Abstract
During primary neurulation, the separation of a single-layered ectodermal sheet into the surface ectoderm (SE) and neural tube specifies SE and neural ectoderm (NE) cell fates. The mechanisms underlying fate specification in conjunction with neural tube closure are poorly understood. Here, by comparing expression profiles between SE and NE lineages, we observed that uncommitted progenitor cells, expressing stem cell markers, are present in the neural plate border/neural fold prior to neural tube closure. Our results also demonstrated that canonical Wnt and its antagonists, DKK1/KREMEN1, progressively specify these progenitors into SE or NE fates in accord with the progress of neural tube closure. Additionally, SE specification of the neural plate border via canonical Wnt signaling is directed by the grainyhead-like 3 (Grhl3) transcription factor. Thus, we propose that the fate specification of uncommitted progenitors in the neural plate border by canonical Wnt signaling and its downstream effector Grhl3 is crucial for neural tube closure. This study implicates that failure in critical genetic factors controlling fate specification of progenitor cells in the neural plate border/neural fold coordinated with neural tube closure may be potential causes of human neural tube defects.
Keywords: Surface ectoderm, Neural tube closure, Neural fold, Uncommitted progenitors, Grainyhead-like family, Canonical Wnt, Wnt antagonists, Neural tube defects
Highlights
-
•
Neural plate border/neural fold possesses stem cell-like characters during primary neurulation.
-
•
Canonical Wnt and its antagonists progressively specify progenitors into surface or neural fates upon neural tube closure.
-
•
Fate specification into surface ectoderm in the neural fold is directed by the Grhl3 transcription factor.
-
•
Fate specification of uncommitted progenitors in the neural plate border is intimately coupled to neural tube closure.
1. Introduction
Neural tube defects are severe developmental disorders of the brain or spinal cord and one of the most common with a frequency of about 1 per 1000 births in humans (Mitchell, 2005). It is still unclear how genetic and environmental factors are associated with human neural tube defects. Neural tube defects arise when the neural tube fails to close completely during early embryonic development. Neural tube closure proceeds progressively at different levels of the anterior–posterior axis during primary neurulation in which the neural plate bends, folds up and fuses along the dorsal midline of neural folds.
Notably, neural ectoderm (NE) and surface ectoderm (SE) initially adjoin within an ectodermal sheet prior to neural tube closure and thereafter the sheet completely separates the two fates by the end of neural tube closure. Thus, it can be assumed that fate specification within the ectodermal sheet into NE or SE should be precisely controlled in conjunction with neural tube closure in both time and space. Indeed, genetic clonal analyses of cell lineage segregations in the mouse embryo have suggested that ectodermal progenitor cells, which are not committed to the NE or SE, are present during early to mid-gastrulation (Tzouanacou et al., 2009, Lawson et al., 1991). However, little is known about what, when, and how molecular mechanisms control the fate specification decision between NE and SE cells in the neural plate border/neural fold, where these cells adjoin.
Moreover, it is still unknown whether uncommitted progenitor cells remain in the neural plate border following NE and SE specification during neurulation in vertebrate embryos. Indeed, much attention has been paid to the neural plate border between the NE and SE in light of the major source of the neural crest and placodes, and for decades many studies have explored the mechanisms underlying neural crest specification in the neural plate border (Patthey and Gunhaga, 2011, Sauka-Spengler and Bronner-Fraser, 2008, Grocott et al., 2011, Steventon et al., 2014, Stuhlmiller and Garcia-Castro, 2012, Weston and Thiery, 2015).
The present study reveals that the neural plate border possesses an uncommitted progenitor state prior to the midline fusion of neural folds during neural tube closure. Our data provide evidence that canonical Wnt signaling promotes SE specification, whereas the Wnt antagonist, Dickkopf1 (Dkk1), induces NE specification in the neural plate border. Additionally, Grainyhead-like 3 (Grhl3) transcription factor, which is essential for neural tube closure, specifies the SE cell fate under the control of canonical Wnt. Thus, we propose that fate specification of the neural plate border/neural fold via canonical Wnt signaling and the Grhl3 factor is crucial for neural tube closure. This study implicates that the canonical Wnt pathway may have an important role in human neural tube closure. Moreover, failure in critical factors controlling fate specification of uncommitted progenitor cells in the neural fold coordinated with neural tube closure may induce human NTDs.
2. Materials and Methods
2.1. Constructions of transgene vectors and generation of transgenic mice
Mouse Dkk1, Wnt8A and Grhl2 cDNA fused to the CAG promoter was ligated to the LacZ gene flanked by two loxP sequences, as described previously (Kimura-Yoshida et al., 2005). Mouse Grhl2 and Grhl3 cDNAs were obtained from EMAGE clone #6306106 and #30607679, respectively. These cDNAs were inserted into the CAG cassettes. These constructs were microinjected into the pro-nucleus of the fertilized eggs from CD-1 as described (Nagy et al., 2003)
2.2. Mouse Genotyping
Genotyping of CAG-Dkk1 transgenic mice obtained using the PCR primers CAG-pro (5′-TAGAGCCTCTGCTAACCATGTTCATGCCTT-3′) and CAG-Dkk1 (5′-TCAGCGCAAGGGTAGGGCTGGTAGTTGTCA-3′), yielding 443 bp. CAG-Wnt8A transgenic mice were genotyped as described (Kimura-Yoshida et al., 2005). Dkk1 null mutant mice were identified with three primers: mdkk1-5′ (5′-GCAACGCTAAAGGGTTAATCAGCAACTATA-3′), mdkk1-frt5′ (5′-CCCCGGGAACTACTGCAAAAATGGT-3′), and mdkk1-frt3′(5′-GTGCTCAAACACAAGCCAGTGACGA-3′), yielding 180 bp as the wild-type allele and 400 bp as the mutant allele. Grhl3-Cre-IRES-nlsLacZ knock-in mice were obtained from the Mutant Mouse Regional Resource Center (MMRRC) and genotyped with the following primers: Grhl3-cre (5′-AATTAAGAGACGAGTGGTCAGCAGCGCCTG-3′), Grhl3-cre wt rev (5′-ACCCTTACAAATTGCCGTGTGAATCCGGGC-3′), and Grhl3-cre mut (5′-GCAGCCCGGACCGACGATGAAGCATGTTTA-3′), yielding 213 bp as the wild-type allele and 370 bp as the mutant allele.
β-Catenin knockout mice were genotyped as described (Kimura-Yoshida et al., 2005). The CAG-Grhl2 allele was genotyped by PCR of genomic DNA using the following primers: CAG-pro primer (described aforementioned) and Grhl2-rev1 5′-CAGATATGACTTCCAGGCCTCATCCTCACT-3′, yielding 330 bp as a PCR fragment. Rosa GNZ knock-in mice (Gt(Rosa)26Sortm1Joe/J) were obtained from the Jackson Laboratory (Stoller et al., 2008). Rosa GNZ mutant mice were identified with these primers: forward 5′-CTCCCAAAGTCGCTCTGAGTTGTTATCAGT-3′, reverse 5′-CAACGCCCACACACCAGGTTAGCCTTTAAG-3′, yielding 265 bp as the wild-type allele and forward 5′-TCGCTACCATTACCAGTTGGTCTGGTGTCA-3′ 443 bp as the mutant allele.
2.3. DNA Microarray
Wild-type embryos were dissected in PBS with calcium and magnesium. The embryos were then incubated in pancreatin/trypsin in calcium- and magnesium-free PBS for dissection. The surface ectoderm and neural ectoderm at the forebrain level, which does not include cephalic mesenchymal cells, were isolated using tungsten needles. The numbers of embryos used for three independent biological replicate experiments were as follows: replicate 1, surface ectoderm 1 (SE1) n = 109, neural ectoderm 1 (NE1) n = 46; replicate 2, SE2 n = 117, NE2 n = 43; and replicate 3, SE3 n = 114, NE3 n = 45. Isolated tissues were stored at − 80 °C after being soaked in RNAlater (Applied Biosystems). Collected tissues were purified using a TRIzol-plus purification kit (Invitrogen, cat. no. 12183-555). The quality of purified RNA samples was confirmed using Experion (BioRad). cRNA was prepared using the illumine Total Prep RNA Amplification Kit (Illumina). Samples were hybridized to a MouseWG6-v2 array (Illumina) and scanned with a BeadArray Reader (Illumina). Raw data were analyzed using Bead Studio software (Illumina), and pairwise comparisons were done between the SE and NE populations for each of the three biological replicates. We noted that there was good correspondence between replicated genes with a two-fold change. The accession number for the microarray data reported in this paper is GSE67977.
2.4. TEM and SEM
TEM and SEM procedures for mouse embryos followed techniques previous described (Kimura-Yoshida et al., 2005, Kimura et al., 2000).
2.5. Whole Embryonic Culture
Wild-type and CAG-Dkk1 embryos at the 4- to 5-somite stage were cultured in DMEM supplemented with 20% fetal bovine serum and 1 × nonessential amino acid in a 5%CO2 incubator at 37 °C.
2.6. Immunohistochemistry
Mouse embryos were dissected and embedded in OCT and frozen overnight at − 80 °C. Cryosections were cut at 10 μm using a Microm HM500 OM cryostat, mounted onto superfrost slides, and stored at − 80 °C until use. After thawing, the slides were fixed in 4% paraformaldehyde in PBS and washed in 0.1% TritonX in PBS (PBT). The sections were then incubated in blocking solution (1% BSA/10% goat serum/PBT) for 1 h. Incubation of the primary antibody diluted in blocking solution was performed overnight, as shown in Fig. S9. Sections were then washed three times in PBT for 5 min per wash and incubated for 2 h at RT with the second antibody in blocking solution. The sections were further washed three times with PBT for 5 min per wash and placed onto slides using a mounting medium. Immunohistochemistry for whole-mount embryos and paraffin embedding sections were performed as described (Shimokawa et al., 2011).
2.7. Histology, electron microscopy, X-gal staining, and in situ hybridization
For the histological analysis, embryos were fixed in Bouin's fixative, dehydrated, and embedded in paraplast. Serial sections (thickness, 7 μm) were generated and stained with hematoxylin and eosin (HE). X-gal staining and in situ hybridization were performed as described (Kimura-Yoshida et al., 2005, Kimura et al., 1997, Kimura et al., 2000). The following template cDNA plasmids were used for the synthesis of the probes: Foxi2 (nt 1554–2267), Grhl2 (nt 203–603), Grhl3 (nt 207–693), Klf5 (nt 986–1590), Kremen1 (nt 3796–4626), Lrp4 (nt 7220–7720), Lrp5 (nt 2888–3444), Lrp6 (nt 2941–3446), Lrp10 (nt 2542–2941), Twist1 (nt 666–1630), and Wnt6 (IMAGE clones 6511061).
2.8. Ethics Statement
All mouse studies followed the fundamental guidelines for proper conduct of animal experiments and related activities in academic research institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology of Japan and were approved by institutional committees at the Osaka Medical Center and Research Institute for Maternal and Child Health for animal and recombinant DNA experiments.
3. Results
3.1. Uncommitted Progenitor Cells are Present in the Neural Plate Border Prior to Neural Fold Fusion
During primary neurulation, NE and SE cells adjoin directly within the same ectodermal sheet, and then the neural plate separates from the SE during the completion of neural tube closure. First, for the determination of how and when the cell fate is specified in conjunction with epithelial fusion during neural tube closure, the expressions of several NE and SE markers were analyzed (Fig. 1). We found that NE markers such as N-cadherin and SOX2 and SE markers such as TROMAI/Keratin8 and E-cadherin are expressed roughly in their respective regions during early neurulation, between E8.0 and E8.5 (somite stages [ss] 1–3 and 5–8, respectively; Fig. 1A–I). However, the uncommitted progenitors between SE and NE in the neural plate border/neural fold — where neither SE nor NE is specified — are present until the completion of epithelial fusion (Fig. 1A–I; dotted lines in C–E, arrowheads in F–I).
Fig. 1.
Comparative expression analysis with NE, SE, and stem cell markers during primary neurulation. (A) Schematic expression patterns in E8.0 (somite stages 1–3) and E8.25 (somite stages 3–5) embryos. (B–M, P–R) Images of section (B–E, P, Q) and whole-mount (F–M, R) immunohistochemistry stained at E8.0 (B, C), E8.25 (D, E) and E8.5 (somite stages 5–8) (F–I, J–M, R) with Sox2/TROMAI (B, C, E), N-cadherin/TROMAI (D, F–H), N-cadherin/E-cadherin (I), OCT4 (J, K), OCT4/TROMAI/N-cadherin (L, M) KLF5 (P, Q) and SSEA4 (R). (N, O) Whole mount in situ hybridization using Klf5 probe at E8.5 (dorsolateral view, N; dorsal view, O). Each panel shows a region where neither SE nor NE markers are expressed as dotted lines (C–E) and arrowheads (F–I). The expression of OCT4, a stem marker, specifically labels the boundary between NE and SE at E8.5, although the expression level is lower than that of primordial germ cells (J–M). Klf5, which is a Krüppel-like transcription factor that plays crucial roles in maintaining embryonic stem (ES) cell pluripotency, is expressed in the neural plate border (arrowheads, N–Q). SSEA4 is a cell surface marker of human ES, embryonic germ (EG), and induced pluripotent stem (iPS) cells and is expressed in the proximity of the neural plate border (arrowheads, R). NPB, neural plate border; PGCs, primordial germ cells. Scale bar represents 300 μm in N; 200 μm in F–J, L, M; 100 μm in B, O, P, R; 50 μm in K, Q; and 20 μm in C–E.
For the molecular characterization of these uncommitted progenitor cells, the expressions of several stem cell markers were examined (Fig. 1J–R). OCT-3/4 expression, a stem cell marker, was detected in the neural plate border/neural fold, although at a lower level than that in primordial germ cells (Fig. 1J–M). Two additional stem cell markers, Klf5 transcripts and its proteins and SSEA-4, were also expressed in the neural plate border (Fig. 1N–R, arrowheads). These findings indicate that a neural plate border region during neurulation possesses a stem cell-like character.
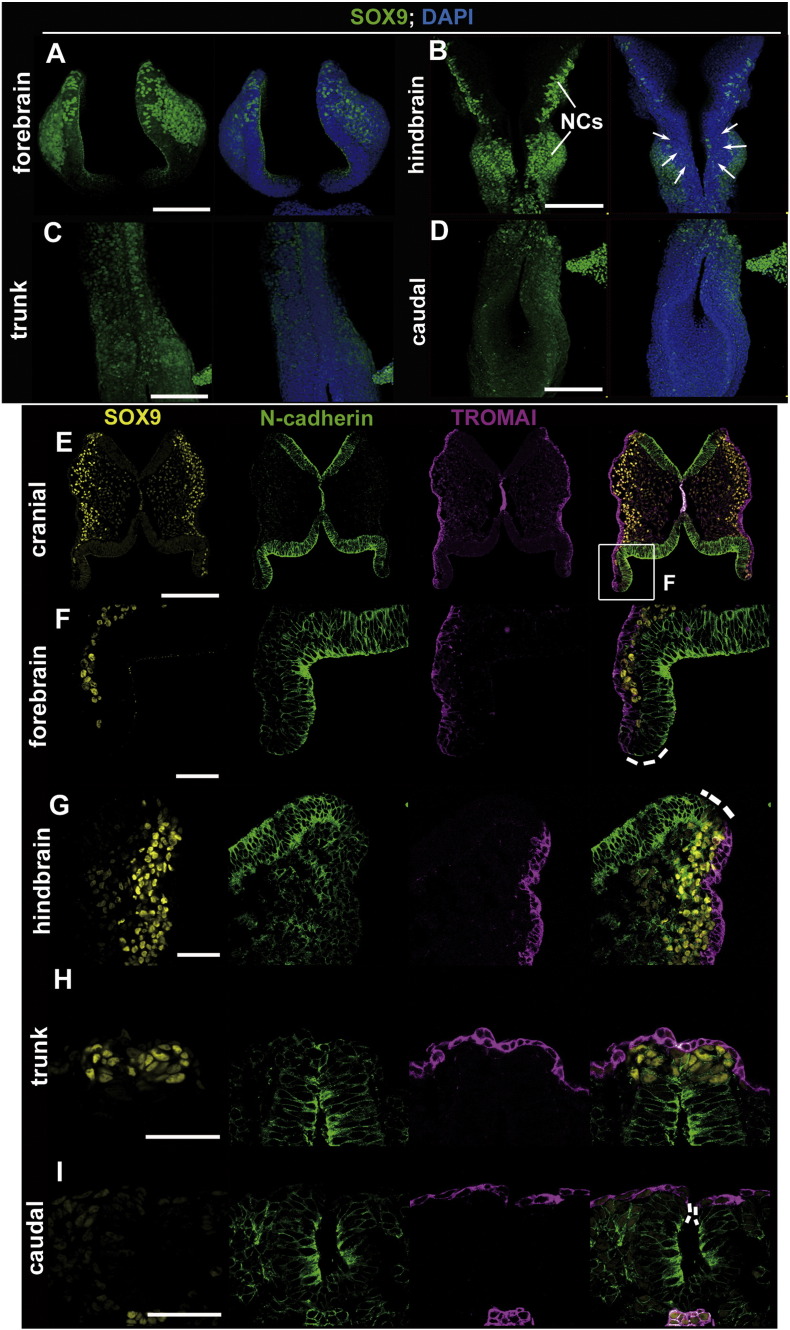
To further assess whether these neural plate border progenitors are relevant to the population of the neural crest, we analyzed the expression of SOX9, a pre-migratory and post-migratory neural crest marker (Fig. 2). A very few SOX9-positive cells were located in the neural plate border at the forebrain (Fig. 2A, E, F). At the hindbrain level, a considerable amount of SOX9-positive cells were delaminated from in the vicinity of the neural plate border (Fig. 2B, G). However, SOX9-negative cells appeared to be present and still remained at an uncommitted state at this stage at both the forebrain and hindbrain levels (Fig. 2F, G, dotted lines). Additionally, given that SOX9-positive cells were localized in the dorsal neural tube at the trunk level and undetectable at the caudal level, neural crest cells are not directly from the uncommitted progenitors in the neural plate border during neurulation at the trunk and caudal levels (Fig. 2C, D, H, I).
Fig. 2.
Behaviors of SOX9-positive neural crest cells during neurulation. (A–D) Whole-mount immunohistochemistry stained at E8.5 with SOX9 antibody. SOX9-positive cells are present in pre- and post-migratory neural crest cells at the forebrain, hindbrain and trunk levels but few were present at the caudal level (A–D). In this study, we defined “trunk” as the anterior spinal cord level in which the neural tube is closed completely, and “caudal” as the caudal spinal cord level in which the neural tube is still open. (E–I) Sectional images of immunohistochemistry at the level of the forebrain (E, F), hindbrain (E, G), trunk (H) and caudal (I) with SOX9 (yellow)/N-cadherin (green)/TROMAI (magenta). At the level of the hindbrain, SOX9-positive cells were present in the proximity of the neural plate border (G; white dotted lines). Dotted white lines indicate the neural plate border. NCs, neural crest cells. Scale bar represents 200 μm in A–E; 50 μm in F–I.
These findings indicate that although uncommitted progenitor cells in the neural plate border contributed to neural crest lineages to some extent (at least at the level of the hindbrain), the decision of fate specification to the neural crest appears to be not intimately linked to neural tube closure. Taken together, the fate specification to SE or NE in the neural plate border appears to be intimately coupled to neural tube closure.
3.2. Wnt Signaling Modulators DKK1 and KREMEN1 are Localized at the Neural Plate Border
To identify what types of signaling pathways are involved in the fate specification between SE and NE cells during neurulation, we carefully separated the SE and NE from the forebrain level of mouse embryos at E8.25–E8.5 (pooled samples from 3- to 5-somite stage embryos; three biological replicates) and compared the whole genome-wide gene expression profiles by means of DNA microarray (Fig. S1). Initially, we surveyed the Wnt, FGF, and BMP ligands, which are thought to be involved in the specification between neural and epidermis fates prior to or during gastrulation in other vertebrates (Murry and Keller, 2008).
The expression profiles of signal transduction-related genes showed that the expression of Wnt-related molecules is upregulated or downregulated between SE and NE, apparently depending on their functions (Fig. S1). In contrast, the expression of other ligands, BMP and FGF, was observed exclusively in the SE and NE, respectively. The subsequent expression analysis with whole-mount in situ hybridization indicated that Wnt ligands, co-receptors, and receptors were actually expressed in the proximity of the neural plate border between the SE and NE (Fig. 3A–W).
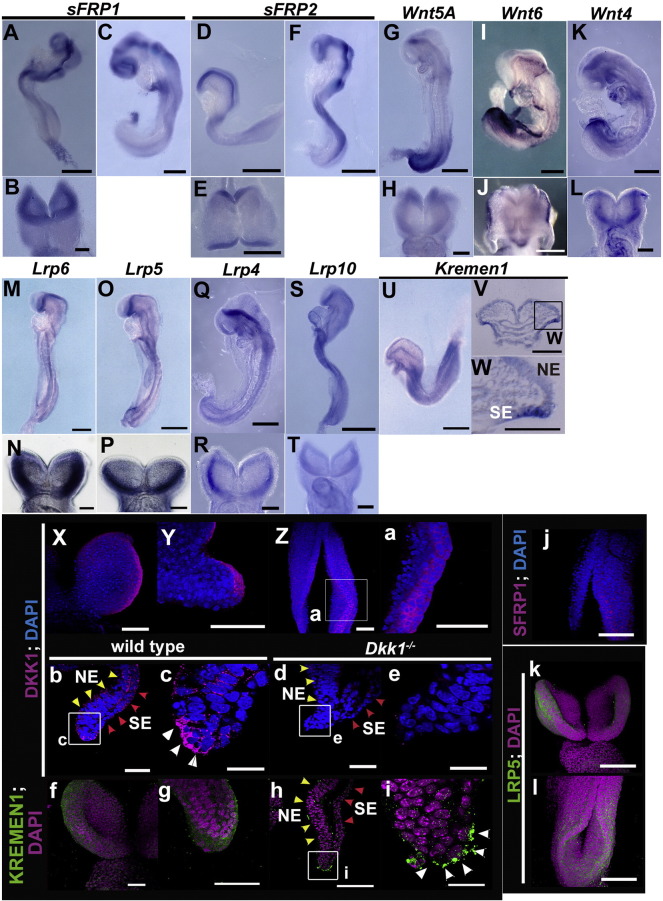
Fig. 3.
Wnt signaling-related genes are expressed in the proximity of the neural plate border. (A–W) Whole-mount in situ hybridization analysis (A–U) and its sectional views (V, W) of wild-type embryos at E8.25 (D, E, U–W), E8.5 (A, B, F, M–P, S, T) and E8.75 (C, G–L, Q, R). Lateral views (A, C, D, F, G, I, K, M, O, Q, S, U), frontal views (B, H, L, N, P, R, T), and dorsal view (E, J). sFRP1 (A–C), sFRP2 (D–F), Wnt5A (G, H), Wnt6 (I, J), Wnt4 (K, L), Lrp6 (M, N), Lrp5 (O, P), Lrp4 (Q, R), Lrp10 (S, T) and Kremen1 (U–W). (X-l) Immunohistochemical analyses of whole-mount embryos (X-a, f, g, j–l) and transverse sections (b–e, h, i) in wild-type (X-c, f–l) and Dkk1−/− embryos (d, e). DKK1 (X-e; magenta), KREMEN1 (f–i; green), SFRP1 (j; magenta) and LRP5 (k, l; green) proteins are localized at the neural plate border (white arrowheads in c and i), and DKK1 expression is not detected in the Dkk1−/− embryos (d, e). NE, neural ectoderm; SE, surface ectoderm. Scale bars: 300 μm in A, C, D, F, G, I, K, M, O, Q, S, U; 200 μm in j–l; 100 μm in B, E, H, J, L, N, P, R, T, V-a, f–h; 50 μm in b, d, e; 20 μm in c, i.
To explore the roles of Wnt signaling in fate specification, we examined the protein expressions of DKK1 and KREMEN1, which are antagonists/modulators for canonical and noncanonical Wnt (Glinka et al., 1998), more precisely (Fig. 3X-i). DKK1 proteins were detected in the neural plate border at both the cranial and caudal levels during neurulation (Fig. 3X-c; arrowheads). This local DKK1 expression was not observed in the Dkk1 knockout mouse embryo (Fig. 3d, e). Concurrently, KREMEN1 proteins as well as its transcripts were localized specifically at the neural plate border (Fig. 3U–W, f–i; arrowheads). Similarly, SFRP1 and LRP5 proteins, an antagonist and co-receptor of canonical Wnt, respectively, were also prominently localized in the neural plate border (Fig. 3j–l). Together, these findings suggested that Wnt signaling is one of the candidate pathways controlling the specification between NE or SE fate in uncommitted progenitors during neural tube closure.
3.3. Canonical Wnt Signaling Promotes the SE Fate in the Neural Plate Border During Primary Neurulation
To determine whether Wnt signaling affects these two cell fates, we generated transgenic mice misexpressing Dkk1 (Fig. 4A). To create Dkk1-misexpressing embryos, we crossed the CAG-loxP-lacZ-loxP-Dkk1 transgenic mouse — in which the lacZ gene is flanked by two loxP sites and the Dkk1 cDNA is misexpressed under the control of the CAG promoter — with the β-actin-cre transgenic mouse (Fig. 4A). The resultant double-transgenic embryos expressed the Dkk1 protein ectopically (CAG-Dkk1) (Kimura-Yoshida et al., 2005). Despite rather ubiquitous Dkk1 misexpression, given that the inhibitory effect on Wnt signaling through Dkk1 appears to require Kremen1/2 co-receptors and Lrp5/6 receptors (Cruciat and Niehrs, 2013), Dkk1 will attenuate Wnt signaling notably in local areas where Kremen1/2 and Lrp5/6 as well as Wnt ligands are expressed during neurulation (Fig. 3).
Fig. 4.
Molecular marker analyses in CAG-Dkk1 and Dkk1−/− mutant embryos. (A) A schematic construct of the CAG-Dkk1 transgene. (B–C′, L) The gross appearances of wild-type (B, C), CAG-Dkk1 (B′, C′), and Dkk1−/− (L) embryos at E8.5 (B, B′, L) and E18.5 (C, C′). (D, P) Transverse sections of embryos. Each section plane is represented by a corresponding line in the scheme in CAG-Dkk1 (D) and Dkk1−/− (P). (E–K′, Q–X) Immunohistochemical analyses with frozen sections of the wild-type (E–K), CAG-Dkk1 (E′–K′), and Dkk1−/− (Q–T) and with whole embryos of the wild-type (U, W) and Dkk1−/− embryos (U′, V, W′, X) at E8.5 (E–K′, Q–T), E8.25 (U–V) and E8.75 (W–X). SOX2 (E, E′, Q), N-cadherin (F–G′, R), Tuj1 (H, H′), E-cadherin (I, I′), TROMAI (J–K′, S–V), and KERATIN17/19 (W, X) merged with nuclei staining (TOTO-3; magenta in E-H′, J–K′, Q–T; blue in U–X). (M–O′) Whole-mount in situ hybridization in the wild-type (M–O) and Dkk1−/− embryos (M′–O′) at E8.5. Lateral and frontal views of Sox2 expression (M–N′) with sectional views and lateral views of Tfap2c expression with their frontal views (O, O′). Scale bars: 3 mm in C, C′; 300 μm in B, B′, L–O′, W–X; 100 μm in E–F′, H, H′, J, J′, Q–U′; 10 μm in I, I′, K, K′.
Morphological abnormalities in CAG-Dkk1 embryos were detectable around E8.5. Notably, the forebrain appeared to be expanded (Fig. 4B′) and the growth of the forelimbs and hindlimbs was greatly diminished in CAG-Dkk1 embryos at E18.5 (Fig. 4C′). Considering the effects of Dkk1 deficiency, which exhibits ectopic digits and forebrain reduction (Mukhopadhyay et al., 2001), the CAG-Dkk1 morphological deformities also support the notion that the effect of Dkk1 misexpression is the inverse of the effect of Dkk1 deficiency. Concurrently, the expression patterns of the canonical Wnt markers β-catenin and Top-gal reporter were down-regulated in Dkk1-misexpressed embryos (Fig. S2A-F, A′–F′).
To explore the possibility that Dkk1 can affect the specification between NE- and SE cell fates, we examined molecular markers in CAG-Dkk1 embryos with high-resolution immunohistochemistry (Fig. 4D–K′). The NE markers SOX2, N-cadherin, and Tuj1 were localized in the NE as they are normally (Fig. 4E–H), but they were also found ectopically in the presumptive SE region in the CAG-Dkk1 embryos (Fig. 4E′–H′; arrowheads). Conversely, the SE markers E-cadherin and TROMAI were severely reduced in the SE region of the CAG-Dkk1 embryos (Fig. 4I′–K′). Together these findings suggested that Dkk1 misexpression promotes the specification of NE fate and inhibits that of SE fate in the neural plate border.
Consistent with the above molecular markers, the presumptive SE of CAG-Dkk1 embryos was transformed from a simple epithelium to a pseudostratified- or stratified-like epithelium (Fig. S3A–C′). To analyze the histological abnormalities of SE and NE in CAG-Dkk1 embryos more precisely, we examined their fine structures by means of conventional scanning electron microscopy (SEM) and by viewing ultra-thin sections using transmission electron microscopy (TEM) (Fig. S3D–H′). In the wild-type embryos at E8.5, the morphological features of the SE, neural plate border and NE regions were distinct, and the neural plate border, previously referred to as “flattened cells” extended to the end of neural folds along with the longitudinal axis (Fig. S3D, E; flattened border cells in blue, NE in pink and SE in yellow, respectively) (Waterman, 1976). In contrast, flattened cells in the neural plate border between the NE and SE of the CAG-Dkk1 embryos became unclear, and the morphological features of the border region were more similar to those of the NE (Fig. S3D′–H′; the border region in green). Together, the fine morphological analyses suggested that in the CAG-Dkk1 embryos, the medial SE region, which is derived from the neural plate border, possessed the characteristics of NE.
In addition, our developmental studies of the CAG-Dkk1 embryos using an in vitro culture system indicated that the timing of the neural tube closure appeared to be delayed in the CAG-Dkk1 embryos (Fig. S3I–K′). The telencephalic neuropore, which closes nearly within 6 h in the wild type (ss 3–4; Fig. S3I–K), was still open and extended laterally after 6 h of culture in the CAG-Dkk1 embryos (Fig. S3I′–K′). This finding suggests the possibility that altered cell fate specification of the neural plate border in CAG-Dkk1 embryos can affect the timing of the neural tube closure.
To investigate whether Dkk1 deficiency may have the opposite effect on the specification between SE and NE, we analyzed Dkk1 null mutant embryos with specific markers (Fig. 4L–X, Fig. S2D′′–F′′) (Pietila et al., 2011). The NE markers SOX2 and N-cadherin were significantly reduced in the Dkk1−/− embryos at E8.5 (Fig. 4M′, N′, Q, R). Conversely, the SE markers Tfap2c and TROMAI were upregulated in the Dkk1−/− embryos, and they were also ectopically found in the presumptive NE region at both E8.25 and E8.5 during neurulation (Fig. 4O′, S, T, U′, V, W′, X; arrowheads). These findings demonstrate that the anterior neural plate border in Dkk1-deficient embryos was specified into an SE cell fate at the expense of an NE cell fate, and that these are the inverse of the ectodermal phenotypes in Dkk1-misexpressing embryos (Fig. 4E′–K′).
To determine whether these two cell fates can be affected by canonical Wnt, we generated transgenic mice in which Wnt8A, a canonical Wnt ligand, is ubiquitously misexpressed (Fig. S2G–P). Indeed, the expression domain of β-catenin and Top-gal reporter expression were greatly expanded to the most anterior end of the SE in Wnt8A-misexpressed embryos compared to the wild-type embryos (Fig. S2A″–C″,D‴–F‴), indicating enhancement of canonical Wnt activity. In accord with the hypothesis that the specification between NE and SE fates is altered, the NE markers Sox2 and N-cadherin were down-regulated (Fig. S2K, L), whereas the SE markers Tfap2c, E-cadherin, and TROMAI were enhanced by Wnt8A misexpression (Fig. S2H′, I′, M–P). Together these findings strongly suggest that canonical Wnt signaling is crucial to specification of the SE fate from uncommitted progenitors in the neural plate border.
3.4. The Grhl3 Gene is Expressed in the Uncommitted Neural Folds Upon Neural Tube Closure
To identify the transcriptional factors that can mediate the fate specification of SE cells, we re-surveyed the expression profiles of the DNA microarrays (Figs. S4, S5). In particular, we focused on SE-specific transcriptional factors under the control of canonical Wnt signaling. In the microarray, 151 genes were more highly expressed in the SE than in the NE (fold change > 3 and p-value < 0.05). Of these 151 genes, 20 are considered transcriptional factors, based on GO slim ontology (Fig. S5). We chose to focus on the Grainyhead-like2 (Grhl2) transcription factor (Tcfcp2I3), which was upregulated over sixfold more in SE than in NE (Fig. S5; red letters).
The Grhl family of CP2 transcription factors consists of important regulators of epidermal development in diverse species: grainyhead (grh) is a key determinant of epidermal barrier formation, wound healing, tube size, and neuroblast development in Drosophila, and Grhl genes play crucial roles in epidermal differentiation as well as neural tube closure in mammals (Pyrgaki et al., 2011, Rifat et al., 2010, Werth et al., 2010). In addition to Grhl2, Grhl3 is also expressed during epidermal maturation (Auden et al., 2006) and genetically interacts with the Grhl2 gene to achieve neural tube closure (Rifat et al., 2010). Hence, Grhl3 is likely to be another candidate for SE specification via Wnt signaling.
To test the possibility that these Grhl genes can contribute to the fate specification of SE cells in conjunction with neural tube closure, we first examined the expression of Grhl2 and Grhl3 transcripts with in situ hybridization from the late gastrula to neurula stages (Fig. 5A–N). At the early to late bud stages, Grhl2 transcripts were detected in the most proximal-embryonic region, and the expression domain became restricted to the prospective border of the NE and SE in the rostral region (Fig. 5A–G), whereas Grhl3 was preferentially expressed in the more caudal border between the NE and SE, where the levels of Grhl2 transcripts were rather low (Fig. 5H–N).
Fig. 5.
Grhl genes are expressed in the prospective SE. (A–N) In situ hybridization of whole-mount embryos (A–D, H–K), sagittal sections (E, L), and transverse sections (F, G, M, N) at E7.25 (A, H), E7.5 (B, I), and E7.8 (C–G, J–N). Lateral view (A–C, H–J), frontal view (D), and caudal view (K). (O-a) Whole-mount immunofluorescent staining (O–V, Y-a) and sectioning image (X) at E8.0 (O, P, R), E8.25 (S, U, Y), and E8.5 (Q, T, V, Z). (W) A section plane (X) is indicated in the schematic. Expression of GRHL2 (O–Q: GRHL2, green; nuclei, magenta) and β-gal antibody (R–T: GRHL3, magenta; nuclei, blue) in wild-type (O–Q) and Grhl3cre-nZ/+ (R-a). (U–X) β-gal (GRHL3; green) and GRHL2 (magenta) merged with DAPI staining (nuclei, blue). (Y-a) DKK1 (magenta; dotted lines), β-gal (GRHL3, green; arrowheads) and nuclei (DAPI, blue). Scale bars: 300 μm in A–E, H–L; 100 μm in F, G, M–V, Y-a; 10 μm in X.
Considering that canonical Wnt promotes the SE fate within the uncommitted neural plate border (Fig. 4, Fig. S2), we tested whether Grhl2 and –3 factors are upregulated in the neural plate border in conjunction with neural tube closure (Fig. 5O–a). Grhl2 proteins were found broadly throughout the cranial SE cells already from the head fold stage (Fig. 5O–Q; green) before the neurulation stage and continuing after the neural tube closure stage, mostly co-localized to the TROMAI-positive cells. On the other hand, Grhl3-β-gal expression, which is marked by Cre-IRES-NLS LacZ driven by the Grhl3 locus (Camerer et al., 2010), was not detected at the early somite stage (Fig. 5R). At the initiation of neural plate folding, Grhl3-β-gal appeared to form a line of dots along the border, and the expression appeared to increase in the midline region from the most rostral to the caudal direction (Fig. 5S, T; magenta). Compared to GRHL2, GRHL3 was evident in the proximity of the neural plate border between SE and NE (Fig. 5U–X).
These findings indicated that GRHL2 is expressed in SE cells from the early stage irrespective of the epithelial fusion of the neural folds and that GRHL3 is upregulated in the neural folds in conjunction with the neural tube closure event. To further address whether the DKK1-positive sharp boundary can demarcate the GRHL3-positive cells, we examined the expression of GRHL3 and DKK1 proteins and found that the Grhl3 products were located consistently on the SE side of the sharp boundary delineated by DKK1 products (Fig. 5Y, Z, a). These expression data suggest the possibility that Grhl3 mediates the SE fate specification of uncommitted progenitor cells via canonical Wnt signaling in coordination with the dorsal midline epithelial fusion of neural folds.
3.5. The Grhl3 Gene is Necessary to Specify the SE Fate of Uncommitted Progenitors
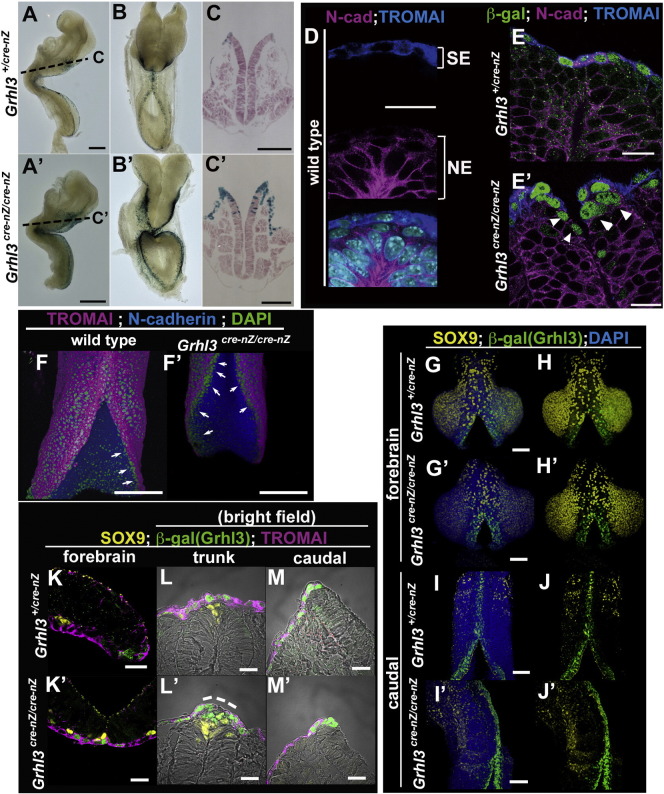
To address whether Grhl3 promotes the SE fate of uncommitted progenitors marked by DKK1/KREMEN1, we examined the lineage of SE cells in the Grhl3 null mutant background (Camerer et al., 2010). Notably, in the absence of Grhl3 function (Grhl3cre-nZ/cre-nZ), the prospective SE cells, which are labeled by IRES β-gal expression driven by the endogenous Grhl3 promoter, were ectopically found within the neural tissue (Fig. 6A′–C′). Next, to examine whether the mis-localized β-gal-positive cells possess NE or SE character, we performed a marker analysis with immunohistochemistry (Fig. 6D–E′). Normally, at E8.5 the neural tube at the levels of the hindbrain and anterior spinal cord is largely closed and TROMAI-positive SE cells and N-cadherin-positive NE cells are exclusively separated into surface and neural tube layers in wild-type embryos (Fig. 6D). However, β-gal-positive Grhl3 heterozygous mutant cells within the SE region ectopically expressed N-cadherin in addition to TROMAI (Fig. 6E).
Fig. 6.
SE in the neural plate border is converted into the NE fate in the Grhl3 mutant embryos. (A–C′) Lineage analysis of Grhl3 expression in Grhl3+/cre-nZ (A–C) and Grhl3 cre-nZ/cre-nZ (A′–C′) embryos at E8.25 with X-gal staining. Transverse sections counterstained with eosin (C, C′). (D, E, E′) Immunohistochemical analyses of whole embryo (D) and frozen section (E, E′) at E8.25 in wild-type (D), Grhl3cre-nZ/+ (E), and Grhl3cre-nZ/cre-nZ (E′) embryos. β-gal (GRHL3, green), N-cadherin (magenta), TROMAI (blue), and DAPI (cyan). β-gal-positive cells express N-cadherin in addition to TROMAI within the SE layer in Grhl3 heterozygous mutant embryos (E). β-gal-positive cells are mislocalized within the NE tissue in Grhl3 null embryos (E′, white arrowheads). (F, F′) Immunohistochemical analyses of whole embryos at E8.5 in wild-type (F) Grhl3cre-nZ/cre-nZ (F′). DAPI (green), N-cadherin (blue), and TROMAI (magenta) in F and F′. N-cadherin-negative and TROMAI-negative cells correspond to uncommitted neural border progenitor cells (green; arrows in F and F′). (G–M′) Immunohistochemical analysis with a neural crest marker, SOX9 using whole-mount embryos (G–J′) and frozen sections (K–M′) in Grhl3cre-nZ/+ (G–M) and Grhl3cre-nZ/cre-nZ (G′–M′). SOX9 (yellow), β-gal (GRHL3, green), TROMAI (magenta) and nuclei (DAPI, blue). Regarding cell behaviors of the SOX9-positive neural crest, a clear difference was not detected between heterozygous and homozygous mutant embryos. Scale bars: 300 μm in A, A′; 200 μm in D–F′; 100 μm in C, C′, G–J′; 20 μm in K–M′.
Moreover, β-gal-positive Grhl3-deficient cells were often observed within NE tissues in Grhl3cre-nZ/cre-nZ embryos and appeared to express N-cadherin but not TROMAI (Fig. 6E′). These results suggest that β-gal-positive cells appeared to specify into NE cells when the Grhl3 gene dosage was reduced, and thus that Grhl3-deficient cells tend to be mis-specified into the NE fate. In addition, uncommitted progenitor cells, which are specified into neither NE nor SE and which are marked by TROMAI-negative and N-cadherin-negative cells, were increased in Grhl3-deficient embryos (Fig. 6F′).
To determine whether GRHL3-positive cells are relevant to neural crest cells, we investigated SOX9 expression (Fig. 6G–M′). Most of the GRHL3/β-gal-positive cells did not contribute Sox9-expressing neural crest cells in the Grhl3 heterozygous or homozygous mutant embryos at the level of the forebrain and caudal spinal cord (Fig. 6G–M′). In addition, these neural crest cells appeared to be unaffected by Grhl3-deficiency (Fig. 6G–M′). Taken together, the present results indicate that Grhl3 primarily participates in SE fate specification in the neural plate border in conjunction with the epithelial fusion of neural folds.
3.6. Grhl3 Interacts Genetically with the Canonical Wnt Pathway in Terms of SE Specification
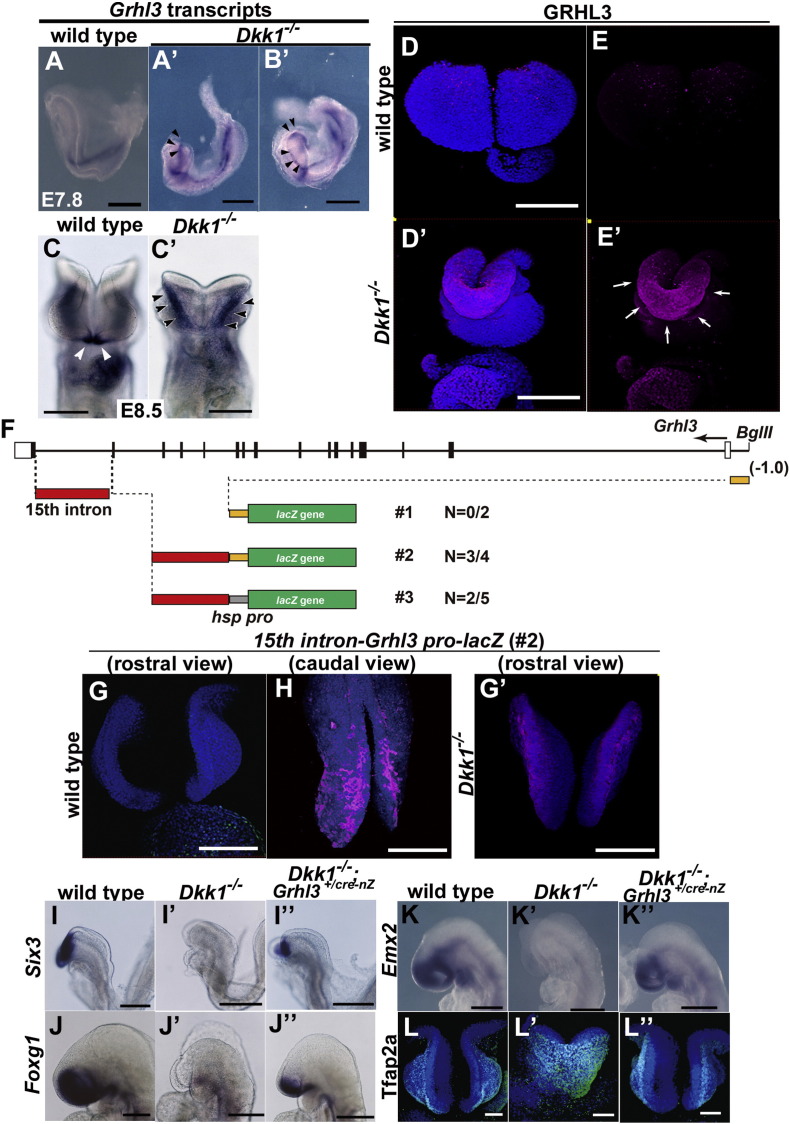
To validate whether Grhl3 expression is controlled by Wnt signaling, we first analyzed this expression in Dkk1-deficient embryos (Fig. 7A–E′). In the Dkk1−/− embryos, the Grhl3 transcripts and protein products were ectopically expanded to the entire neural plate border at the forebrain level (Fig. 7A–E′; arrowheads in A′–C′; arrows in E′). Next, to investigate whether the regulatory mechanism by Wnt signaling depends on the transcription level through cis-elements of the Grhl3 gene, we surveyed the mouse Grhl3 intron 15th, which is highly conserved among vertebrates and carries the enhancer activity in the zebrafish periderm cells at the early gastrula stage (de la Garza et al., 2013).
Fig. 7.
The expression of the Grhl3 gene in the neural plate border is controlled by Dkk1 during primary neurulation. (A–C′) In situ hybridization of whole-mount embryos at E7.8 (A–B′) and E8.5 (C, C′). Expression of Grhl3 transcripts in wild-type (A, C) and Dkk1−/− (A′–C′). (D, E′) Immunohistochemistry of whole-mount wild-type (D, E) and Dkk1−/− embryos (D′, E′). GRHL3 (magenta) and nuclei (DAPI, blue). Both Grhl3 transcripts and proteins are ectopically expressed in the rostral neural plate border (A′–E′). (F) Schematic diagram of the mouse Grhl3-endogeneous (from − 1.0 kb to 0)/or hsp promoter construct. The 15th intron region (2.35 kb; SalI–BglII), which is conserved among vertebrates, fused with the β-gal cassette. (G, G′, H) The expression of β-gal proteins carrying the transgenic #2 construct appears to recapitulate the endogenous Grhl3 expression in the wild-type (G, H) and Dkk1−/− (G′) embryos. (I–L″) The reduction of Grhl3 gene dosage can partly rescue the forebrain defects in Dkk1−/− embryos at E8.25 (I–I″) and E8.5 (J–L″). Whole-mount in situ hybridization (I–K″) and immunohistochemistry (L–L″) in wild-type (I–L), Dkk1−/− (I′–L′), and Dkk1−/−;Grhl3+/cre-nZ (I″–L″). Six3 (I–I″), Foxg1 (J–J″), Emx2 (K–K″), and TFAP2A (L–L″). Scale bars: 300 μm in A–C′, I–K″; 200 μm in D–E′, G, G′, H; 100 μm in L–L″.
The transgene construct that harbors the conserved Grhl3 15th intron region linked to a reporter gene, lacZ, was constructed, after which transgenic mouse lines were generated and the β-gal reporter activity of F1 transgenic embryos was analyzed (Fig. 7F–G′). Consequently, the transgene displayed the enhancer activity in the SE at the levels of the hindbrain and spinal cord, but not at that of the forebrain, which appears to recapitulate the endogenous Grhl3-lacZ knocked-in expression patterns at E8.25 (Figs. 6A–C, 7G, H). To further test whether this enhancer activity can be affected by Dkk1 expression, we examined the reporter activity in the Dkk1-deficient-background and found that it was enhanced in the SE of the forebrain as well as in those of the hindbrain and spinal cord (Fig. 7G′). These precise expression studies together suggest that Grhl3 expression in the neural plate border during neural tube closure is regulated by canonical Wnt signaling at the level of transcription.
To further investigate whether phenotypic defects in the Dkk1-deficient embryos are partly brought about by the ectopic expression of the Grhl3 gene due to increased canonical Wnt signaling, we crossed Grhl3 mutant mice with Dkk1 mutant mice and examined whether a reduction in the Grhl3 gene dosage could rescue the anterior defects of Dkk1-deficient embryos (Fig. 7I–L″). Consequently, anterior NE marked by Six3, Foxg1, and Emx2 was partly restored in Dkk1−/− embryos by reducing one copy of the Grhl3 gene (Fig. 7I″–K″). Concurrent with the alteration in NE markers, the expanded expression of Tfap2a, an SE marker at the level of the forebrain (Fig. 7L′), was reduced toward the normal condition in the Dkk1−/−; Grhl3+/cre-nZ embryos (Fig. 7L″). These findings indicate that the reduction in Grhl3 activity can partly compensate for a failure in anterior development due to Dkk1 deficiency. They also support the idea that Dkk1 promotes NE specification by repressing Grhl3 expression in the neural plate border.
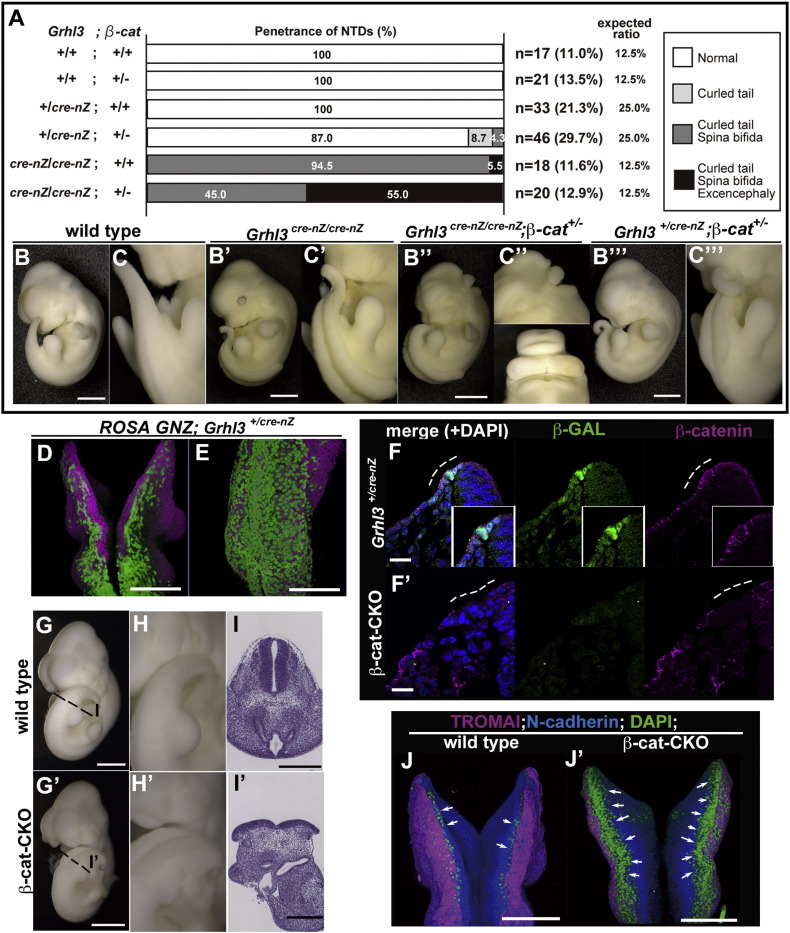
To test the relationship between canonical Wnt signaling and Grhl3 genetically in terms of neural tube closure, we generated the compound mutant Grhl3; β-catenin by crossing double heterozygous mice (Fig. 8A–C‴). The Grhl3+/cre-nZ embryos did not exhibit neural tube defects, whereas the Grhl3cre-nZ/cre-nZ embryos displayed neural tube closure defects; all embryos had thoraco-lumbo-sacral spina bifida and a curled tail (100%), but only a small fraction of embryos also exhibited exencephaly (5.5%) (Fig. 8A, B′, C′) (Rifat et al., 2010, Ting et al., 2003). The Grhl3cre-nZ/cre-nZ; β-catenin+/− embryos exhibited fully penetrant spina bifida, similar to Grhl3cre-nZ/cre-nZ alone, but more than half of the embryos displayed exencephaly (55.0%) (Fig. 8A, B″, C″). In addition, Grhl3+/cre-nZ; β-catenin+/− mutant embryos showed the thoraco-lumbo-sacral spina bifida phenotype, although penetrance was not high (curled tail 8.7%; curled tail and spina bifida 4.3%) (Fig. 8A, B‴, C‴). The enhanced manifestations of neural tube defects in the Grhl3 and β-catenin double-mutant embryos provide compelling evidence of a positive genetic interaction between the canonical Wnt pathway and Grhl3. Our data further support the notion that canonical Wnt signaling is epistatic to the Grhl3 gene.
Fig. 8.
Grhl3 genetically interacts with β-catenin for the SE specification. (A) The number of E11.5 embryos of each genotype from mating crosses between Grhl3+/cre-nZ; β-catenin+/− and Grhl3+/cre-nZ mice and the frequency with which penetrance shows neural tube defects, respectively (n = 155). (B–C‴) Neural tube defects of the compound mutants of Grhl3; β-catenin. Gross appearance of Grhl3 mutant and Grhl3; β-catenin double-mutant embryos. Wild-type (B, C), Grhl3cre-nZ/cre-nZ (B′, C′), Grhl3cre-nZ/cre-nZ; β-catenin+/− (B″, C″), and Grhl3+/cre-nZ; β-catenin+/− (B‴, C‴). Grhl3; β-catenin double heterozygous mutant embryos exhibit spina bifida (B‴, C‴), whereas the Grhl3 single heterozygous mutant has no defects by appearance (A). Moreover, the Grhl3cre-nZ/cre-nZ; β-catenin+/− embryos display exencephaly in addition to spina bifida and curled tail (A, B″, C″). (D, E) Detection of Grhl3-cre recombinase-active cells labeled by GFP protein expression (green) with whole-mount immunohistochemistry. Grhl3-cre-nZ mice, which were produced by the targeted insertion of the Cre gene into the Grhl3 locus, are crossed with ROSA-GNZ mice (Stoller et al., 2008). Dorsal views of doubly transgenic offspring showing GFP expression only in the SE, not in the NE at the hindbrain (D) and trunk (E) levels. (F, F′) The production of β-catenin deletion in Grhl3-positive SE by crossing β-cateninflox/+ or β-cateninflox/flox with Grhl3-cre-nZ mice. β-gal (GRHL3, green), β-catenin (magenta), and nuclei (DAPI, blue). The expression of β-catenin proteins is specifically lost in the Grhl3-β-gal-positive cells of the conditional knockout embryo (F, F′; dotted line). (G–I′) The gross morphology (G–H′) and histology (I, I′) in the wild-type (G–I) and conditional β-catenin knockout (G′–I′) embryos at E10.5. β-catenin conditional knockout embryos show spina bifida (G′–H′). (J, J′) Immunohistochemistry of β-catenin conditional knockout embryos. TROMAI (magenta), N-cadherin (blue), and nuclei (DAPI, green). Progenitor cells, which express neither NE nor SE markers (green), are expanded by β-catenin conditional mutation (J′; arrows). Scale bars: 1 mm in B–C‴; 500 μm in G, G′; 300 μm in I, I′; 200 μm in D, E, J, J′; 20 μm in F, F′.
To further confirm whether β-catenin itself plays crucial roles in neural tube closure specifically within the GRHL3-positive uncommitted progenitor cells, we analyzed the conditional knockout (CKO) mutation of floxed-β-catenin driven by Cre recombinase from the Grhl3 locus (Fig. 8D–J′). Consistent with the notion that Grhl3 is expressed only in the prospective SE of the neural plate border at E8.5 (Fig. 5, Fig. 8D–F), β-catenin expression was specifically lost in the SE but not the NE of the neural plate border (Fig. 8F′). β-catenin-CKO embryos displayed spina bifida closely resembling that of the Grhl3 cre-nZ/cre-nZ embryos (Fig. 8B′, C′, G′–I′).
In agreement with the above hypothesis that canonical Wnt signaling regulates the fate specification of uncommitted progenitors in the neural plate border, in the β-catenin-CKO embryos these progenitor cells, which are marked as TROMAI-negative and N-cadherin-negative cells, appeared to be increased compared to the wild type (Fig. 8J′). Together with the above two lines of evidence that Grhl3 expression in the neural plate border is regulated at the transcriptional level by the canonical Wnt signaling (Fig. 7A–G′) and that the defects in fate specification observed in the Dkk1-deficient embryos can be restored by the removal of Grhl3 gene dosage (Fig. 7I–L″), these findings strongly suggested that Grhl3 and β-catenin genetically interact to achieve correct closure of the neural tube primarily in the fate specification of neural plate border cells. These results also support that the Wnt antagonists DKK1 and KREMEN1 may be required to maintain an uncommitted progenitor state and/or to keep away from SE specification for the neural plate border, whereas canonical Wnt participates in SE specification by activating Grhl3 expression (Fig. 9, see Discussion).
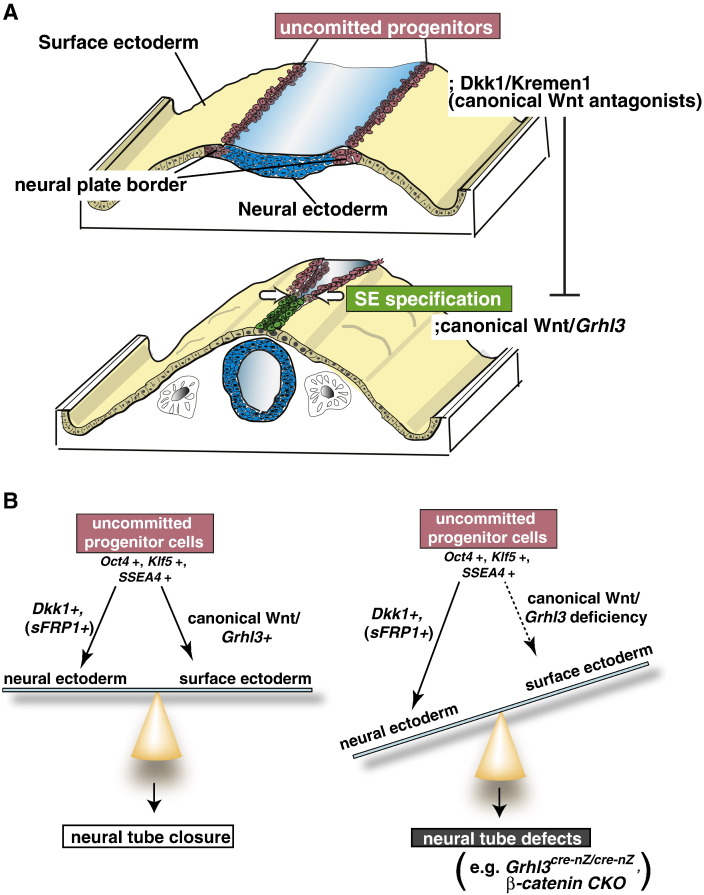
Fig. 9.
A schematic model of fate specification of uncommitted progenitors in the neural plate border/neural folds being coordinately coupled to neural tube closure. (A) A schematic model illustrating an intimate link between fate specification of the uncommitted progenitor cells and neural tube closure. Prior to the fusion of neural folds, the neural plate border cells between the NE (in blue) and SE (in yellow), which express DKK1/KREMEN1 (in red), are not specified into either the NE or SE fate as an uncommitted progenitor state marked by OCT4, KLF5 and SSEA4 (upper illustration). During the progression of neural tube closure, GRHL3-positive cells (in green) directed by canonical Wnt emerge coordinately in the vicinity of the neural plate border toward the identical direction of the neuropore closure, and then DKK1/KREMEN1-positive cells (in red) become restricted to the opposite side, where the neuropore is still open (lower illustration). (B) At the midline fusion of the neural folds, neural plate border cells lose the stem cell-like character and progressively specify into either SE or NE, regulated by canonical Wnt and its antagonists, respectively. In the Grhl3cre-nZ/cre-nZ and β-catenin conditional knockout embryos, uncommitted progenitor cells are expanded in the neural plate border and consequently they result in hyperplasia of NE with hypoplasia of SE, and consequently, the neural tube fails to close correctly.
4. Discussion
In this study, we observed genetic mechanisms underlying the fate specification of uncommitted progenitor cells in the neural plate border/neural fold coordinately coupled to neural tube closure in both time and space (Fig. 9). Notably, the Grhl3 transcription factor can promote the SE fate as one of the downstream effectors of canonical Wnt signaling. Given that Grhl3 directly contributes to neural tube closure genetically interacting with β-catenin, we propose that the fate specification of uncommitted progenitor cells in the neural plate border by canonical Wnt signaling and Grhl3 is crucial for neural tube closure (Fig. 9).
The results of the present study showed that the specification of progenitor cells into the NE or SE fate is progressively controlled in both time and space by canonical Wnt and its antagonists during neural tube closure (Fig. 9A). Prior to the fusion of neural folds, the neural plate border possesses an uncommitted progenitor character such as Oct4, Klf5 and SSEA4 expression, which is specified into neither NE nor SE, and loses this undifferentiated character at the time of fusion (Fig. 1). Wnt antagonists DKK1/KREMEN1 contribute to the maintenance of the uncommitted progenitor character and/or to the avoidance of the SE specification by canonical Wnt during neurulation (Fig. 1, Fig. 3, Fig. 4). The properly balanced cell fate specification between NE and SE cells within the neural plate border during neurulation might lead to a progressive midline fusion of the neural folds and coincidental separation of the neural tube and SE, whereas a failure in cell fate specification in the neural plate border/neural fold might lead to neural tube defects (Fig. 9B).
β-Catenin-CKO mutation within the neural plate border, where the SE fate specification is altered, results in neural tube defects (Fig. 8G′–J′). Additionally, the gain and loss of Dkk1 expression appear to affect the timing of neural tube closure (Fig. S2; unpublished data; C.K.-Y. and I.M.). Thus, we propose that the precise progressive mechanisms of fate specification in uncommitted progenitors involving canonical Wnt and its antagonists may determine the correct neural tube closure via epithelial development in the vertebrate embryo (Fig. 9).
Considering that cranial neural crest cells are delaminating from the non-neural ectoderm in the vicinity of the neural plate border/neural fold prior to neural tube closure in the mouse (Nichols, 1987, Weston and Thiery, 2015), it can be questioned whether the neural crest is relevant to uncommitted progenitor cells in the neural plate border and whether its population is primarily affected in our studies. Our study also supports the previous finding that a very few cranial neural crest cells appear to delaminate from uncommitted progenitors within the neural plate border at the forebrain level and from a considerable amount of neural crest cells at the hindbrain level (Fig. 2F, G). However, given that SOX9-negative uncommitted progenitor cells are still present at both the forebrain and hindbrain levels and that trunk neural crest cells emigrate from the dorsal neural tube after the completion of the fate specification of uncommitted progenitors (Fig. 2E–I), the decision of fate specification into neural crest cells appears to be not directly linked to neural tube closure. Additionally, Grhl3-positive cells apparently contribute to few neural crest cells in the Grhl3 heterozygous and homozygous mutant embryos (Fig. 6G–M′).
Concurrent with these cell behaviors, the formation of neural crest cells appeared not to be affected by Dkk1-misexpression or by Dkk1 and Grhl3 deficiency (Fig. 6G′–M′, Figs. S6, S7). Hence, although the fate decisions of neural crest cells appear to be controlled by stage-dependent differential responsiveness to canonical Wnt signaling activation after emigration in the mouse embryo (Hari et al., 2012), it is likely that the altered fate specification through canonical Wnt observed in this study is not due primarily to alterations of the fate decision of the neural crest.
Grhl3 has been shown to play crucial roles in neural tube closure (Gustavsson et al., 2008, Rifat et al., 2010). However, there is little understanding of how Grhl3 expression is controlled during primary neurulation. In the present study, Grhl3 was shown to be one of the pivotal downstream effectors of canonical Wnt signaling for SE specification in uncommitted progenitors of the neural plate border/neural fold (Fig. 6, Fig. 7, Fig. 8, Fig. 9). This is in good agreement with the notion that Grainyhead transcription factors play conserved roles in epidermal differentiation in the animal kingdom (Wang and Samakovlis, 2012). Disruption of Xenopus Grhl1 leads to defects in epidermal differentiation, as evidenced by the loss of expression of the keratin gene (Tao et al., 2005). Similarly, Xenopus Grhl3 activates specific markers of superficial epidermal cells in deep proliferating progenitor cells (Chalmers et al., 2006). Mouse Grhl1-deficient embryos display defects in skin and hair, partly due to the reduction of desmosomal cadherins (Wilanowski et al., 2008). Consistent with that finding, in the present study the reduction of Grhl3 activity appeared to inhibit SE specification (Fig. 6, Fig. 7, Fig. 8); i.e., neural plate border cells marked by Grhl3-expression tend to transform into NE cells in Grhl3-mutant embryos. These convergent lines of evidence support the idea that Grhl3-positive cells will differentiate into an SE fate by trans-activating SE-specific genes in a cell autonomous fashion and, conversely, that Grhl3-negative cells will tend to become NE cells in the neural plate border.
Grhl3 plays crucial roles in the specification of SE fate of the uncommitted neural plate border for neural tube closure. The epithelial fusion and remodeling allow the reorganization of two multicellular structures spanning an anatomical and physiological gap to form a new, unified structure. The process of epithelial fusion involves cellular protrusions that extend from the leading edges of apposing SE cells (Waterman, 1976, Geelen and Langman, 1979, Pyrgaki et al., 2010). Indeed, fine structures of SE cells of the neural plate border appeared to be affected in the Dkk1-misexpressing embryos (Fig. S3). Given that apposing epithelial contact seems to be specific within neural folds, these cellular behaviors may be initiated by Grhl3 as a crucial downstream effector of canonical Wnt signaling.
Considering our hypothesis that the fate specification of uncommitted progenitors is crucial for neural tube closure, it can be argued that Grhl3-deficient embryos display neural tube defects solely at the level of the caudal spinal cord and not at the forebrain and trunk levels (Rifat et al., 2010, Ting et al., 2003). We assume that the roles of Grhl3 in fate specification might be complemented by Grhl2 expression redundantly at the forebrain level (Fig. S8). To explore this possibility, we examined Grhl2 expression in the Grhl3 mutant background and found that at the forebrain level, Grhl2 expression extended locally to the most medial SE (i.e., the neural plate border/neural fold) in the Grhl3-deficient embryos (Fig. S8A–D, E–H′), whereas at the caudal level the compensatory Grhl2 expression in the neural plate border/neural fold is not evident (Fig. S8A–H).
Consistent with our hypothesis, the Grhl3-deficient embryos displayed cranial neural tube defects by the removal of one copy of the Grhl2 gene dosage (Rifat et al., 2010). In addition, our misexpression studies with Grhl2 revealed that Grhl2 was able to induce the SE fate ectopically in the NE region (Fig. S8H′–P′), confirming the compensatory function of Grhl2 in place of Grhl3 for fate specification during neurulation. Consequently, we consider that the absence of both Grhl2 and Grhl3 expression in the neural plate border will cause neural tube defects.
Thus, together with the expression of stem cell markers and Wnt antagonists in the neural plate border (Fig. 1, Fig. 3), these findings lead us to propose that the properly balanced fate specification of uncommitted progenitor cells in the neural plate border/neural fold by canonical Wnt and one of its downstream effectors, Grhl3, may be crucial for correct neural tube closure (Fig. 9).
Funding
This work was supported in part by a grant-in-aid for Scientific Research on Priority Areas and on Innovative Areas and Scientific Research (B) and (C) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, KAKENHI Grant Numbers, 24116713 26291053, 25461720; and by the Naito Foundation, the Mitsubishi Foundation, the Uehara Foundation and the Takeda Science Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' Contributions
CK-Y and IM initiated the research and planned most of the experiments. KE and CN generated the mouse Dkk1flox/flox strain. CK-Y and KM performed most of the experiments. CK-Y, CN, and IM contributed to the writing of the manuscript.
Conflict of Interest Disclosure
The authors have declared that they have no conflict of interest.
Acknowledgments
We are grateful to Dr. L. Niswander for her critical reading of our manuscript; to Drs. G. Martin, B. Hogan, H. Sasaki, A. McMahon, P. Gruss, C. Leimeister, R. Lovell-Badge, H. Clever, P. Koopman, K.A. Mahon, R. Grosschedl, H. Niwa and S. Vainio for the plasmids; and to the Research Support Center, Graduate School of Medical Sciences, Kyushu University for the technical support.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.04.012.
Contributor Information
Chiharu Kimura-Yoshida, Email: chiharu@mch.pref.osaka.jp.
Isao Matsuo, Email: imatsuo@mch.pref.osaka.jp.
Appendix A. Supplementary data
Supplementary material.
References
- Auden A., Caddy J., Wilanowski T., Ting S.B., Cunningham J.M., Jane S.M. Spatial and temporal expression of the Grainyhead-like transcription factor family during murine development. Gene Expr. Patterns. 2006;6:964–970. doi: 10.1016/j.modgep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Camerer E., Barker A., Duong D.N., Ganesan R., Kataoka H., Cornelissen I., Darragh M.R., Hussain A., Zheng Y.W., Srinivasan Y., Brown C., Xu S.M., Regard J.B., Lin C.Y., Craik C.S., Kirchhofer D., Coughlin S.R. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev. Cell. 2010;18:25–38. doi: 10.1016/j.devcel.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers A.D., Lachani K., Shin Y., Sherwood V., Cho K.W., Papalopulu N. Grainyhead-like 3, a transcription factor identified in a microarray screen, promotes the specification of the superficial layer of the embryonic epidermis. Mech. Dev. 2006;123:702–718. doi: 10.1016/j.mod.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Cruciat C.M., Niehrs C. Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza G., Schleiffarth J.R., Dunnwald M., Mankad A., Weirather J.L., Bonde G., Butcher S., Mansour T.A., Kousa Y.A., Fukazawa C.F., Houston D.W., Manak J.R., Schutte B.C., Wagner D.S., Cornell R.A. Interferon regulatory factor 6 promotes differentiation of the periderm by activating expression of Grainyhead-like 3. J. Invest. Dermatol. 2013;133:68–77. doi: 10.1038/jid.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen J.A., Langman J. Ultrastructural observations on closure of the neural tube in the mouse. Anat. Embryol. 1979;156:73–88. doi: 10.1007/BF00315716. [DOI] [PubMed] [Google Scholar]
- Glinka A., Wu W., Delius H., Monaghan A.P., Blumenstock C., Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Grocott T., Johnson S., Bailey A.P., Streit A. Neural crest cells organize the eye via TGF-beta and canonical Wnt signalling. Nat. Commun. 2011;2:265. doi: 10.1038/ncomms1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson P., Copp A.J., Greene N.D. Grainyhead genes and mammalian neural tube closure. Birth Defects Res. A Clin. Mol. Teratol. 2008;82:728–735. doi: 10.1002/bdra.20494. [DOI] [PubMed] [Google Scholar]
- Hari L., Miescher I., Shakhova O., Suter U., Chin L., Taketo M., Richardson W.D., Kessaris N., Sommer L. Temporal control of neural crest lineage generation by Wnt/beta-catenin signaling. Development. 2012;139:2107–2117. doi: 10.1242/dev.073064. [DOI] [PubMed] [Google Scholar]
- Kimura C., Takeda N., Suzuki M., Oshimura M., Aizawa S., Matsuo I. Cis-acting elements conserved between mouse and pufferfish Otx2 genes govern the expression in mesencephalic neural crest cells. Development. 1997;124:3929–3941. doi: 10.1242/dev.124.20.3929. [DOI] [PubMed] [Google Scholar]
- Kimura C., Yoshinaga K., Tian E., Suzuki M., Aizawa S., Matsuo I. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev. Biol. 2000;225:304–321. doi: 10.1006/dbio.2000.9835. [DOI] [PubMed] [Google Scholar]
- Kimura-Yoshida C., Nakano H., Okamura D., Nakao K., Yonemura S., Belo J.A., Aizawa S., Matsui Y., Matsuo I. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev. Cell. 2005;9:639–650. doi: 10.1016/j.devcel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Lawson K.A., Meneses J.J., Pedersen R.A. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- Mitchell L.E. Epidemiology of neural tube defects. Am. J. Med. Genet. C: Semin. Med. Genet. 2005;135C:88–94. doi: 10.1002/ajmg.c.30057. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M., Shtrom S., Rodriguez-Esteban C., Chen L., Tsukui T., Gomer L., Dorward D.W., Glinka A., Grinberg A., Huang S.P., Niehrs C., Izpisua Belmonte J.C., Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev. Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- Murry C.E., Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nagy A., Gertsenstein M., Vinterstein K., Behringer R.R. 3rd edition. Cold Spring Harbor Laboratory Press; 2003. Manipulating the Mouse Embryo — A Laboratory Manual. [Google Scholar]
- Nichols D.H. Ultrastructure of neural crest formation in the midbrain/rostral hindbrain and preotic hindbrain regions of the mouse embryo. Am. J. Anat. 1987;179:143–154. doi: 10.1002/aja.1001790207. [DOI] [PubMed] [Google Scholar]
- Patthey C., Gunhaga L. Specification and regionalisation of the neural plate border. Eur. J. Neurosci. 2011;34:1516–1528. doi: 10.1111/j.1460-9568.2011.07871.x. [DOI] [PubMed] [Google Scholar]
- Pietila I., Ellwanger K., Railo A., Jokela T., Barrantes Idel B., Shan J., Niehrs C., Vainio S.J. Secreted Wnt antagonist Dickkopf-1 controls kidney papilla development coordinated by Wnt-7b signalling. Dev. Biol. 2011;353:50–60. doi: 10.1016/j.ydbio.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Pyrgaki C., Trainor P., Hadjantonakis A.K., Niswander L. Dynamic imaging of mammalian neural tube closure. Dev. Biol. 2010;344:941–947. doi: 10.1016/j.ydbio.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrgaki C., Liu A., Niswander L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev. Biol. 2011;353:38–49. doi: 10.1016/j.ydbio.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifat Y., Parekh V., Wilanowski T., Hislop N.R., Auden A., Ting S.B., Cunningham J.M., Jane S.M. Regional neural tube closure defined by the Grainy head-like transcription factors. Dev. Biol. 2010;345:237–245. doi: 10.1016/j.ydbio.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T., Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Shimokawa K., Kimura-Yoshida C., Nagai N., Mukai K., Matsubara K., Watanabe H., Matsuda Y., Mochida K., Matsuo I. Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev. Cell. 2011;21:257–272. doi: 10.1016/j.devcel.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Steventon B., Mayor R., Streit A. Neural crest and placode interaction during the development of the cranial sensory system. Dev. Biol. 2014;389:28–38. doi: 10.1016/j.ydbio.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller J.Z., Degenhardt K.R., Huang L., Zhou D.D., Lu M.M., Epstein J.A. Cre reporter mouse expressing a nuclear localized fusion of GFP and beta-galactosidase reveals new derivatives of Pax3-expressing precursors. Genesis. 2008;46:200–204. doi: 10.1002/dvg.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmiller T.J., Garcia-Castro M.I. Current perspectives of the signaling pathways directing neural crest induction. Cell. Mol. Life Sci. 2012;69:3715–3737. doi: 10.1007/s00018-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Kuliyev E., Wang X., Li X., Wilanowski T., Jane S.M., Mead P.E., Cunningham J.M. BMP4-dependent expression of Xenopus Grainyhead-like 1 is essential for epidermal differentiation. Development. 2005;132:1021–1034. doi: 10.1242/dev.01641. [DOI] [PubMed] [Google Scholar]
- Ting S.B., Wilanowski T., Auden A., Hall M., Voss A.K., Thomas T., Parekh V., Cunningham J.M., Jane S.M. Inositol- and folate-resistant neural tube defects in mice lacking the epithelial-specific factor Grhl-3. Nat. Med. 2003;9:1513–1519. doi: 10.1038/nm961. [DOI] [PubMed] [Google Scholar]
- Tzouanacou E., Wegener A., Wymeersch F.J., Wilson V., Nicolas J.F. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev. Cell. 2009;17:365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Wang S., Samakovlis C. Grainy head and its target genes in epithelial morphogenesis and wound healing. Curr. Top. Dev. Biol. 2012;98:35–63. doi: 10.1016/B978-0-12-386499-4.00002-1. [DOI] [PubMed] [Google Scholar]
- Waterman R.E. Topographical changes along the neural fold associated with neurulation in the hamster and mouse. Am. J. Anat. 1976;146:151–171. doi: 10.1002/aja.1001460204. [DOI] [PubMed] [Google Scholar]
- Werth M., Walentin K., Aue A., Schonheit J., Wuebken A., Pode-Shakked N., Vilianovitch L., Erdmann B., Dekel B., Bader M., Barasch J., Rosenbauer F., Luft F.C., Schmidt-Ott K.M. The transcription factor grainyhead-like 2 regulates the molecular composition of the epithelial apical junctional complex. Development. 2010;137:3835–3845. doi: 10.1242/dev.055483. [DOI] [PubMed] [Google Scholar]
- Weston J.A., Thiery J.P. Pentimento: neural crest and the origin of mesectoderm. Dev. Biol. 2015;401:37–61. doi: 10.1016/j.ydbio.2014.12.035. [DOI] [PubMed] [Google Scholar]
- Wilanowski T., Caddy J., Ting S.B., Hislop N.R., Cerruti L., Auden A., Zhao L.L., Asquith S., Ellis S., Sinclair R., Cunningham J.M., Jane S.M. Perturbed desmosomal cadherin expression in grainy head-like 1-null mice. EMBO J. 2008;27:886–897. doi: 10.1038/emboj.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.