TABLE 2.
New Individualized Posttransplant Monitoring Schedule for 12 Tests (October 2008)
| Test | Frequency |
|---|---|
| Liver profile | (Not on statin) at 6 mo, 1 y, 2 y, and every 2 y |
| (On a statin) before statin, every 3 mo ×2,a then every 6 mo thereafter | |
| Creatine kinase (only if on statin) | See liver profile “on a statin” |
| Uric acid | At 6 mo, 1 y, 2 y, and every 2 y |
| Fasting lipid profile | (Not on sirolimus) at 6 mo, 1 y, then yearly thereaftera |
| (On sirolimus) at initiation, every 6 mo | |
| (On statin) at initiation, every 3 mo ×2, then every 6 moa | |
| Nuclear glomerular filtration rate | At 6 mo, 1 y, 2 y, and every 2 y |
| Cystatin C | At 6 mo, 1 y, 2 y, and every 2 y |
| Urine BK virus | At 1 mo, at 6 mo, at 1 y, and yearly |
| Testosterone, LH, FSH (only if on sirolimus) | At time of sirolimus initiation, every 6 mo while on sirolimus |
| Echocardiogram | At 1 y, 2 y, and every 2 y |
| Transplant ultrasound | At 3 mo, 1 y, 2 y, and every 2 y |
Indicates a new frequency of monitoring. FSH, follicle stimulating hormone; LH, luteinizing hormone.
TABLE 3.
Frequency of Cholesterol Monitoring by Dyslipidemia Risk
| Risk | Low | Moderate | High |
|---|---|---|---|
| Criteria | • >1 y posttransplant | • <1 y posttransplant | • On statin <6 mo |
| • No dyslipidemia | • On sirolimus | ||
| • Not on sirolimus or statin | • On statin >6 mo | ||
| Cholesterol monitoring frequency | Yearly | Every 6 mo | Every 3 mo |
Our second intervention was to develop a decision-support report automatically generated from our EMR to (1) identify all KTRs coming to clinic in the upcoming week, (2) assign 1 of the new 18 unique testing schedules to each patient according to dyslipidemia risk, and (3) report the most recent test results, whether additional testing was due, and the next due date for each test. This report was tested iteratively to refine it for accuracy and ease of use. Our head transplant nurse coordinator assessed accuracy by comparing the report with data gathered manually. The same nurse coordinator initially determined ease of use subjectively, then all nurse coordinators in our group trialed the report to ensure that any of them could reproducibly interpret it and order needed tests appropriately.
Subsequent interventions focused on developing reliable systems to use this report. We assigned a nurse to review the report weekly. We initially trialed entering reminders for indicated testing into the EMR, but quickly learned that providers did not always review this information. Educating physicians about this process resulted in modest improvement. We then modified our process so that orders for indicated testing were pended in the EMR by the individual reviewing the report and forwarded to the responsible physician for signature before the patient would come to clinic. This same team member would then contact the patient and/or family and give any special instructions and required education (eg, come fasting for lipid profile). The EMR was configured to automatically forward laboratory results to the ordering physician after the patient’s visit. If cholesterol results were abnormal, the patient was contacted by the provider or nurse to verify they had fasted.
There were no specific interventions focused on reliable therapy for dyslipidemia. As had been the practice before our interventions, treatment was at the discretion of the patient’s nephrologist, according to published guidelines.3
Data Collection and Measures
Cholesterol Monitoring
We measured the proportion of visits for which indicated monitoring was performed within 1 week. We also monitored the total time per week allotted to ensuring testing was performed and the percentage of visits for which all indicated testing (in addition to cholesterol) was performed within 1 week. Internal laboratory results were available immediately in our EMR and external laboratory results were faxed to our center and entered into the EMR on the day received. This process did not change throughout our study. KT nurse coordinators collected data weekly as part of their standardized work flow. They recorded the number of indicated tests each week in a spreadsheet and then reviewed the EMR the following week to determine whether indicated testing was performed. Data collection was started at the time of EMR roll-out, before project initiation, and continued through September 2010 when we had sufficient belief that our system had experienced sustained improvement.
Control of Dyslipidemia
After implementing our monitoring system, we assessed whether monitoring, treatment, and control of dyslipidemia improved in our population. Our primary measure was the proportion of KTRs who had LDL <130 mg/dL (3.3 mmol/L) documented within the previous 12 months. We included the most recent LDL value of patients >7 months posttransplant (when cholesterol monitoring begins) and who had been seen within the previous year. Patients who transferred care or experienced graft loss were removed from the population at the time of event. Other measures included the proportion of patients with a lipid profile recorded in the previous 12 months and the percentage of KTRs aged ≥10 years with dyslipidemia who were on statin therapy. We defined dyslipidemia as LDL ≥130 mg/dL (3.3 mmol/L) or on statin therapy. For outcome measures, we recorded the most recent value for each patient and carried them forward for each subsequent time point until a new assessment was performed.16 We extracted data quarterly from our EMR using automated reports to create statistical process control charts17 from October 2008 through January 2012. We validated data by manual chart review.
Methods of Analysis
We plotted the percentage of visits where indicated cholesterol testing was performed over time on a run chart and applied run chart rules18 to detect improvements in the process. We evaluated population-based measures (control of dyslipidemia, treatment of dyslipidemia, and cholesterol monitoring; see previous section “Control of Dyslipidemia”) using statistical process control.17 Data from the baseline period (October 2008–April 2009, during improvement activities) were compared with data from July 2009 to January 2012. We identified data points outside the control limits (±3 SDs)17 as special cause variation (apparent improvement) and adjusted the centerline mean and control limits accordingly. We generated charts in Microsoft Excel (2007) by using templates developed at our center.
Because patients entered (new KTR) and exited (transfer or graft loss) our cohort over time, changes in case mix might have affected our time-series results. To account for this, we performed a before/after analysis of cholesterol control in a cohort of 62 patients who had fasting cholesterol measured at 2 time points: (1) within the year before our QI initiative, and (2) at least 6 months after the full implementation of our improvement bundle. By using SAS 9.2 statistical software (SAS Institute, Cary, NC) and McNemar’s test for paired data, we compared the percentage of patients with LDL <130 mg/dL (3.3 mmol/L) at each time point. We considered P < .05 to be statistically significant.
Results
Study Population
Seventy-eight patients met inclusion criteria at the start of our project in October 2008 and there were 69 by January 2012. Over this time span, 36 patients left the population (26 transitioned to adult care, 5 went on dialysis, 2 died, 2 were lost to follow-up, 1 moved) and 27 new patients entered it (24 new transplants, 3 transferred care). Demographics and clinical characteristics for patients at each time point and for the 62 patients in the before/after analysis are listed in Table 4. Patients were predominantly male and white and ranged from 3 to 26 years old. The most common underlying disorders were aplasia/hypoplasia/dysplasia, followed by “other,” obstructive/reflux, and focal segmental glomerulosclerosis.
TABLE 4.
Patient Demographics
| Oct. 2009 | Jan. 2012 | Before/After Cohort | |
|---|---|---|---|
| n | 78 | 69 | 62 |
| Male, n (%) | 52 (67) | 43 (62) | 39 (63) |
| Median years old (range) | 18 (3–26) | 18 (3–25) | 16 (2–24) |
| Median years since transplant (range) | 4.9 (0.7–14.4) | 5.3 (0.6–16) | 4.6 (0.6–13.8) |
| Race/Ethnicity, n (%) | |||
| White non-Hispanic | 60 (77) | 52 (75) | 45 (73) |
| Black non-Hispanic | 16 (21) | 13 (19) | 15 (24) |
| Hispanic | 0 (0) | 1(1) | 0 (0) |
| Other | 2 (3) | 3 (4) | 2 (3) |
| Primary diagnosis | |||
| Aplasia/Hypoplasia/Dysplasia | 20 (26) | 17 (25) | 14 (23) |
| Obstructive/Reflux Nephropathy | 13 (17) | 11 (16) | 9 (15) |
| FSGS | 13 (17) | 9 (13) | 12 (19) |
| Chronic glomerulonephritis and nephrotic syndrome | 10 (13) | 7 (10) | 10 (16) |
| Cystic disease | 7 (9) | 5 (7) | 5 (8) |
| Other | 15 (19) | 20 (29) | 12 (19) |
| On sirolimus, n (%) | 37 (47) | 38 (55) | |
| On statin, n (%) | 15 (19) | 26 (38) |
Reliability of Cholesterol Monitoring
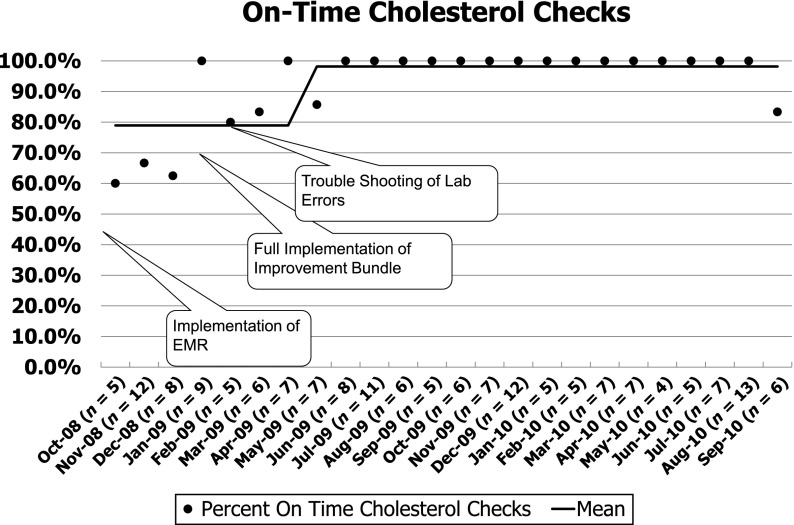
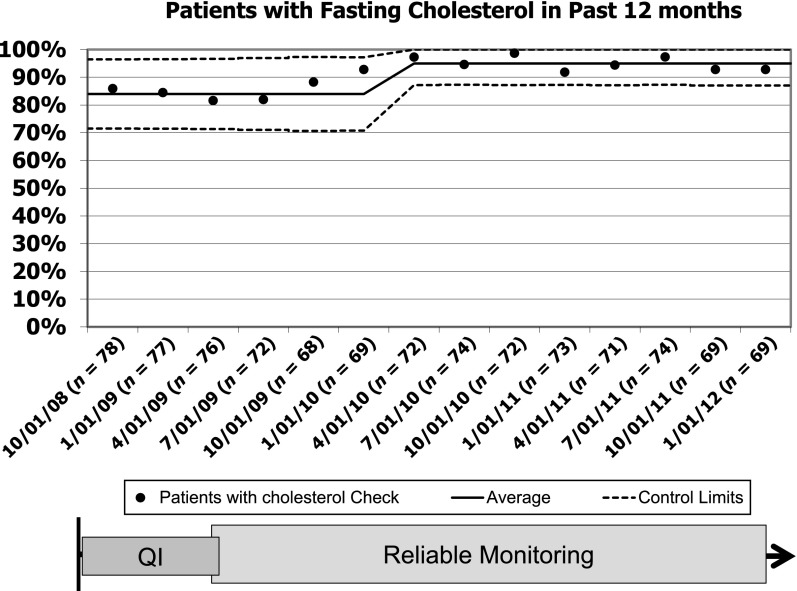
During the baseline period, cholesterol testing was performed correctly ∼80% of the time (Fig 2). By January 2009, we had completely implemented our new process, and reliability of cholesterol monitoring started to improve. Thereafter, our new tracking process exposed a problem in the clinical laboratory: even when orders were entered and signed appropriately, the laboratory did not always process them appropriately. Accordingly, we initiated regular performance feedback to the clinical laboratory that resulted in improvement of their processes. By 8 months, the new process had achieved 98% reliability that was sustained for more than a year (Fig 2). The reliability of monitoring all other tests was similar (95%) for the same period. Within our entire population, the percentage of patients with cholesterol documented in the previous 12 months increased from 84% at the start of our project to 95% and has been sustained for nearly 2 years (Fig 3).
FIGURE 2.
Annotated Run chart of our primary measure for improved cholesterol monitoring. Each point represents the percentage of visit where indicated cholesterol testing was performed. The line indicates the mean. The annotations mark interventions and external factors that may influence our system.
FIGURE 3.
Statistical process control chart of the percentage of patients with a fasting cholesterol documented in the previous 12 months. Baseline median and 3-σ control limits were based on data from October 2008 through April 2009 and were readjusted when special cause was achieved.
Treatment of Dyslipidemia
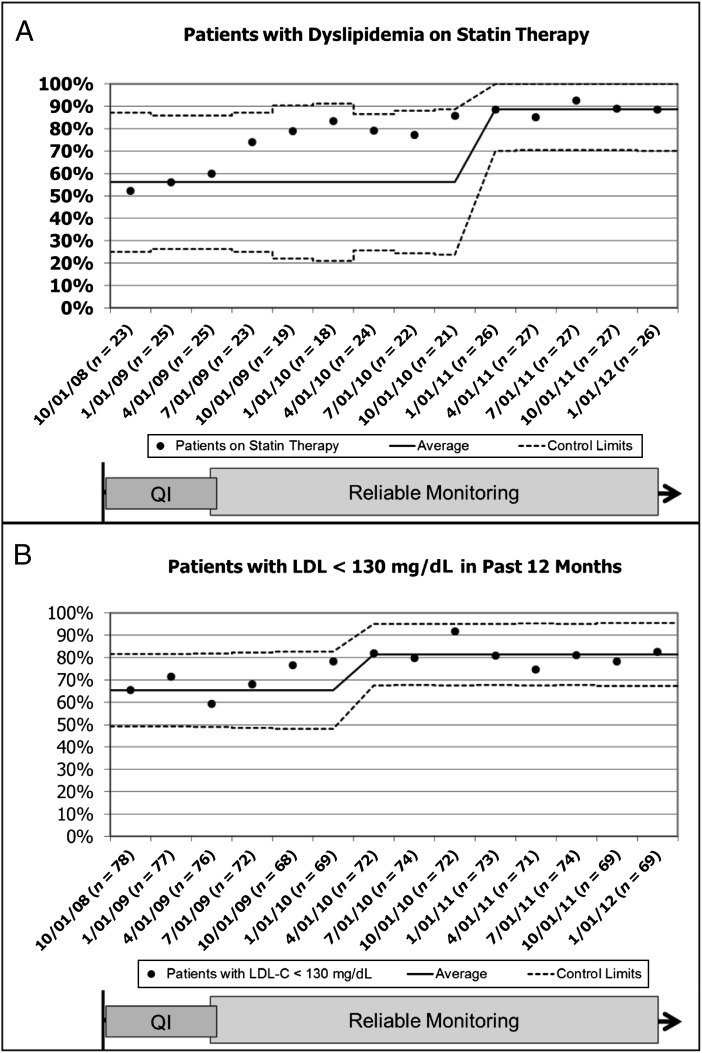
At the initiation of our project, 23 (28%) of 81 patients had dyslipidemia. Of these, 12 (52%) were on statin therapy. Over the next 3 years, statin prescriptions for patients with dyslipidemia increased to 23 (88%) of 26 patients (Fig 4A). Improved treatment of dyslipidemia was accompanied by an increase in the percentage of patients with LDL <130 mg/dL (3.3 mmol/L) in the previous 12 months, from 51 (65%) of 78 at baseline to 57 (83%) of 69 in January 2012 (Fig 4B).
FIGURE 4.
Statistical process control chart of (A) the percentage of dyslipidemic patients >10 years treated with a statin and (B) the percentage of patients in our cohort with LDL <130 mg/dL (3.3 mmol/l) documented in the previous 12 months. Data are limited to patients ≥7 months out from kidney transplant. Baseline median and 3-σ control limits were based on data from October 2008 through April 2009 and were readjusted when special cause was achieved.
Before/After Analysis
Sixty-two (95%) of 65 patients with a fasting cholesterol in the year before January 2009 also had another fasting lipid profile documented at least 6 months (June 2009) after sustained improvement in cholesterol monitoring. Before our interventions, 44 (71%) of 62 patients had an LDL <130 mg/dL (3.3 mmol/L), compared with 58 (94%) of 62 (P = .002) after a median follow-up of 24 months.
Discussion
This study demonstrates rapid achievement of efficient and reliable individualized laboratory screening by implementing components of the Chronic Care Model20 using structured QI methodology. Our improved system requires less time and no longer relies on the specialized knowledge of an experienced nurse coordinator. Moreover, we observed associated improvement in treatment and control of dyslipidemia within the first year of reliable, risk-based monitoring.
The importance of screening for and controlling modifiable cardiovascular risk factors in KTRs is well recognized,2,3,11,12 including in the recent report from the Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents at the National Heart, Lung and Blood Institute.4 Pediatric KTRs are among the patients at highest risk for CVD, with substantially increased cardiovascular mortality in adulthood.1 Yet, recent data indicate that only about 50% of adolescents with advanced kidney disease or after KT have even a single cholesterol test documented before transferring to an adult-focused provider and, of those with uncontrolled cholesterol, fewer than half were on statin therapy.13 This is consistent with other research findings that indicate preventive care is delivered less than half the time in the pediatric ambulatory setting23 and that fewer than 40% of adult transplant patients have controlled LDL cholesterol, with indicated therapy prescribed in only about 60%.24
More than a decade ago, Wagner and colleagues21 identified the potential to improve outcomes for patients with chronic disease by developing new systems capable of delivering reliable evidence-based and individualized care. Their Chronic Care Model20 called for planned care according to evidence-based guidelines supported by clinical information systems, decision support, and delivery system design. Unfortunately, with the exception of relatively few group practices, widespread reliable chronic disease management for both children and patients with kidney disease has yet to be achieved.23,24 Our study demonstrates the potential for such systems to achieve desired therapeutic targets in most patients.
This QI initiative did not focus specifically on treating cholesterol, but, rather, on monitoring. Consequently, our measure of reliable monitoring is only one indicator of this system that has ultimately improved patient care. Other key drivers also affected this system. For example, our use of health information technology enabled each patient to be assigned an individualized cholesterol-monitoring schedule determined by dyslipidemia risk. We configured the EMR to forward results automatically to responsible providers. Care delivery team design including assigned individual roles ensured that tests were ordered and forwarded to physicians before the clinic visit. Education of patients, providers, and staff helped ensure that each knew his or her responsibility within the system. Ultimately, these interventions combine to create a system that has resulted in improved care and is now part of our routine.
There are several approaches for managing dyslipidemia25 in high-risk patients. Current guidelines2,3 recommend assessment by a trained dietician and a trial of TLC before initiating statin therapy. Additional interventions include minimizing dyslipidemia-causing medications, such as steroids26 or sirolimus,9 and/or treating with statins.27,28 Despite the resulting substantial improvement in cholesterol control, our project did not focus on treating dyslipidemia, but, rather, on improving our process for monitoring this and other important risks. As such, treatment of dyslipidemia was at the discretion of the patient’s physician, according to published guidelines.2 Thus, our results suggest that reliable monitoring and forwarding of results to clinicians can have substantial effects on treatment and control of dyslipidemia. Future efforts should focus on developing and testing more sophisticated risk-stratification (eg, proteinuria, other dyslipidemia-causing medications) and standardizing therapy for dyslipidemic patients, including dietary assessment and TLC. Such approaches should emphasize the essential role of trained dieticians as part of a multidisciplinary team.29
Our findings should be interpreted in light of their limitations. First, to most effectively demonstrate improvement in our process, we would have, ideally, collected several months of baseline cholesterol monitoring data before intervening. However, even without a large amount of baseline data, we decided to begin improvement because the initial data were consistent with our knowledge of the system’s performance. Second, the time series analysis of cholesterol control in our population may have been affected by higher- or lower-risk patients entering or leaving the population over time. To account for this, we performed a before/after analysis on a discrete cohort of patients who had cholesterol testing both before and after our QI interventions. Finally, our project did not address patient behavior, such as medication adherence, clinic “no shows,” and patients who had not been seen in more than 12 months. Accordingly, a small proportion of our population was not reached by our new system. Comprehensive chronic disease management will require self-management support and strategies to enhance the patient interaction with our system, including specific interventions focused on adherence. Notwithstanding these limitations, our report adds to the small, but accumulating, literature supporting the use of QI methods to drive improved clinical outcomes in pediatric patients with chronic illness.
Conclusions
We used QI methods to rapidly implement a series of changes that created a reliable system for individualized monitoring of cholesterol and 11 other tests in KTRs who are at exceedingly high risk for CVD in adulthood. This reliable, risk-based monitoring was followed by improved treatment and control of dyslipidemia in our population. Our interventions were informed by the Chronic Care Model20 and reliability science and hold promise for improving care and outcomes for patients with other chronic conditions.
Acknowledgments
We acknowledge Julie Ross, RN, Diane Miller, and James Brown, MS, for their contributions to improvement work. We also acknowledge Pamela J. Schoettker, MS, for her assistance editing and submitting this manuscript.
Glossary
- CVD
cardiovascular disease
- EMR
electronic medical record
- KT
kidney transplantation
- KTR
kidney transplant recipient
- LDL
low-density lipoprotein cholesterol
- QI
quality improvement
- TLC
therapeutic lifestyle change
Footnotes
Drs Hooper and Goebel contributed substantially to the conception and design of the study, acquisition of data, analysis and interpretation of the data, drafting the article, and revising it critically for important intellectual content; Ms Kirby contributed substantially to the acquisition of data, interpretation of data, and drafting and revising of the article; Dr Margolis contributed to the conception and design of the study, analysis and interpretation of the data, drafting the article, and revising it critically for important intellectual content; and all authors approved the final version to be published.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: No external funding.
References
- 1.Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141(2):191–197 [DOI] [PubMed] [Google Scholar]
- 2.Kavey RE, Allada V, Daniels SR, et al. American Heart Association Expert Panel on Population and Prevention Science. American Heart Association Council on Cardiovascular Disease in the Young. American Heart Association Council on Epidemiology and Prevention. American Heart Association Council on Nutrition, Physical Activity and Metabolism. American Heart Association Council on High Blood Pressure Research. American Heart Association Council on Cardiovascular Nursing. American Heart Association Council on the Kidney in Heart Disease. Interdisciplinary Working Group on Quality of Care and Outcomes Research . Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114(24):2710–2738 [DOI] [PubMed] [Google Scholar]
- 3.Kasiske B, Cosio FG, Beto J, et al. National Kidney Foundation . Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: a report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am J Transplant. 2004;4(suppl 7):13–53 [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213–S256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson AC, Greenbaum LA, Barletta GM, et al. High prevalence of the metabolic syndrome and associated left ventricular hypertrophy in pediatric renal transplant recipients. Pediatr Transplant. 2010;14(1):52–60 [DOI] [PubMed] [Google Scholar]
- 6.Newman WP, III, Freedman DS, Voors AW, et al. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med. 1986;314(3):138–144 [DOI] [PubMed] [Google Scholar]
- 7.Sheth RD, Perez MD, Goldstein SL. Cardiovascular calcifications in pediatric patients receiving maintenance dialysis. Pediatr Nephrol. 2003;18(8):810–813 [DOI] [PubMed] [Google Scholar]
- 8.Filler G. Challenges in pediatric transplantation: the impact of chronic kidney disease and cardiovascular risk factors on long-term outcomes and recommended management strategies. Pediatr Transplant. 2011;15(1):25–31 [DOI] [PubMed] [Google Scholar]
- 9.Kasiske BL, de Mattos A, Flechner SM, et al. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant. 2008;8(7):1384–1392 [DOI] [PubMed] [Google Scholar]
- 10.Daniels SR, Greer FR, Committee on Nutrition . Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208 [DOI] [PubMed] [Google Scholar]
- 11.KDIGO Kideney disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(suppl 3):S1–S157 [DOI] [PubMed] [Google Scholar]
- 12.NKF. K/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney disease. Am J Kidney Dis. 2003;41(4 suppl 3):I-IV, S1-91 [PubMed]
- 13.Patel U, Williams J, Patel H, et al. Variation in cardiovascular risk assessment among adolescents with kidney disease [abstract F-PO1255]. Paper presented at: Renal Week 2009, American Society of Nephrology; October 30, 2009; San Diego, CA [Google Scholar]
- 14.Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296(4):427–440 [DOI] [PubMed] [Google Scholar]
- 15.Bravata DM, Gienger AL, Holty JE, et al. Quality improvement strategies for children with asthma: a systematic review. Arch Pediatr Adolesc Med. 2009;163(6):572–581 [DOI] [PubMed] [Google Scholar]
- 16.Crandall WV, Margolis PA, Kappelman MD, et al. ImproveCareNow Collaborative . Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics. 2012;129(4). Available at: www.pediatrics.org/cgi/content/full/129/4/e1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46–51 [DOI] [PubMed]
- 19.Langley GJMR, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA: Jossey-Bass; 2009 [Google Scholar]
- 20.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4 [PubMed] [Google Scholar]
- 21.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544 [PubMed] [Google Scholar]
- 22.Luria JW, Muething SE, Schoettker PJ, Kotagal UR. Reliability science and patient safety. Pediatr Clin North Am. 2006;53(6):1121–1133 [DOI] [PubMed] [Google Scholar]
- 23.Mangione-Smith R, DeCristofaro AH, Setodji CM, et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357(15):1515–1523 [DOI] [PubMed] [Google Scholar]
- 24.Marcén R, del Castillo D, Capdevila L, et al. Achieving chronic kidney disease treatment targets in renal transplant recipients: results from a cross-sectional study in Spain. Transplantation. 2009;87(9):1340–1346 [DOI] [PubMed] [Google Scholar]
- 25.Butani L. Dyslipidemia after renal transplantation: a cause for concern? Pediatr Transplant. 2008;12(7):724–728 [DOI] [PubMed] [Google Scholar]
- 26.Li L, Chang A, Naesens M, et al. Steroid-free immunosuppression since 1999: 129 pediatric renal transplants with sustained graft and patient benefits. Am J Transplant. 2009;9(6):1362–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butani L. Prospective monitoring of lipid profiles in children receiving pravastatin preemptively after renal transplantation. Pediatr Transplant. 2005;9(6):746–753 [DOI] [PubMed] [Google Scholar]
- 28.Butani L, Pai MV, Makker SP. Pilot study describing the use of pravastatin in pediatric renal transplant recipients. Pediatr Transplant. 2003;7(3):179–184 [DOI] [PubMed] [Google Scholar]
- 29.Filler G, Lipshultz SE. Why multidisciplinary clinics should be the standard for treating chronic kidney disease. Pediatr Nephrol. 2012;27(10):1831–1834 [DOI] [PubMed] [Google Scholar]