Abstract
Background:
Dysmenorrhea is a common gynecological condition with painful menstrual cramps of uterine origin. Prevalence of primary dysmenorrhea is not yet clearly studied in central India.
Objective:
To study prevalence of primary dysmenorrhea in young girls and to evaluate associated clinical markers of dysmenorrhea.
Materials and Methods:
In a cross-sectional study, data was collected among 310 girls (18–25 years) on age at menarche, presence and absence of dysmenorrhea, dysmenorrhea duration, pre-menstrual symptoms (PMS), family history, menses irregularities, menstrual history, severity grading using visual analogue scale (VAS) using a semi-structured questionnaire.
Results:
Dysmenorrhea was reported in 84.2% (261) girls and 15.8% (49) reported no dysmenorrhea. Using VAS, 34.2% of girls experienced severe pain, 36.6% moderate and 29.2% had mild pain. Bleeding duration was found to be significantly associated with dysmenorrhea (χ2 = 10.5; P < 0.05), girls with bleeding duration more than 5 days had 1.9 times more chance of getting dysmenorrhea (OR: 1.9; 95% CI: 1.7–3). Moreover, girls with the presence of clots had 2.07 times higher chance of having dysmenorrhea (OR: 2.07; 95% CI: 1.04–4.1) (P < 0.05). Almost 53.7% girls who had some family history of dysmenorrhea, 90.9% experience the condition themselves (χ2 = 11.5; P < 0.001). Girls with family history of dysmenorrhea had three times greater chance of having the same problem (OR: 3.0; 95% CI: 1.5–5.8; P = 0.001).
Conclusion:
Dysmenorrhea is found to be highly prevalent among college going girls. Family history, bleeding duration and presence of clots were significant risk factors for dysmenorrhea.
Keywords: Dysmenorrhea, prevalence, young girls
Introduction
Dysmenorrhea is a common gynecological condition with painful menstrual cramps of uterine origin. Two categories of dysmenorrhea are primary and secondary dysmenorrhea. Primary dysmenorrhea refers to menstrual pain without any pelvic pathology. These symptoms have underlying cause of elevated endometrial prostaglandins and their metabolites.[1] Primary dysmenorrhea is an important clinical as well as social problem affecting more than 50% of menstruating women.
The prevalence of dysmenorrhea is difficult to determine because of different definitions of the condition, the estimates varying from 45% to 95%.[2] A recent systematic review of the world literature on chronic pelvic pain reports prevalence of dysmenorrhea ranging between 17% and 80%.[3] Prevalence studies also have shown several other factors that are associated with dysmenorrhea like body mass index (BMI), smoking, early menarche, prolonged menstrual flow and psychological disturbances.[4]
The true prevalence of primary dysmenorrhea is not yet clearly established in India. A dysmenorrhea prevalence of 33.5% among adolescent girls in India was reported by Nag.[5] George and Bhaduri found dysmenorrhea to be a common problem in India with prevalence of 87.7%.[6] Similar findings had been reported by Jayashree and Jayalakshmi in rural married women of Andhra Pradesh.[7]
Although dysmenorrhea is a common gynecological problem in young females but there are limited studies in this subject especially in central India. It is unclear the extent to which young girls are incapacitated each month due to the severity of dysmenorrhea. Hence, this raises a need to evaluate the menstrual characteristics and prevalence of dysmenorrhea in young college going females in Indore to provide evidences of the severity of the problem.
Thus, a cross-sectional study was conducted among college going females at Indore city of central India with following objective: To study prevalence of primary dysmenorrhea in young females and to evaluate associated clinical markers of dysmenorrhea in them.
Materials and Methods
The cross-sectional study was conducted by the department of obstetrics and gynecology, Index Medical College, Hospital and Research Centre Indore, Madhya Pradesh, India for a period of 3 months (June 2014 to August 2014). The study proposal was cleared from Scientific evaluation board of the Institute. The study was performed on a total of 310 girls who agreed to voluntarily participate in the study. All the girls belonged to the same socio-economic and dietary background and were of the same age group which constituted a homogenous group. Written informed consent was obtained from all the girls before commencement of the study.
Three hundred and ten young girls aged between 18 and 25 years participated in the study. A questionnaire regarding details of menstrual cycle was filled up by the participants in the presence of members of the study team. Prior to the distribution of the questionnaire, a brief orientation lecture was conducted in local language.
Study parameters
Information regarding current age, education, anthropometric data (height, weight) was recorded. BMI was then calculated by the formula: Weight (kg)/Height2 (meter).[2] Asian criteria for BMI have been taken in analysis: <18, 18–22.99 and >23 were taken as cut off for underweight, normal and overweight, respectively.[8] Data on age at menarche, presence and absence of dysmenorrhea, duration of dysmenorrhea, details of pain (onset, location, type, duration etc), pre-menstrual symptoms, family history, menses irregularities, menstrual history, and severity grading using visual analogue scale (VAS) was collected using the standardized questionnaire.
The VAS using a 10-cm line represented the continuum of the female student's opinion of the degree of pain. One extremity of the line represented “unbearable pain,” and the other extremity represented “no pain at all.” The participants were asked to rate the degree of pain by making a mark on the line. The scores received from the scale were classified into mild dysmenorrhea if it was between 1 and 3 points moderate between 4 and 7 points, and severe between 8 and 10 points.[9]
Following inclusion criteria was used to categorize females under primary dysmenorrhea:
Onset of pain within 6–12 hours after onset of menses
Lower abdominal and pelvic pain associated with onset of menses and lasting for 9–72 hours
Lower back pain
Medial or anterior thigh pain.
Participants both with primary menstrual pain without significant pathology and without menstrual pain were recruited.
Participant with any endocrine disorders, chronic disease or who had undergone major surgery were excluded from the study.
Statistical analyses
An analysis was performed using SPSS software for Windows (version 11.0, 2001, SPSS Inc., Chicago, IL). All the variables were tested for normality by the Kolmogorov–Smirnov test prior to statistical comparisons. Chi-square test and logistic regression were used for the analyses. To avoid the effect of multi co-linearity, separate models were be fitted for different parameters. Adjusted odds ratio (OR) s were calculated in the regression of the outcome events. A P value less than 0.05 was considered to be significant.
Results
Baseline characteristics
The average age of the participants was 20.4 ± 1.8 years, ranging from 17 to 25 years. Around 57% (175) were in the age range of 17 to 20 years and 43% (132) were between age ranges of 21 and 25 years. Majority fall below 23 years, only 17.1% were above the age of 23 years. Mean BMI of the participants was 20.5 ± 3.6 kg/m2. More than half of the participants (57.7%) had a normal BMI (18-23 kg/m2), whereas underweight and overweight categories had almost equal distribution with 20.5% and 21.8%, respectively. Dysmenorrhea was reported by 84.2% (261) of the total girls, whereas only 15.8% (49) reported no dysmenorrhea. Mean age, BMI and age at menarche was found to be similar in both the groups (dysmenorrhea and no dysmenorrhea) (P > 0.1) [Table 1].
Table 1.
Baseline characteristics of the study population
Menstrual characteristics
Age of menarche
The average age of menarche was reported as 13.8 ± 1.6 years (ranging from 9 to 19), majority of the participants (97.6%) fall between 10 and 17 years, remaining 1.6% had started menstruating after the age of 18 and only 0.7% were had started menstruating below 10 years. Majority of girls (162) fall under the reference category of 12–14 yrs for age of menarche; therefore, the difference between age at menarche and dysmenorrhea was not significant (P > 0.1).
Details of pain namely onset, duration, type and location has been presented as follows:
Onset of pain
Majority of girls (61.5%) have reported onset of pain on day 1, 23.5% reported prior to menses cycle remaining, 14.2% stated on either day 2 or 3 and only 0.8% of the girls have said even after cessation of menses. Thus, maximum numbers of girls were suffering on day 1 of menstruation in the present study.
Duration of pain
Regarding the duration of pain, almost one third of the girls (37%) have informed pain duration for one day only, 39.8% reported 2 days, followed with 15.4% who stated pain for 3 days moreover, 5.4% girls have reported pain duration for 4 days and 2.3% reported even after cessation of menses.
Location of pain
Though dysmenorrhea is largely associated with abdominal pain, other locations of pain were also asked in the present study. One third of the girls (34%) reported location of pain as diffused lower abdomen followed with that was supra-pubic (22.8%), lower back (16%) and thighs (3.4%). Besides, 24% of the girls have reported option “all,” i.e., they suffered from pain at all these locations.
Type of pain
As one would expect, majority of girls have stated pain type as spasmodic (68%) but few of them also have reported shooting (15.4%), piercing (11.9%) and stabbing (4.7%).
Visual analog scale
Measuring pain is very difficult however, using VAS gradient of pain was asked to all the study girls who reported dysmenorrhea.
The prevalence of dysmenorrhea was very high with 84.2%, with around 34.2% of these girls who had experienced “severe pain,” i.e., visual analog score between 7 and 10. Around 36.6% reported the pain score as moderate, i.e. between 4 and 7 and remaining 29.2% had mild pain (2–4).
Length of menstrual cycle
Among the 310 participants, the average duration of the menstrual cycle was 29.8 ± 3.3 days. A large chunk of students (86.7%) had menstrual cycle duration of 28 to 35 days, which is considered as normal. Since a very small number (1.5%) had cycle length >35 days and remaining 11.8% had cycle length between 22 and 27 days, no significance could be established with dysmenorrhea.
Bleeding duration
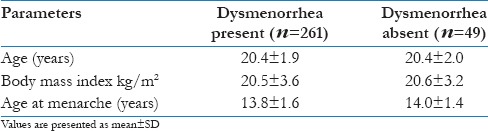
Majority of participants (73.9%) reported bleeding duration as 4–5 days which is normal only, 7.7% reported less than 3 days and in 18.4% participants the duration was more than 5 up to 7 days. Of the 84% girls who had dysmenorrhea, 20% had bleeding duration more than 5 days; when bleeding duration was studied across the presence of dysmenorrhea, bivariate analysis was found to significant (χ2 = 10.5; df = 2 and P = 0.005), thus showing association of dysmenorrhea with bleeding duration. Further logistic regression analyses depict participants with bleeding duration more than 5 days had 1.9 times more chance of getting dysmenorrhea (OR: 1.9; 95% CI: 1.7–3) [Figure 1].
Figure 1.
Association of bleeding duration with dysmenorrhea. Black: Bleeding duration <3 days; light gray: Bleeding duration 4–5 days; and dark gray: Bleeding duration >5 days
On the other hand, bleeding duration showed an increasing trend across the VAS. Participants with bleeding duration greater than 5 days had significantly higher pain score (6.1 ± 0.5) as compared to other two categories (4.6 ± 0.5) for <3 days and (5.7 ± 0.2) for 4–5 days (P < 0.05).
Presence of clots
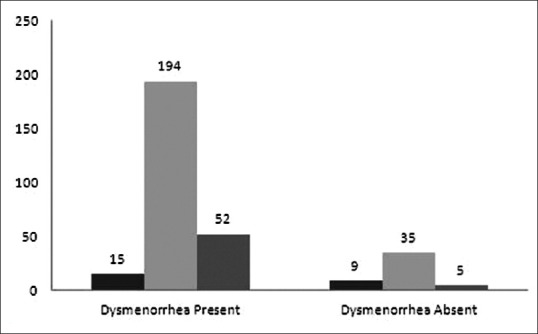
Majority of girls (80.4%) have reported the presence of clots during menstruation, size of the clot was mostly small in all these girls. Of these 80.4% girls, 82.5% had dysmenorrhea thus, there is strong association between dysmenorrhea and presence of clots (χ2 = 4.4; df = 1; P = 0.03). Moreover, girls with the presence of clots had 2.07 times higher chance of having dysmenorrhea (OR: 2.07; 95% CI: 1.04–4.1) (P < 0.05) [Figure 2].
Figure 2.
Association of the presence of clots with dysmenorrhea. Black: Clots present; and gray: Clots absent
Family history
Almost half of the participants (53.7%) had complaints of dysmenorrhea among their immediate family members remaining 46.3% had no complaints.
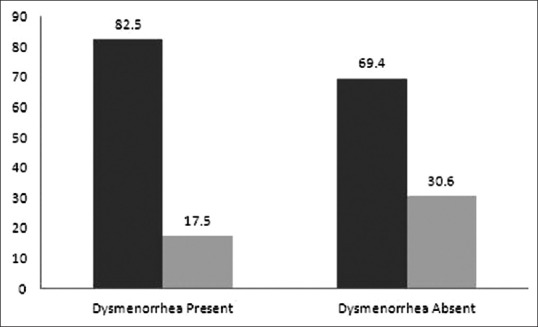
Of the 53.7% participants who had some family history of dysmenorrhea, 90.9% experiences the condition themselves. The bivariate analysis performed proved to be statistically significant (χ2 = 11.5 df = 1 and P = 0.001). Thus, participants whose family members (sisters or mother) had a history of dysmenorrhea had 3 times greater chance of having the same problem when compared to participants without family history of the same (OR: 3.0; 95% CI: 1.5–5.8; P = 0.001) [Figure 3].
Figure 3.
Association of family history with dysmenorrhea. Dark gray: Family history Yes; and light gray: Family history No
Pre-menstrual symptoms
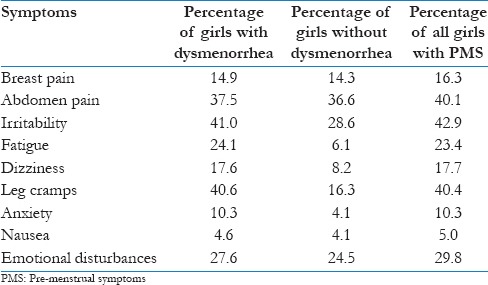
Premenstrual symptoms (PMS) were classified as physical symptoms and psychological symptoms. Nausea, leg cramps, dizziness, fatigue, abdominal pain, and breast pain were considered as physical symptoms, while anxiety, irritability and emotional instability were considered under psychological symptoms.
Dysmenorrhea is usually associated with pre-menstrual symptoms; however, in the present study we have observed that majority of girls (91%) have reported PMS even in the absence of dysmenorrhea only 9% of the girls didn’t report any such symptom. Of these 91% girls, majority of them (64.2%) have reported multiple symptoms therefore the total percentage doesn’t come to 100. Table 2 depicts clear presentation of PMS symptoms in the study population.
Table 2.
Percentage of girls with premenstrual symptoms
Discussion
It is estimated that prevalence of dysmenorrhea varies from 20% to 95%.[10,11] The results of the present study confirms the same, as dysmenorrhea was seen in majority of the young girls (84%). The variation of prevalence was due to a different diagnostic tool or different attitude toward menstruation. Similar high prevalence (67.2%) was reported by Sharma et al.,[12] and Harlow and Park (71.6%).[2] A Cochrane systematic review of studies in developing countries reported prevalence of dysmenorrhea in 25% to 50% of adult women.[13] On the other hand, studies from the developed countries also reported a wide range of 60% to 73%.[14]
The highest number of girls (42.6%) having dysmenorrhea was observed at the age of 19–20 yrs. More than two third of the girls (70.2%) described their dysmenorrhea as moderate to severe. Pain is extremely subjective symptom and it has been very difficult to quantify pain. Researchers have, therefore, found out a way to measure pain by various scoring systems like VAS.[9] In our study, it was revealed that 29.2%, 36.6% and 34.2% of girls had mild, moderate and severe pain, respectively. In a study conducted by Ortiz in 1539 students of Mexican University, author concluded that dysmenorrhea was mild in 36.1%, moderate in 43.8% and severe in 20.1%.[15] Maitri shah et al., have found that 18%, 40% and 42% of students had mild, moderate and severe pain (dysmenorrhea), respectively.[16] This indicates dysmenorrhea is still an important public health problem which may have a negative impact on health, social environment, work and psychological status.
Some of the studies have determined that prevalence of dysmenorrhea decrease with increasing age thus indicating that primary dysmenorrhea peaks in late adolescent by 20s and then the incidence falls with increasing age.[17] The present study also showed more number of girls with dysmenorrhea at 19–20 and then the number falls; however, the difference was not significant (P > 0.05) data was not reported across age groups.
Several studies have shown a significant association between early age at menarche and dysmenorrhea; the underlying reason could be the fact that girls who attend menarche early have longer exposure to uterine prostaglandins leading to higher prevalence of dysmenorrhea.[18] However, since majority of girls were in reference category of 12–14 years for age of menarche, we found no significant difference in the mean age of menarche between the two groups (presence and absence of dysmenorrhea). Our data differs from other studies where age of menarche is an important factor.[17] One of the reasons could be less number of girls below 12 and above 15 years for age at menarche. Our findings are on par with results observed by Pawlowski et al., who did not find any difference in the ages of menarche between dysmenorrheic and non-dysmenorrheic girls.[19]
Epidemiological studies have shown link between dysmenorrhea and several environmental risk factors, including cigarette smoking and consumption of coffee; however, no such linkages were observed in the present study and none of the girls have reported smoking. Similarly, linkages of BMI with dysmenorrhea were observed in few studies;[18] moreover, these findings are contradictory with other Indian study.[20] Present study findings are consistent with this and showed no association with BMI.
Average length of menstrual cycle was reported to be normal in majority (86.7%) of girls (28–35 days). Normal length of the cycle is considered as 21–35 days.[21] This is in view of fact that the cycles are already regularized by this age. We did not find the association between length of cycle and dysmenorrhea. Different studies have suggested that dysmenorrhea is more prevalent in women with longer cycles.[22]
On the other hand, menstrual bleeding duration of 5 days and over was an important risk factor for dysmenorrhea. Bleeding duration was found to be significantly associated with dysmenorrhea in the present study. Girls who had bleeding duration more than 5 days had more chance of getting dysmenorrhea. This finding is compatible with the result showing that the risk of dysmenorrhea is higher in women with long menstrual flows.[14]
Also, bleeding duration was significant across the VAS. The intensity of pain which was measured by VAS was significantly higher for girls with bleeding duration more than 5 in comparison with those less than 3 and 4—5 days. Girls with the presence of clots had two times more chance of getting dysmenorrhea as compared to girls who did not report clots. This finding has been rarely reported by other studies.
Dysmenorrhea seems to be familial problem similar conclusion was made by the present study. Around 53.7% participants had positive familial correlation, i.e., either mother or sibling had similar complaints. History of dysmenorrhea seems to be an important risk factor for women with dysmenorrhea. The results are in accordance with previous studies[18,23] that have found correlation between familial predisposition and dysmenorrhea. As an explanation for this some of the researchers have reported that daughters of mothers who have menstrual complaints also experienced menstrual discomfort, which is related to behavior that is learned from the mother.[24] Women of reproductive age experience symptoms during late luteal phase of their menstrual cycle, and collectively these complaints are termed premenstrual symptoms and typically include both psychiatric and physical symptoms.[25] The present study reports a presence of pre-menstrual symptoms in majority of girls despite of the absence of dysmenorrhea. Most frequent occurring symptoms were irritability, leg cramps and abdominal pain. An Indian study in 2012 also reported similar symptoms like irritability, breast pain, emotional disturbances etc.[18,26]
There are certain limitations of the study firstly, it has been conducted in single college in single district, and therefore the sample may not be representative of all colleges in Indore, Madhya Pradesh. And since the nature of data was self-reporting, it may have resulted in under-reporting of the conditions in few cases.
Conclusion
To conclude, dysmenorrhea is found to be highly prevalent among college going girls. Findings suggest family history, bleeding duration and presence of clots as significant risk factors for dysmenorrhea in these girls. Majority of girls were suffering from pre-menstrual symptoms indicating the magnitude of problem and thus, need an appropriate intervention through a change in lifestyle.
Acknowledgements
We would like to express our profound gratitude to all the participants (medical college, nursing college and dental college girls) for their co-operation and contribution toward this study. Our sincere thanks to Index Medical College and Research Centre, Indore, India for funding this study.
Footnotes
Source of Support: Index Medical College and Research Centre, Indore, India.
Conflict of Interest: None declared.
References
- 1.Zaiei S, Faghihzadeh S, Sohrabvand F, Lamyian M, Emamgholy T. A randomised placebo-controlled trial to determine the effect of vitamin E in treatment of primary dysmenorrhoea. BJOG. 2001;108:1181–3. doi: 10.1111/j.1471-0528.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 2.Harlow SD, Park M. A longitudinal study of risk factors for the occurrence, duration and severity of menstrual cramps in a cohort of college women. Br J Obstet Gynaecol. 1996;103:1134–42. doi: 10.1111/j.1471-0528.1996.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 3.Latthe P, Latthe M, Say L, Gülmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: Neglected reproductive health morbidity. BMC Public Health. 2006;6:177. doi: 10.1186/1471-2458-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latthe P, Mignini L, Gray R, Hills R, Khan K. Factors predisposing women to chronic pelvic pain: Systematic review. BMJ. 2006;332:749–55. doi: 10.1136/bmj.38748.697465.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nag RM. Calcutta: Medical Allied Agency; 1982. Adolescent in India; pp. 18–26. [Google Scholar]
- 6.George A, Bhaduri A. Dysmenorrhea among adolescent girls-symptoms experienced during menstruation. Health Promot Educ. 2002;17:4. [Google Scholar]
- 7.Jayashree R, Jayalakshmi VY. Socio-cultural dimensions of menstrual problems. Health Educ South East Asia. 1997;12:21–6. [Google Scholar]
- 8.Organization WH. Geneva, Switzerland: World Health Organization; 2000. The Asia-Pacific perspective: Redefining obesity and its treatment. [Google Scholar]
- 9.Larroy C. Comparing visual-analog and numeric scales for assessing menstrual pain. Behav Med. 2002;27:179–81. doi: 10.1080/08964280209596043. [DOI] [PubMed] [Google Scholar]
- 10.Harel Z. A contemporary approach to dysmenorrhea in adolescents. Paediatr Drugs. 2002;4:797–805. doi: 10.2165/00128072-200204120-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CS, Yang JK, Yang LL. Effect of a dysmenorrheal Chinese medicinal prescription on uterus contractility in vitro. Phytother Res. 2003;17:778–83. doi: 10.1002/ptr.1235. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Malhotra C, Taneja DK, Saha R. Problems related to menstruation amongst adolescent girls. Indian J Pediatr. 2008;75:125–9. doi: 10.1007/s12098-008-0018-5. [DOI] [PubMed] [Google Scholar]
- 13.Harlow SD, Campbell OM. Epidemiology of menstrual disorders in developing countries: A systematic review. BJOG. 2004;111:6–16. doi: 10.1111/j.1471-0528.2004.00012.x. [DOI] [PubMed] [Google Scholar]
- 14.Unsal A, Ayranci U, Tozun M, Arslan G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci. 2010;115:138–45. doi: 10.3109/03009730903457218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortiz MI. Primary dysmenorrhea among Mexican university students: Prevalence, impact and treatment. Eur J Obstet Gynecol Reprod Biol. 2010;152:73–7. doi: 10.1016/j.ejogrb.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Shah M, Monga A, Patel S, Shah M, Bakshi H. A study of prevalence of primary dysmenorrhea in young students-A cross-sectional study. Healthline. 2013;4:30–4. [Google Scholar]
- 17.Patel V, Tanksale V, Sahasrabhojanee M, Gupte S, Nevrekar P. The burden and determinants of dysmenorrhea: A population-based survey of 2262 women in Goa, India. BJOG. 2006;113:453–63. doi: 10.1111/j.1471-0528.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 18.Shrotriya C, Ray A, Ray S, Thomas GA. Menstrual characteristics and prevalence and effect of dysmenorrhea on quality of life in medical students. Int J Collab Res Intern Med Public Health. 2012;4:276–94. [Google Scholar]
- 19.Pawlowski B. Prevalence of menstrual pain in relation to the reproductive life history of women from the Mayan rural community. Ann Hum Biol. 2004;31:1–8. doi: 10.1080/03014460310001602072. [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Kiran D, Singh H, Nel B, Singh P, Tiwari P. Prevalence and severity of dysmenorrhea: A problem related to menstruation, among first and second year female medical students. Indian J Physiol Pharmacol. 2008;52:389–97. [PubMed] [Google Scholar]
- 21.Munster K, Schmidt L, Helm P. Length and variation in the menstrual cycle: A cross-sectional study from a Danish county. Br J Obstet Gynaecol. 1992;99:422–9. doi: 10.1111/j.1471-0528.1992.tb13762.x. [DOI] [PubMed] [Google Scholar]
- 22.El-Gilany AH, Badwai J, El-Fedway S. Epidemiology of dysmenorrhea among adolescent students in Mansoura, Egypt. East Mediterr Health J. 2005;11:155–63. [PubMed] [Google Scholar]
- 23.Gagua T, Tkeshelashvili B, Gagua D. Prevalence and risk factors of primary dysmenorrhea. J Turk Ger Gynecol Assoc. 2012;13:162–8. doi: 10.5152/jtgga.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polat A, Celik H, Gurates B, Kaya D, Nalbant M, Kavak E, et al. Prevalence of primary dysmenorrhea in young adult female university students. Arch Gynecol Obstet. 2009;279:527–32. doi: 10.1007/s00404-008-0750-0. [DOI] [PubMed] [Google Scholar]
- 25.Schorge JO, Schaffer JI, Halvorson LM, Bradshaw KD, Cunningham FG, Calver LE, editors. 2nd ed. Printed in China: McGraw-Hill; 2008. Williams's gynecology; p. 296. [Google Scholar]
- 26.Agarwal AK, Agarwal A. A Study of Dysmenorrhea during Menstruation in Adolescent Girls. Indian J Community Med. 2010;35:159–64. doi: 10.4103/0970-0218.62586. [DOI] [PMC free article] [PubMed] [Google Scholar]


