Abstract
Key features of the metabolic syndrome are insulin resistance and diabetes. The liver as central metabolic organ is not only affected by the metabolic syndrome as non-alcoholic fatty liver disease (NAFLD), but may contribute to insulin resistance and metabolic alterations. We aimed to identify potential associations between liver injury markers and diabetes in the population-based Heinz Nixdorf RECALL Study. Demographic and laboratory data were analyzed in participants (n = 4814, age 45 to 75y). ALT and AST values were significantly higher in males than in females. Mean BMI was 27.9 kg/m2 and type-2-diabetes (known and unkown) was present in 656 participants (13.7%). Adiponectin and vitamin D both correlated inversely with BMI. ALT, AST, and GGT correlated with BMI, CRP and HbA1c and inversely correlated with adiponectin levels. Logistic regression models using HbA1c and adiponectin or HbA1c and BMI were able to predict diabetes with high accuracy. Transaminase levels within normal ranges were closely associated with the BMI and diabetes risk. Transaminase levels and adiponectin were inversely associated. Re-assessment of current normal range limits should be considered, to provide a more exact indicator for chronic metabolic liver injury, in particular to reflect the situation in diabetic or obese individuals.
The liver as central organ for glucose and lipid metabolism is strongly affected by the metabolic syndrome. Thus, non-alcoholic fatty liver disease (NAFLD) represents the hepatologic consequence of Western lifestyles. NAFLD is the most common chronic liver disease in industrialized nations with a prevalence of up to 30% and probably the most common cause of elevated liver enzymes1,2. According to the National Health and Nutrition Examination Survey, the prevalence of major causes of chronic liver diseases remained stable from the years 1988 to 2008 except for NAFLD. Incidence of NAFLD increased steadily during this time, contributing to the burden of chronic liver disease in the United States3. NAFLD is associated with obesity as well as diabetes and could be not only a result of insulin resistance (IR) and metabolic syndrome but rather a major contributor to systemic IR4,5. NAFLD ranges from simple hepatic steatosis (NAFL) to non-alcoholic steatohepatitis (NASH) with hepatocyte ballooning and inflammatory components. Simple hepatic steatosis generally has a good prognosis, however NASH can lead to cirrhosis and hepatocellular carcinoma (HCC) in up to 15% of patients6. Metabolic injury to the liver will probably constitute a major burden for health care systems worldwide in the near future.
By now the metabolic syndrome is a common term and high cholesterol, high blood pressure, or obesity raise the attention of internists regarding the risk for cardiovascular or metabolic complications. Though, despite the very obvious link of NAFLD and metabolic syndrome, the awareness of metabolic liver injury and its connection to cardiovascular risk remains low. Several studies have shown, that normal transaminase levels do neither exclude NAFLD (or NASH) nor progression to advanced fibrosis7,8. However, aminotransferases are regarded as the main alarm signal for liver diseases or injury before enrolling further diagnostic. Previous studies already discuss the idea to lower normal values and to take metabolic factors into account, especially body mass index (BMI) and sex, which have a significant effect on ALT values9,10,11. Since the origin of obesity may be based in early childhood, hepatologists already claim to revise the values in pediatrics11. Still the classic liver serum parameters (ALT, AST, GGT), elevated in most chronic and acute liver diseases, may not be ideal markers for liver injury in NAFLD.
Liver function is crucial for glucose- and fatty acid metabolism and vice versa12. Enzymes and signaling pathways involved in hepatic glucose homeostasis contribute to insulin sensitivity. Reciprocally peripheral IR and lipolysis contribute to hepatic steatosis13,14. Other factors known to contribute to systemic IR and to development of NAFLD are adipokines such as adiponectin. Low adiponectin is associated with obesity, IR, and severity of NAFLD15,16,17. Vitamin D (VD) is also discussed to play an important role in the pathophysiology of IR and VD serum concentrations above 25 ng/ml were associated with a lower risk of type 2 diabetes18.
In the present study we aimed to investigate in a large population based cohort, whether serum transaminase levels within normal ranges are associated with metabolic risk and prevalence of diabetes. Associations of adiponectin, systemic inflammation, and VD levels with transaminases and diabetes were analyzed. Previously we were able to generate effective classification models by machine learning techniques19,20,21. Thus, we also aimed to build a model for diabetes prediction from non-invasive parameters.
Results
Demographic data
The initial cohort included 4814 participants (2419 female), aged 45 to 75 years (female 59.6 ± 7.8y, male 59.7 ± 7.8y). Detailed demographics are given in Table 1. Men exhibited significantly higher weight (86.2 ± 13.2 kg; p < 0.0001) and waist circumference (100.3 ± 10.8 cm; p < 0.0001) than women. The mean BMI of the cohort was 27.9 kg/m2, suggesting large parts of this population to be overweight or obese. Both women and men were considered overweight by BMI, with men reaching higher values (m: 28.2 ± 4.0 kg/m2 vs. w: 27.7 ± 5.2 kg/m2; p < 0.0001). Type 2 diabetes was present in 656 individuals of the HNR cohort (13.7%; Fig. 1). Thereof 397 (8.2%) had previously known type 2 diabetes and 259 (5.4%) had unknown diabetes. Type 2 diabetes was more common in men with 418 (17.5%) male subjects affected compared to 238 female subjects (9.8%). The highest proportion of diabetes was found in subjects with BMI above 40 (approx. 45%).
Table 1. Demographic data of the analyzed study cohort (Heinz Nixdorf Recall).
| Male (n = 2395) | Female (n = 2419) | |
|---|---|---|
| age (y) | 59.7 ± 7.8 | 59.6 ± 7.8 |
| height (cm) | 174.8 ± 6.8 | 162.1 ± 6.2*** |
| weight (kg) | 86.2 ± 13.2 | 72.6 ± 13.8*** |
| BMI (kg/m2) | 28.2 ± 4.0 | 27.7 ± 5.2*** |
| waist circumference (cm) | 100.3 ± 10.8 | 88.5 ± 12.9*** |
| Diabetes n (%) | 418 (17.5) | 238 (9.8)*** |
| smoking status | ||
| current | 614 (25.6%) | 514 (21.3%) |
| former | 1105 (46.1%) | 557 (23.0%) |
| never | 669 (27.9%) | 1345 (55.6%) |
***p < 0.0001 vs. males.
Figure 1. Prevalence of diabetes in the Heinz Nixdorf Recall study by BMI groups.
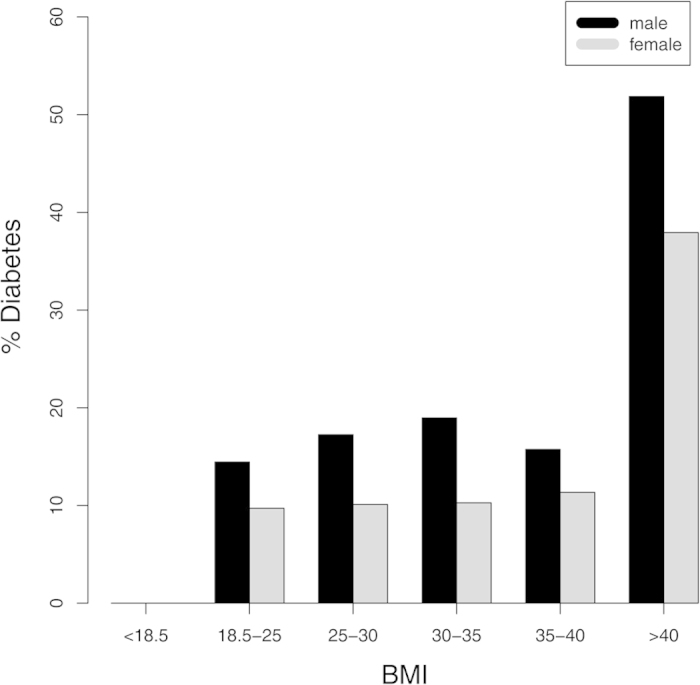
Diabetes was present in below 20% of males and below 10% in females of all BMI groups up to 40 kg/m2. In the highest BMI group (obesity grade III; >40 kg/m2) more than 50% male subjects and almost 40% female subjects had overt diabetes.
Transaminase levels in the HNR Study Cohort were within normal limits, but gradually increase with BMI
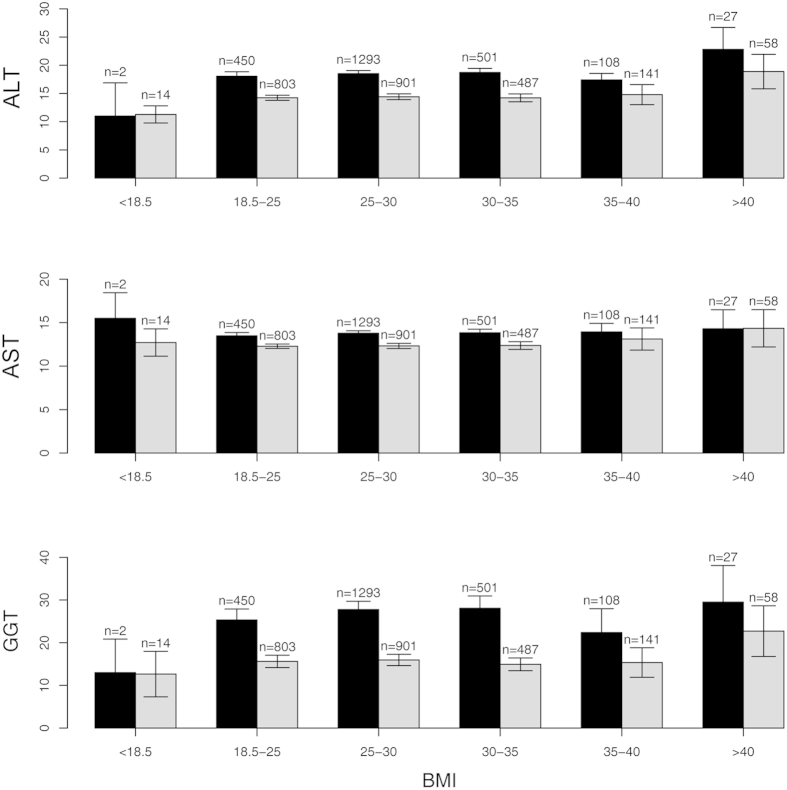
Mean ALT (16 ± 8.8 U/l) and mean AST (13 ± 4.6 U/l) remained both well below the common threshold for normal values (<50 U/l for males; <35 U/l for females). ALT was significantly higher in male subjects than in females (19 ± 9.7 U/l vs. 14 ± 7.1 U/l; p < 0.0001), which was also observable for AST (14 ± 4.8 U/l vs. 12 ± 4.2 U/l; p < 0.0001; Fig. 2).
Figure 2. Distribution of classic serum liver parameters in the Heinz Nixdorf Recall study by BMI groups.
Alanine aminotransferase (ALT/GPT) and gamma Glutamyltransferase (GGT) exhibited a trend of increased concentrations with higher BMI. Aspartate aminotransferase (AST/GOT) showed no differences between the BMI groups.
Subjects were grouped by BMI according to common ranges for underweight, normal weight, overweight, and obesity grades I-III. Serum concentrations of ALT and GGT tended to be higher in higher BMI ranges, though this trend did not reach significance (Fig. 2). Moreover, even in the highest BMI group (>40 kg/m2) the mean concentrations were still within normal ranges. A comparison of demographic and metabolic data of individuals with ALT in normal ranges and those with elevated ALT is given in Supplementary Table 1. AST levels were similar in all BMI groups.
Adiponectin and vitamin D levels are inversely correlated with BMI
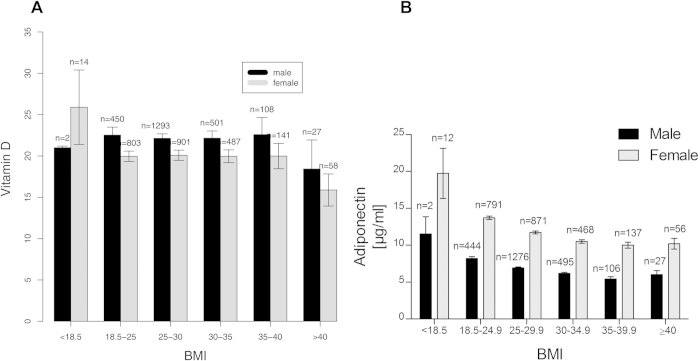
For all BMI groups VD was around the threshold for a deficit. Only in the highest BMI category (above 40 kg/m2) the mean VD values indicated a true deficit (<20 ng/ml; Fig. 3A). Slightly higher VD levels were found in males compared to females. Serum adiponectin, an adipocytokine with known hepatoprotective and insulin sensitizing properties22,23,24, was low (<15 μg/ml) in all BMI groups. The lowest concentrations were again observed in the highest BMI group (Fig. 3B). Female subjects exhibited higher adiponectin levels than males. While no significant differences between BMI groups were observed for adiponectin and VD, both parameters correlated inversely with BMI (Table 2).
Figure 3. Distribution of metabolic serum markers in the Heinz Nixdorf Recall study by BMI groups.
(A) Vitamin D serum levels were close to the threshold to insufficiency (20 ng/ml) in all BMI groups <40 kg/m2. In the highest BMI group (>40 kg/m2) the lowest vitamin D values were observed, with a mean concentration indicating insufficiency. (B) Adiponectin was distributed in a similar way as vitamin D with lowest serum concentrations found in the highest BMI group.
Table 2. Correlations of classic liver serum markers and metabolic parameters in the Heinz Nixdorf Recall study population.
| Parameters | r | p | |
|---|---|---|---|
| BMI | Adiponectin | −0.2195 | <0.0001 |
| BMI | Vitamin D | −0.1243 | <0.0001 |
| BMI | ALT/GPT | 0.2399 | <0.0001 |
| BMI | AST/GOT | 0.127 | <0.0001 |
| BMI | GGT | 0.1221 | <0.0001 |
| HbA1c | ALT/GPT | 0.1237 | <0.0001 |
| HbA1c | AST/GOT | 0.0448 | <0.01 |
| HbA1c | GGT | 0.0907 | <0.0001 |
| HbA1c | Adiponectin | −0.1456 | <0.0001 |
| HbA1c | Vitamin D | −0.0678 | <0.0001 |
| GGT | CRP | 0.0798 | <0.0001 |
| Diabetes | ALT/GPT | 0.1394 (rpb) | <0.0001 |
| Diabetes | AST/GOT | 0.0973 | <0.0001 |
| Diabetes | GGT | 0.1362 | <0.0001 |
Transaminase levels correlate with BMI and HbA1c, while adiponectin was inversely correlated with transaminase levels
ALT, AST, and GGT correlated positively with BMI (Table 2). To link transaminase levels with a surrogate parameter of IR, HbA1c was quantified in this cohort. ALT, AST, and GGT significantly correlated with HbA1c. In contrast, adiponectin was inversely correlated with AST, ALT, and GGT. Interestingly, GGT was significantly associated with CRP, a marker of systemic inflammation and associated with obesity, linking hepatocellular injury to systemic inflammation.
HbA1c, adiponectin, and BMI are efficient predictors of diabetes in the HNR study cohort
Utilizing machine learning techniques a computational model was built to identify the most important parameters for prediction of diabetes from the available serum parameters. The model identified HbA1c, adiponectin, and BMI as highly important for the prediction of diabetes (Table 3). These were followed by GGT, vitamin D, and transaminases. Logistic regression models were built, using HbA1c, adiponectin, and/or BMI to predict diabetes from the presented cohort. The model using all three parameters (HbA1c, adiponectin and BMI) as well as the reduced models reached AUC values of 0.85. A model using only HbA1c reached an AUC of 0.83. The ROC curves of the models HbA1c and BMI, HbA1c and adiponectin and HbA1c only are shown in Fig. 4. Comparing the AUC values by the method of De Long et al.25, it turned out that both, the model of HbA1c + BMI and HbA1c + adiponectin have significant higher AUC values compared to the model that uses only HbA1c (p < 0.001). The differences between the AUC values of the models HbA1c + BMI and HbA1c + adiponectin were not significant (p = 0.7044). By comparing the models at FPRs of 0.1, 0.05, and 0.001 using McNemar’s tests, both, the model using HbA1c + adiponectin as well as the model using HbA1c + BMI showed significant differences at FPR = 0.05 (p < 0.001 and p = 0.002, respectively) compared to the model using only HbA1c. However, only the model using HbA1c and adiponectin showed significant differences for very low FPRs (FPR = 0.01, p = 0.005). A model using HbA1c, adiponectin and BMI did not significantly improve the overall performance compared to the models that use only two parameters. Adding the parameters GGT and vitamin D did not significantly improve the models further (not shown).
Table 3. Importance of parameters for diabetes prediction from the Heinz Nixdorf Recall cohort.
| Parameter | Mean decrease of Gini impurity* | SD4 |
|---|---|---|
| HbA1c | 189.69 | 5.01 |
| Adiponectin | 67.72 | 3.43 |
| BMI1 | 63.49 | 3.11 |
| GGT2 | 55.47 | 2.70 |
| Vitamin D | 50.98 | 1.63 |
| CRP3 | 46.43 | 1.65 |
| ALT | 41.43 | 1.42 |
| AST | 34.07 | 1.40 |
*The Gini impurity gives an estimate, which parameters are most important for the random forest to predict the condition of interest (in this case: diabetes). A higher decrease of the Gini impurity represents a higher importance for this parameter. 1: Body mass index; 2: gamma-Glutamyltranerase; 3:C-reactive protein; 4: standard deviation.
Figure 4. ROC curves of the logistic regression models generated from non-invasive markers.
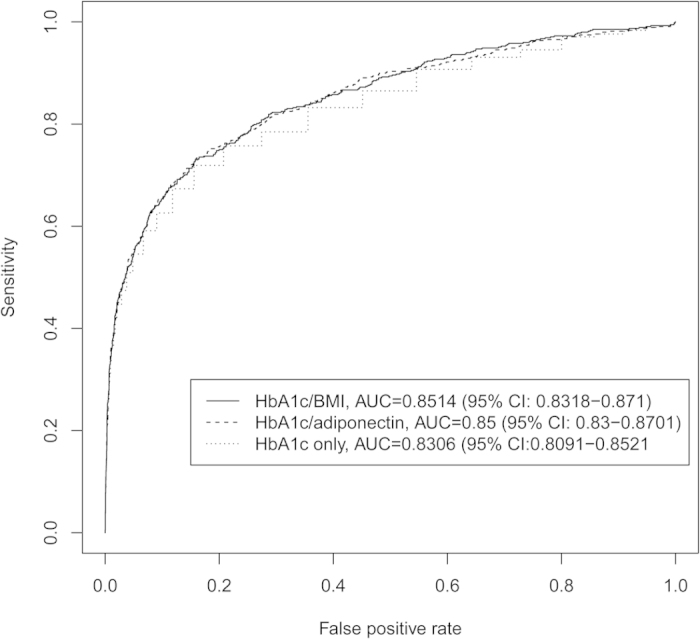
The models generated from serum markers and BMI (see table 3 for list) were able to predict diabetes with a high accuracy.
Discussion
Metabolic syndrome is alongside the obesity epidemic of industrialized countries increasingly common. It is hardly surprising that in parallel NAFLD has become the most predominant chronic liver disease in Europe26 with up to 75% of NAFLD patients suffering from diabetes27. Mild elevations of aminotransferases are a common finding in NAFLD28, though in over 25% of patients with advanced NAFLD and NASH, transaminase levels remain within normal levels7. In the presented cohort a very low proportion of transaminase levels above normal limits were detected (<2% for ALT). Though, with increasing BMI a parallel increase in ALT and GGT levels was observed. Mean BMI of the cohort suggests a substantial proportion of the population is overweight or obese. Moreover, overt type 2 diabetes was found in roughly 14%, while current German cross-sectional studies indicate a prevalence of approximately 7.2% in the general population29,30, though in these cases only known diabetes was taken into account. This data suggests that a relevant proportion of the analyzed collective may have undiagnosed metabolic syndrome. These individuals are at a greater risk to develop NASH and consecutively cirrhosis or HCC31. The main indicator to enroll further diagnostics for liver injury in clinician’s daily routine are liver transaminases. Thus, current normal values might miss a significant amount of individuals already developing chronic metabolic liver disease and presenting with transaminase values in the upper normal levels32,33.
Despite mostly marginal elevations in patients with overweight and obesity, serum liver enzymes have previously been associated to metabolic syndrome or its components. In the Cyprus Metabolism study, Liu et al. demonstrated a clear association between ALT and GGT with metabolic risk factors, including IR34. Similar associations between transaminase levels and diabetes have been shown, however, data is still scarce35. ALT, AST, and GGT in the presented cohort were each correlated with HbA1c levels and BMI. Additionally, GGT was associated with CRP, suggesting a connection to systemic inflammation beyond metabolic associations. This may support previous suggestion of GGT as biomarker for atherosclerosis36. Although classic serum markers for liver damage were not elevated above normal ranges, higher values may still indicate metabolic alterations or even injury by IR and diabetes.
Apart from the classic serum parameters for liver injury, markers related to the metabolic syndrome might be more valuable to assess damage to the liver in this particular setting. Among possible candidates is adiponectin, an adipose tissue derived adipokine with insulin sensitizing properties, found reduced in obesity. Several study groups elucidated the importance of adiponectin for diabetes37,38. Adiponectin was shown to increase the insulin-induced tyrosine phosphorylation of the insulin receptor in skeletal muscle as well as to increase whole-body sensitivity to insulin39. Hui et al. found that hypoadiponectinemia predicts carotid intima media thickness progression, independent of known predictive factors such as age, smoking, hyperlipidemia, and hypertension40. Carotid intima thickness is associated with diabetes and NAFLD41,42. In the present study serum adiponectin concentrations were inversely correlated to BMI and classic parameters of liver damage. Moreover, adiponectin was one of three most important factors for random forests to predict diabetes from serum derived markers. Interestingly performance of logistic regression models to predict diabetes with simple parameters was almost similar for HbA1c + adiponectin and HbA1c + BMI. Addition of both parameters to the model (HbA1c, adiponectin, and BMI) did not further improve the performance. This result suggests, that adiponectin may represent an objective marker for adipocyte function, with similar relevance as BMI. Moreover, the differences between the models HbA1c and HbA1c + adiponectin were also significant at very low FPRs, while this was not true for HbA1c + BMI. Thus the HbA1c + adiponectin model should be preferred in settings where a very high specificity is needed. Generally BMI can be determined easier, faster and cheaper than adiponectin. Though, there are cases (i.e. in highly trained athletes, very small or very large individuals), where BMI is not a reliable estimate for body composition (or adipocyte function)43,44, although it is a valid estimate in most situations. This might explain the slightly better performance of adiponectin, which needs confirmation in larger studies. Collection of adiponectin data in large cohorts may also enhance our understanding of this marker for adipocyte function and mechanisms leading from obesity to insulin resistance and metabolic syndrome.
Another factor associated with metabolic syndrome is vitamin D, which was found reduced in obesity and has been linked to type 1 and 2 diabetes45,46,47. Histological and clinical stages of NAFLD have also been associated with VD levels in several studies48. However, these observations could not be confirmed as causal relationship in some studies49,50. Among other factors this might be due to the highly complex interaction of the VD and the TGF-β pathway51. In particular polymorphisms in the VD receptor gene might impact the influence of VD on liver disease progression52. Additionally VD supplementation has been shown to affect adipocytokine levels53. This effect might contribute indirectly to the association of low VD with adverse metabolic profiles. In the HNR cohort we were able to show an association between VD levels and serum ALT and AST. VD was also among possible relevant predictors of diabetes. Taken together serum markers related to metabolic syndrome, as adiponectin or VD, can predict prevalence of diabetes in a population based cohort. Moreover, these parameters might also represent candidates for non-invasive markers of NAFLD or NASH. Due to the correlation to classic liver damage markers, even within normal ranges, and the connection to the metabolic syndrome further studies are warranted to elucidate the potential of adipokines and VD to assess metabolic liver injury.
In summary a strong association between transaminase levels, BMI, adiponectin, VD, and diabetes was found in the HNR study. This association was present despite transaminase concentrations within normal range. Adiponectin and HbA1c can predict diabetes with high accuracy in this population based cohort. Re-assessment of current normal range limits should be considered for classic liver transaminases to provide an improved focus concerning chronic metabolic liver injury. Adipokines or other markers related to the metabolic syndrome should be evaluated as possible NAFLD-specific liver injury markers, especially in individuals at risk for metabolic syndrome.
Patients and Methods
Study population
The Heinz Nixdorf Recall (HNR) Study is an ongoing population-based prospective cardiovascular cohort study of the Ruhr area in Germany. Random samples of the general population were drawn from residents’ registration offices including both genders aged 45–75 years. People were invited by mail (one invitational letter plus a maximum of two reminder letters) and phone calls to participate. Most people decided to participate after one invitational letter (52.6%)54. Blood tests were performed in fasting state, risk factors for coronary artery disease were analyzed by standardized questionnaires. A detailed description of the study design and population has been published previously55. Informed consent was obtained from the included participants. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institution’s human research committee (Ethikkommission am Universitätsklinikum Essen).
Diabetes mellitus was defined as a prior physician diagnosis of diabetes or taking an anti-diabetic drug. Unknown diabetes was considered when (1) fasting glucose was ≥7.0 mmol/L (60% of study participants with fasting status) or random blood glucose ≥11.1 mmol/L (remaining subjects with less than 8 hours non-fasting status) and (2) subjects had not reported a diagnosed diabetes or antidiabetic medication. For the purpose of the present study known diabetes and unknown diabetes were grouped together as “diabetes”.
Statistical data analysis and mathematic models
For correlation analyses Pearson product-moment correlation r, point-biserial correlation coefficient rpb, and the phi coefficient ϕ were employed, depending on the type of parameters used (quantitative or dichotomous).
Random forests (RFs) we used to identify the most important parameters for the prediction of diabetes. We used the RF implementation in the R package randomForest (http://www.r-project.org/). Earlier studies have shown that RFs are excellent non-linear classifiers, which are highly stable and robust in comparison to other classifiers19,20. Additionally, RFs provide an importance analysis, which can be used to identify the most important positions for the classification process. The theoretical complexity of the random forest is Θ(MKÑlog2Ñ), which is based on the complexity of building single trees (Θ(KNlog2N)), with K: the number of variables at each node, N: the number of samples. Due to the fact that the random forests use bootstrap replicates, N is reduced to Ñ = 0.632N. However, a random forest uses M randomized trees (bagging). In the current study, we used the random forests only for variable importance measurement, thus they have only been calculated once and are not used for prediction. For the importance analysis in our dataset the random forest needed 4 seconds on an Intel-Core i7-4700MQ CPU @ 2.40 Ghz. To reduce the bias due to the class imbalance in the dataset, we repeated sub-sampling for 100 times56. The most important parameters were then used to build logistic regression models to identify patients with diabetes (as defined above) within the analyzed cohort. For evaluation of the models, we calculated the Receiver Operating Characteristics (ROC) and the corresponding Area Under the Curve (AUC) from a leave-one-out cross-validation. For comparisons between the different models we used the method of De Long et al. on the AUC values as well as McNemar’s tests at certain false-positive rates (FPR), namely 0.01, 0.05, and 0.1.
Additional Information
How to cite this article: Kälsch, J. et al. Normal liver enzymes are correlated with severity of metabolic syndrome in a large population based cohort. Sci. Rep. 5, 13058; doi: 10.1038/srep13058 (2015).
Supplementary Material
Footnotes
Author Contributions J.K., L.P.B., J.P.S., S.M., K.H.J., R.E. and A.C. designed the study. J.K., J.B., P.M. and H.K. collected data. L.P.B., D.H., J.P.S., S.M., U.S. and K.H.J. performed data analyses. D.H., S.M., U.S., K.H.J., R.E. and G.G. provided material or technical support. J.K., D.H., J.B., P.M. and H.K. drafted the manuscript. L.P.B., J.P.S., K.H.J., R.E. and A.C. revised the manuscript for important intellectual content. S.M., U.S., K.H.J., R.E., G.G. and A.C. supervised the study. All authors approved the final version of the manuscript.
References
- Clark J. M. & Diehl A. M. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA 289, 3000–3004 (2003). [DOI] [PubMed] [Google Scholar]
- Ruhl C. E. & Everhart J. E. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 124, 71–79 (2003). [DOI] [PubMed] [Google Scholar]
- Younossi Z. M. et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin. Gastroenterol. Hepatol. 9, 524–530.e1 (2011). [DOI] [PubMed] [Google Scholar]
- Dietrich P. & Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol 28, 637–653 (2014). [DOI] [PubMed] [Google Scholar]
- Vanni E. et al. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis 42, 320–330 (2010). [DOI] [PubMed] [Google Scholar]
- Kim C. H. & Younossi Z. M. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med 75, 721–728 (2008). [DOI] [PubMed] [Google Scholar]
- Verma S., Jensen D., Hart J. & Mohanty S. R. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 33, 1398–1405 (2013). [DOI] [PubMed] [Google Scholar]
- Torres D. M. & Harrison S. A. NAFLD: Predictive value of ALT levels for NASH and advanced fibrosis. Nat Rev Gastroenterol Hepatol 10, 510–511 (2013). [DOI] [PubMed] [Google Scholar]
- Al-hamoudi W. et al. Revising the upper limit of normal for levels of serum alanine aminotransferase in a Middle Eastern population with normal liver histology. Dig. Dis. Sci. 58, 2369–2375 (2013). [DOI] [PubMed] [Google Scholar]
- Park H. N. et al. Upper normal threshold of serum alanine aminotransferase in identifying individuals at risk for chronic liver disease. Liver Int. 32, 937–944 (2012). [DOI] [PubMed] [Google Scholar]
- Schwimmer J. B. et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 138, 1357–1364 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann L. P. et al. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 56, 952–964 (2012). [DOI] [PubMed] [Google Scholar]
- Bechmann L. P. et al. Glucokinase links Krüppel-like factor 6 to the regulation of hepatic insulin sensitivity in nonalcoholic fatty liver disease. Hepatology 55, 1083–1093 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wree A. et al. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metab. Clin. Exp. 63, 1542–1552 (2014). [DOI] [PubMed] [Google Scholar]
- Cheung O. & Sanyal A. J. Abnormalities of lipid metabolism in nonalcoholic fatty liver disease. Semin. Liver Dis. 28, 351–359 (2008). [DOI] [PubMed] [Google Scholar]
- Albano E., Mottaran E., Occhino G., Reale E. & Vidali M. Review article: role of oxidative stress in the progression of non-alcoholic steatosis. Aliment. Pharmacol. Ther. 22 Suppl 2, 71–73 (2005). [DOI] [PubMed] [Google Scholar]
- Day C. P. From fat to inflammation. Gastroenterology 130, 207–210 (2006). [DOI] [PubMed] [Google Scholar]
- Mitri J., Muraru M. D. & Pittas A. G. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr 65, 1005–1015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa J.-P. et al. Non-invasive separation of alcoholic and non-alcoholic liver disease with predictive modeling. PLoS ONE 9, e101444 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa J.-P. et al. Novel Algorithm for Non-Invasive Assessment of Fibrosis in NAFLD. PLoS ONE 8, e62439 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertle J. M. et al. A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion 87, 121–131 (2013). [DOI] [PubMed] [Google Scholar]
- Bechmann L. P. et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology 57, 1394–1406 (2013). [DOI] [PubMed] [Google Scholar]
- Berg A. H., Combs T. P., Du X., Brownlee M. & Scherer P. E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7, 947–953 (2001). [DOI] [PubMed] [Google Scholar]
- Cantley J. The control of insulin secretion by adipokines: current evidence for adipocyte-beta cell endocrine signalling in metabolic homeostasis. Mamm. Genome 25, 442–454 (2014). [DOI] [PubMed] [Google Scholar]
- DeLong E. R., DeLong D. M. & Clarke-Pearson D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988). [PubMed] [Google Scholar]
- Blachier M., Leleu H., Peck-Radosavljevic M., Valla D.-C. & Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J. Hepatol. 58, 593–608 (2013). [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med 346, 1221–1231 (2002). [DOI] [PubMed] [Google Scholar]
- Torres D. M. & Harrison S. A. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology 134, 1682–1698 (2008). [DOI] [PubMed] [Google Scholar]
- Müller G. et al. Regional and neighborhood disparities in the odds of type 2 diabetes: results from 5 population-based studies in Germany (DIAB-CORE consortium). Am. J. Epidemiol. 178, 221–230 (2013). [DOI] [PubMed] [Google Scholar]
- Rathmann W., Scheidt-Nave C., Roden M. & Herder C. Type 2 diabetes: prevalence and relevance of genetic and acquired factors for its prediction. Dtsch Arztebl Int 110, 331–337 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto E. & Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol. Res. 42, 1–14 (2012). [DOI] [PubMed] [Google Scholar]
- Siddiqui M. S. et al. Association between high-normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology 145, 1271–1279.e1–3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. et al. Alanine transferase: An independent indicator of adiposity related comorbidity risk in youth. J Diabetes (2014). 10.1111/1753-0407.12221. [DOI] [PubMed] [Google Scholar]
- Liu X. et al. Circulating alanine transaminase (ALT) and γ-glutamyl transferase (GGT), but not fetuin-A, are associated with metabolic risk factors, at baseline and at two-year follow-up: the prospective Cyprus Metabolism Study. Metab. Clin. Exp. 63, 773–782 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunutsor S. K., Apekey T. A. & Walley J. Liver aminotransferases and risk of incident type 2 diabetes: a systematic review and meta-analysis. Am. J. Epidemiol. 178, 159–171 (2013). [DOI] [PubMed] [Google Scholar]
- Bradley R. D. et al. Associations between γ-glutamyltransferase (GGT) and biomarkers of atherosclerosis: the Multi-ethnic Study of Atherosclerosis (MESA). Atherosclerosis 233, 387–393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001). [DOI] [PubMed] [Google Scholar]
- Pham M. N. et al. Serum adipokines as biomarkers of beta-cell function in patients with type 1 diabetes: positive association with leptin and resistin and negative association with adiponectin. Diabetes Metab. Res. Rev. 29, 166–170 (2013). [DOI] [PubMed] [Google Scholar]
- Stefan N. et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes 51, 1884–1888 (2002). [DOI] [PubMed] [Google Scholar]
- Hui E. et al. Hypoadiponectinemia as an independent predictor for the progression of carotid atherosclerosis: a 5-year prospective study. Metab Syndr Relat Disord 12, 517–522 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakurt F. et al. Relationship between cerebral arterial pulsatility and carotid intima media thickness in diabetic and non-diabetic patients with non-alcoholic fatty liver disease. J. Endocrinol. Invest. 32, 63–68 (2009). [DOI] [PubMed] [Google Scholar]
- Sookoian S. & Pirola C. J. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J. Hepatol. 49, 600–607 (2008). [DOI] [PubMed] [Google Scholar]
- Roberts C. K. et al. Strength Fitness and Body Weight Status on Markers of Cardiometabolic Health. Med Sci Sports Exerc 47, 1211–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Marken Lichtenbelt W. D., Hartgens F., Vollaard N. B. J., Ebbing S. & Kuipers H. Body composition changes in bodybuilders: a method comparison. Med Sci Sports Exerc 36, 490–497 (2004). [DOI] [PubMed] [Google Scholar]
- Frederiksen B. N. et al. Association between vitamin D metabolism gene polymorphisms and risk of islet autoimmunity and progression to type 1 diabetes: the diabetes autoimmunity study in the young (DAISY). J. Clin. Endocrinol. Metab. 98, E1845–1851 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonchaya-anant P., Holick M. F. & Apovian C. M. Serum 25-hydroxyvitamin D levels and metabolic health status in extremely obese individuals. Obesity (Silver Spring) 22, 2539–2543 (2014). [DOI] [PubMed] [Google Scholar]
- Forouhi N. G. et al. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia 55, 2173–2182 (2012). [DOI] [PubMed] [Google Scholar]
- Dasarathy J. et al. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int. 34, e118–127 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril F. et al. Relationship of vitamin D with insulin resistance and disease severity in non-alcoholic steatohepatitis. J. Hepatol. 62, 405–411 (2015). [DOI] [PubMed] [Google Scholar]
- Ye Z. et al. Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol 3, 35–42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N. et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 153, 601–613 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilfuss A. et al. Vitamin D counteracts fibrogenic TGF-β signalling in human hepatic stellate cells both receptor-dependently and independently. Gut 64, 791–9 (2015). [DOI] [PubMed] [Google Scholar]
- Tabesh M., Azadbakht L., Faghihimani E., Tabesh M. & Esmaillzadeh A. Calcium-vitamin D cosupplementation influences circulating inflammatory biomarkers and adipocytokines in vitamin D-insufficient diabetics: a randomized controlled clinical trial. J. Clin. Endocrinol. Metab. 99, E2485–2493 (2014). [DOI] [PubMed] [Google Scholar]
- Stang A. et al. Baseline recruitment and analyses of nonresponse of the Heinz Nixdorf Recall Study: identifiability of phone numbers as the major determinant of response. Eur. J. Epidemiol. 20, 489–496 (2005). [DOI] [PubMed] [Google Scholar]
- Schmermund A. et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am. Heart J. 144, 212–218 (2002). [DOI] [PubMed] [Google Scholar]
- Fithian W. & Hastie T. Local case-control sampling: efficient subsampling in imbalanced data sets. Ann Stat 42, 1693–1724 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.