Abstract
Background:
Dengue is the most rapidly spreading mosquito-borne viral disease in the world with a 30-fold increase in incidence in the last 50 years. Approximately, 50 million dengue infections occur annually.
Aim:
To study the various clinical and laboratory manifestations of dengue in the elderly and observe for any variations in IgM titer elevation with progression of age.
Design:
Retrospective observational study.
Subjects and Methods:
Medical charts of all patients admitted to the Division of Geriatrics of the institute during study period were reviewed for collection of demographic, clinical, and laboratory information. The diagnosis of dengue was made based on positive dengue IgM ELISA. An elderly patient referred to one whose age was ≥60 years.
Results:
Fever and myalgia were noted to be the most common clinical manifestation with only four patients presenting with overt bleeding manifestations. Only one patient presented in delirium and there was no case fatality. Thrombocytopenia was the single most common hematological abnormality noted. Hyponatremia was found to be prevalent in a majority of the patients and were symptomatic in more than half of them. There have been very few studies done worldwide on the varied clinical manifestations of dengue in the elderly.
Keywords: Dengue, elderly, geriatric, India, Kerala
Introduction
Dengue is the most rapidly spreading mosquito-borne viral disease in the world with a 30-fold increase in incidence over the past 50 years. An estimated 50 million infections occur annually, and approximately 2.5 billion people live in dengue endemic countries.[1] Statistics released by the National Vector Borne Disease Control Programme of India,[2] total number of cases reported have shot up significantly from 3306 in 2001 to 75,454 in 2013 with mortality also increasing significantly from 53 in 2001 to 242 in 2012. The first major epidemic of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) was reported in 1996, with four serotypes in circulation, and another one in 2012, where thrice the number of previous year were reported,[3] which makes it imperative to study various characteristics and atypical presentation of this disease.
Dengue virus (DEN) is a small single-stranded RNA virus comprising of four distinct serotypes, (DEN 1–4). Various serotypes are transmitted to humans through the bites of infected Aedes mosquitoes, principally Aedes aegypti. Studies were done in Kerala by Anoop et al.,[4] reveal hyperendemicity of dengue in the region with the presence of multiple serotypes and high rates of co-infection. Individual risk factors determine the severity of the disease and include secondary infection, age, ethnicity, and possibly chronic diseases.
After the onset of illness, the virus can be detected in serum, plasma, circulating blood cells and other tissues for 4–5 days. As per the diagnostic tests criteria established by the dengue and control study, IgM positivity in a single serum sample and IgG in a single serum sample with a high titer of 1280 or greater is highly suggestive of dengue fever.
This study focuses on its varied clinical and laboratory manifestations, in the elderly – and how severe and gargantuan can its manifestations be when the elderly is afflicted. A study of this magnitude about dengue's manifestations in the aged has not been studied much in India – considering their inherently fragile immunity and multiple co-existing co-morbid illnesses.
Subjects and Methods
We conducted a retrospective observational study of elderly patients admitted under the Division of Geriatric Medicine with dengue fever admitted between June 1, 2010 and May 31, 2012 at a 1500 bed tertiary care hospital, catering to the central and Southern parts of Kerala state, India. The medical charts of the included patients were reviewed for the collection of demographic, clinical, and laboratory information. The diagnosis of dengue was made based on positive enzyme-linked immunosorbent assay result for specific IgM antibody for dengue in acute phase serum.
An elderly patient is referred to one whose age was >60 years. Concurrent bacteremia was defined as a positive bacterial growth from the blood that was sampled for culture within 72 h after the patient was hospitalized for dengue fever.
Patients were managed as per a standard hospital protocol, which included supportive measures (intravenous fluids and symptomatic management), after addressing them in the context of background comorbidities. Antibiotics were initiated, based on the latest institution antibiogram, if secondary infections were suspected or if culture and sensitivity sent on the basis of clinical suspicion was reported as positive for microbes.
Acute renal failure (ARF) in adults with dengue fever was defined as a rapid increase in serum creatinine up to >1.4 mg/dL in a patient with original normal kidney function or doubling of the baseline serum creatinine value within 3 days if he or she has an underlying chronic renal disease.
Fatality referred to all-cause death within 1-week in patients with dengue fever.
The following study protocol was cleared by the Institutional Ethics Committee.
Results
Fifty elderly patients (22 women and 31 men, mean age 66.1 ± 4.7 standard deviation [SD] years) were included in the study, of which 87% belonged to the age group of 60–70 years, 11% were in the age group 70–80 and 2% of the patients were 80+ years old.
Fever (ear temperature >38°C) was found in 98.1% patients. The two leading symptoms other than fever and myalgia (43.4%) among the 53 elderly were headache (18.9%) and arthralgia (13.2%). Only four patients (7.5%) of these patients presented with overt bleeding manifestations and only one patient presented in a state of delirium, and no fatality was reported in these patients.
The most common co-morbid illness affecting these patients were systemic hypertension (60.4%) and type 2 diabetes mellitus (39.6%).
At presentation, thrombocytopenia (platelet count <150 K/μL) (77.4%) (mean of 68.5 K/μL with SD of 49.6) was the major hematological abnormality followed by leukopenia (total white blood cell count <4 K/μL) (mean 5.2 K/μL with SD 2.3) (52.8%) and anemia (hemoglobin <12 g/dL) (mean 13.5 g/dL with SD of 1.6) (13.2%). Hepatic derangement, as signified by the elevation of serum glutamic oxaloacetic transaminase (more than 35 IU/L) (mean 254.03 IU/L with SD of 655.5) (92.5%) and serum glutamic pyruvic transaminase (>45 IU/L) (mean 196.6 IU/L with a SD of 423.5) (71.7%). Renal dysfunction was observed in (15.1%).
Among electrolyte abnormalities, incidence of hyponatremia (serum sodium <135 mEq/L) was higher (50.9%) (mean 129.8 with SD of 14.66) with seven patients (13.2%) of the patients developing significant hyponatremia (<125 mEq/L). IgM dengue titers showed no correlation, positive or negative, with an increase in age (P = 0.089).
The mean duration of IP admission was 5.6 ± SD of 2.8 days.
Co-morbid illnesses in the patient were studied in relation to their in-hospital admission duration, and it was found that co-morbid illness did not influence IP duration (mean IP 5.21).
Being a retrospective study, the severity of dengue fever could not be assessed in the background of co-morbid illnesses as standardization of the severity of comorbidity would not be possible.
Discussion
Due to the lack of studies of varied clinical manifestations of diseases in India and across the world, especially tropical diseases like dengue, there has been a paucity of adequate studies to compare our results with. To our knowledge, there have been very few studies done in the elderly regarding varied and myriad presentations and manifestations, especially ones comparing with a younger populations.[5,6]
Out of the 53 patients, which were part of the study, it was observed that 87% of the patients affected were in the age group of 60–70 years. The male:female ratio was 1.5:1, which was slightly below the male:female ratio observed by Agarwal et al.[7] who observed it to be at 1.9:1. Fever was a prominent clinical manifestation in all the patients, except for one. This is of significance, in the background that an elderly is often found to not mount a febrile reaction against an active infection or systemic illness and is often seen to be in delirium – hypoactive, mostly. As compared to similar studies from the same part of the world, fever and lethargy were the most common of clinical manifestations in the elderly afflicted with DHF [Figure 1].[8] Uncommon presentations of dengue fever need to be expected such as acalculous cholecystitis, hepatitis, edematous gall bladder wall, serositis, ARF or neurological manifestations.[9,10]
Figure 1.
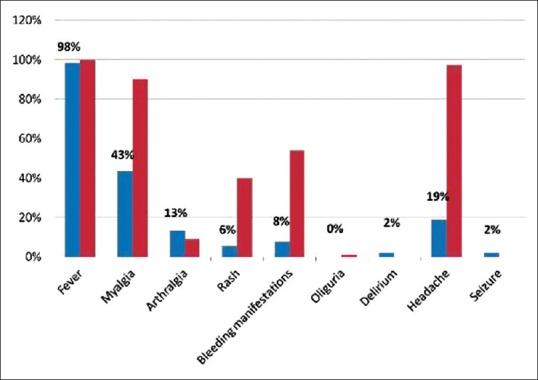
Comparison of clinical features with other studies done in India in dengue fever
Only one patient among this study population, presented to us in a state of delirium in the absence of fever. Myalgia and headache were the other clinical presentations that were found to be at par with symptoms observed in younger adult population.
Among the laboratory profile, thrombocytopenia was observed in 90% of the patients, and a platelet value <100,000 in 78% of the patients. This again was comparable to the prevalence of thrombocytopenia observed by Khan et al.[11] This raises the possibility of another catastrophic outcome – hemorrhagic stroke. Though gastrointestinal bleeds have been most documented, the risk of hemorrhagic stroke appears to be increasingly recognized across the world.[8]
Hepatic function derangement was noted in 92% of the patients and renal derangement in 15% of the patients, all of whom were asymptomatic and subsequently improved.
It was noted that 73% of the patients had hyponatremia, and 22 of the 32 hypertensives were also noted to be hyponatremic [Figure 2]. 58% of the hyponatremic patients were found to be symptomatic.
Figure 2.
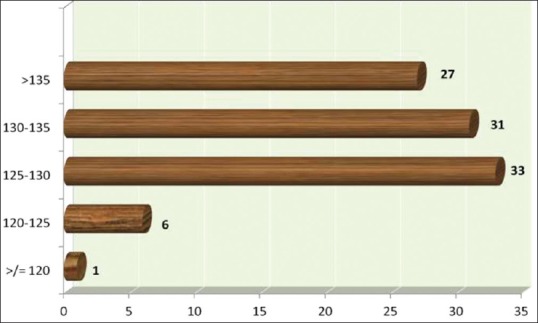
Classification of patients with dengue fever based on severity of hyponatremia
Since the majority of the hyponatremia patients were hypertensives, concurrent diuretic usage (e.g., thiazides) and dietary restriction of salt can also be attributed to be causing the same. Hyponatremia is not a well-documented complication in elderly patients afflicted by dengue fever except by Mekmullica et al.[12] who documented increase urine sodium in elderly dengue patients.
Renal dysfunction was documented in 15.1% of the patients, which was comparable to previous studies revealing ARF in elderly patients with DHF.[5] DSS is an independent risk factor for the development of ARF in patients with DHF and that the fatality rate is high once ARF developed. No case mortality was reported among our sample population as against the 3.8% mortality observed by Agarwal et al. in 1996, but in our case, the outcome was relatively different with no case mortality in the study period. This could be explained by the higher threshold for shock in elderly adults as compared to children, due to a lower tendency for increased capillary permeability. Another possibility could be that older patients, due to their previous exposure and immunity to multiple prior dengue infections.[13,14] At the same time, studies from Latin America[6,15] have revealed a higher incidence of more serious clinical conditions, higher hospitalization rates, and significant mortality, primarily due to previous infections by other serotypes, presence of chronic diseases, immunosenescence, and high drug consumption.
Being a retrospective study, certain limitations need to be given its due diligence. Study was conducted at a single tertiary care referral center and patients thus referred may be biased based on the pattern of referral. Due to the lack of a standard protocol for the management of DHF, patient management and detection of early complications may have also resulted in the varied outcomes. A prospective study with a larger population, involving multiple centers would provide a much more enlightening picture of the varied presentations of dengue in the elderly.
Conclusion
About 87% of the geriatric patients studied were in the 60–70 years age group with a male preponderance. Fever, myalgia, and headache were the most common presenting symptoms in the elderly. Thrombocytopenia and hepatic function derangement occurred in 90% of the patients with renal function compromise occurring in only 15% of the patients. Zero mortality was reported in this study when compared to more catastrophic eventualities in the younger population. Being a retrospective study, limitations included being a single center tertiary care hospital based study. A prospective study with a larger population would prove more useful in reaching far-fetched conclusions with regard to dengue in the elderly in India.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.WHO. Dengue and dengue hemorrhagic fever. Health in Asia and the Pacific. 2008:244–255. [Google Scholar]
- 2.Mariappan T. Current emerging situation of dengue in India. Trop Doct. 2013;43:116–9. doi: 10.1177/0049475513491944. [DOI] [PubMed] [Google Scholar]
- 3.Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res. 2012;136:373–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Anoop M, Issac A, Mathew T, Philip S, Kareem NA, Unnikrishnan R, et al. Genetic characterization of dengue virus serotypes causing concurrent infection in an outbreak in Ernakulam, Kerala, South India. Indian J Exp Biol. 2010;48:849–57. [PubMed] [Google Scholar]
- 5.Lee IK, Liu JW, Yang KD. Clinical and laboratory characteristics and risk factors for fatality in elderly patients with dengue hemorrhagic fever. Am J Trop Med Hyg. 2008;79:149–53. [PubMed] [Google Scholar]
- 6.Gorzoni ML, Massaia IF, Pires SL. Dengue in an elderly patient. Rev Inst Med Trop Sao Paulo. 2010;52:163–7. doi: 10.1590/s0036-46652010000300010. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Kapoor S, Nagar R, Misra A, Tandon R, Mathur A, et al. A clinical study of the patients with dengue hemorrhagic fever during the epidemic of 1996 at Lucknow, India. Southeast Asian J Trop Med Public Health. 1999;30:735–40. [PubMed] [Google Scholar]
- 8.Low JG, Ooi EE. Dengue in an ageing population. Ann Acad Med. 2013;42:373–5. [PubMed] [Google Scholar]
- 9.Ahluwalia G, Sharma SK. Dengue: Current trends and challenges – An Indian perspective. J Assoc Physicians India. 2004;52:561–3. [PubMed] [Google Scholar]
- 10.Bhaskar ME, Moorthy S, Kumar NS, Arthur P. Dengue haemorrhagic fever among adults – an observational study in Chennai, South India. Indian J Med Res. 2010;132:738–40. [PMC free article] [PubMed] [Google Scholar]
- 11.Khan R, Zaheer MS, Khan T, Quaiser S, Rabbani MU. Profile of dengue patients in a North Indian referral hospital. J Assoc Clin Med. 2011;12:179–81. [Google Scholar]
- 12.Mekmullica J, Suwanphatra A, Thienpaitoon H, Chansongsakul T, Cherdkiatkul T, Pancharoen C, et al. Serum and urine sodium levels in dengue patients. Southeast Asian J Trop Med Public Health. 2005;36:197–9. [PubMed] [Google Scholar]
- 13.Lye DC, Lee VJ, Sun Y, Leo YS. The benign nature of acute dengue infection in hospitalized older adults in Singapore. Int J Infect Dis. 2010;14:e410–3. doi: 10.1016/j.ijid.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhuri M. What can India do about dengue fever? BMJ. 2013;346:f643. doi: 10.1136/bmj.f643. [DOI] [PubMed] [Google Scholar]
- 15.García-Rivera EJ, Rigau-Pérez JG. Dengue severity in the elderly in Puerto Rico. Rev Panam Salud Publica. 2003;13:362–8. doi: 10.1590/s1020-49892003000500004. [DOI] [PubMed] [Google Scholar]


