Abstract
Background
Accelerated telomere shortening may cause cancer via chromosomal instability, making it a potentially useful biomarker. However, publications on blood telomere length (BTL) and cancer are inconsistent. We prospectively examined BTL measures over time and cancer incidence.
Methods
We included 792 Normative Aging Study participants with 1–4 BTL measurements from 1999 to 2012. We used linear mixed-effects models to examine BTL attrition by cancer status (relative to increasing age and decreasing years pre-diagnosis), Cox models for time-dependent associations, and logistic regression for cancer incidence stratified by years between BTL measurement and diagnosis.
Findings
Age-related BTL attrition was faster in cancer cases pre-diagnosis than in cancer-free participants (pdifference = 0.017); all participants had similar age-adjusted BTL 8–14 years pre-diagnosis, followed by decelerated attrition in cancer cases resulting in longer BTL three (p = 0.003) and four (p = 0.012) years pre-diagnosis. Longer time-dependent BTL was associated with prostate cancer (HR = 1.79, p = 0.03), and longer BTL measured ≤ 4 years pre-diagnosis with any (OR = 3.27, p < 0.001) and prostate cancers (OR = 6.87, p < 0.001).
Interpretation
Age-related BTL attrition was faster in cancer cases but their age-adjusted BTL attrition began decelerating as diagnosis approached. This may explain prior inconsistencies and help develop BTL as a cancer detection biomarker.
Keywords: Telomere, Longitudinal study, Cancer incidence
Highlights
-
•
Normal, age-related telomere attrition is faster in subjects who later developed cancer.
-
•
Telomere attrition began decelerating before cancer diagnosis, resulting in longer telomeres 3–4 years pre-diagnosis.
-
•
Longer telomeres measured within four years of diagnosis were associated with all-cancer incidence.
1. Introduction
Telomeres are tandem repeats of TTAGGG nucleotides at the ends of eukaryotic chromosomes that, along with telomere binding proteins, help maintain genomic stability (Ma et al., 2011). Studies show that blood telomere length (BTL) decreases with age and that environmental exposures causing oxidative stress and chronic inflammation accelerate this process (Jennings et al., 2000, von Zglinicki, 2002). Shortened telomeres are often involved in cellular senescence or apoptosis. However, if their shortening becomes critical, such biological responses can be inhibited, resulting in genomic instability (Kong et al., 2013, Frias et al., 2012) including chromosomal rearrangements, and both gains and losses of chromosomal segments (Lundblad and Szostak, 1989), all essential steps in carcinogenesis. For these reasons, telomeres have long been an object of study for potential early involvement in cancer development (Londono-Vallejo, 2008, DePinho, 2000). One major weakness to tissue-specific telomere length in tumors is that it is only measurable after disease development, and thus can be affected by both cancer and treatment.
Blood leukocytes play an important role in carcinogenesis via inflammatory response and pro-apoptotic processes. Leukocyte infiltration is critical early in carcinogenesis and has been linked to many cancers including pancreatic (Schnekenburger et al., 2008) and colorectal (Ichikawa et al., 2011). Thus, studying BTL in DNA collected before cancer development can provide important information on its role in cancer etiology and serve a valuable predictive purpose. However, BTL has been extensively studied in relation to cancer risk with inconsistent results (Hou et al., 2012a, Willeit et al., 2010). One possible explanation is that most studies reporting shorter BTL in cancer patients relative to controls are retrospective studies in which BTL was measured post-diagnosis, a finding which could be a consequence of cancer development or treatment, not a cause (Hou et al., 2012a). For example, Unryn et al. showed that patients with neck and head tumors who went through eight weeks of chemotherapy had a mean telomere loss of 660 base pairs (Unryn et al., 2006). Results have also been inconsistent in prospective studies where BTL was measured pre-diagnostically, some reporting increased cancer risk in participants with shorter BTL, and others with longer (Hou et al., 2012a). Most studies examined BTL at a single time point only, and none to our knowledge measured BTL more than once before cancer diagnosis, making it difficult to examine the causal relationship between BTL attrition and cancer risk. Longitudinal studies of BTL with multiple pre-diagnostic measurements may be more informative about how BTL contributes to cancer risk, and provide critical information on the relationship between BTL and cancer development and diagnosis. Our objective is to examine BTL attrition over time in relation to risk of developing cancer, specifically: 1) How BTL changes with time affect, and are affected by, cancer development and 2) whether BTL measured prior to clinical diagnosis is associated with risk of developing cancer.
2. Methods
2.1. Study Design and Participants
The Normative Aging Study (NAS) was established by the US Department of Veteran Affairs (VA) in 1963 with an initial cohort of 2280 healthy men. Initial eligibility criteria at enrollment included veteran status, residence in the Boston area, ages 21–80, and no history of hypertension, heart disease, cancer, diabetes, or other chronic health conditions. From 1963 to 1999, 981 participants died and 470 were lost to follow up. Statistical comparisons between the remaining 829 participants and those lost to follow up revealed no significant differences in subject characteristics (age, BMI, etc.). Participants were recalled for clinical examinations every 3–5 years. Starting in 1999, these included 7-ml blood samples for DNA analysis. Between January 1st, 1999 and December 31st, 2012, 802/829 (96.7%) of active participants agreed to donate blood. Our study population included participants who had 1–4 clinical visits during which blood was collected, and non-missing data for BTL from at least one of those visits, resulting in a total population of 792. Of these, 227 (28.7%) participants had data from one visit, 202 (25.5%) from two, 229 (28.9%) from three, and 134 (16.9%) from four. This study was approved by the Institutional Review Boards of all participating institutions, and all participants provided written consent.
2.2. Identification of Cancer Cases
Information on cancer diagnosis was obtained from questionnaires and confirmed via review of medical records. Among the 792 participants, 213 were diagnosed with cancer (75 prostate, 97 skin, 41 other) before their first blood draw (baseline). After examining associations between BTL and prevalent cancers, these participants were excluded, and subsequent analyses only examined pre-diagnostic BTL measurements. Among the remaining 579 participants free of cancer at baseline, 135 new cancer cases occurred (53 prostate, 42 skin, 10 lung, nine leukemia, five bladder, four colon, two stomach, two liver, two pancreas, and six unspecified) during median 10.6 year follow-up. Participants' mean age at diagnosis was 75.9 ± 6.6 years. Participants who were cancer-free for the full duration of the study were censored after their last recorded visit.
2.3. Telomere Measurement
BTL was measured using quantitative real-time polymerase chain reaction (qPCR) (Cawthon, 2002). Relative BTL was measured by the ratio of the telomere (T) repeat copy number to single-copy gene (S) copy number (T:S ratio) in a given sample and reported as relative units expressing the ratio between test DNA BTL and reference pooled DNA BTL. The latter was created using DNA from 475 participants randomly selected (400 ng per sample) and used to generate a fresh standard curve from 0.25 to 20 ng/μL in every T and S PCR run. qPCR primer sets for T and human beta-globin, taken as the reference S, as well as qPCR mix composition were previously described (Hou et al., 2009). We ran all samples in triplicate, and the average of the three T measurements was divided by the average of the three S measurements to calculate the average T:S ratio. The intra-assay coefficient of variation for the T/S ratio was 8.1%. The average coefficient of variation for the T reaction was 8%, and for the S reaction was 5.6%. When the coefficient of variation for T or S reactions was higher than 15%, the measurement was repeated.
2.4. Statistical Analysis
After our initial descriptive analysis of BTL and subject characteristics by visit, we performed a second descriptive analysis using a repeated measures study to examine associations between participant characteristics at baseline and cumulative mean BTL (BTL averaged across all visits) among cancer-free participants only. Next, we used linear mixed-effects models to compare rates of BTL attrition over time by cancer status (those who developed cancer at some point during follow-up, and those who did not). BTL attrition rate was examined relative to increasing age, and age-adjusted BTL attrition was also examined relative to decreasing years pre-diagnosis (pre-censoring in the case of cancer-free subjects). Next, we used Cox proportional hazards regression models to estimate time-dependent associations between BTL and time to diagnosis/censoring. Finally, based on our above analysis, we performed logistic regression of BTL and cancer stratified by time between BTL measurement and diagnosis/censoring (≤ 4, 4–8 and > 8 years).
All multivariable models adjusted for age at baseline, race, education, BMI, smoking status, pack-years of smoking, and alcohol consumption. For ease of tabular presentation, continuous variables were categorized into tertiles but retained in continuous form for all analyses. We examined the effect of adjusting for white blood cell count and proportion neutrophils, but including these variables did not appreciably affect our results, prompting their exclusion. We also excluded participants missing any data for outcome, BTL, or covariates. Figures were generated using R v3.0.2 and all other analyses used SAS (version 9.3, SAS Institute). We used two-sided tests to compare means and BTL attrition rates, and set a statistical significance threshold of p = 0.05.
2.5. Funding Source
This study was funded by the Epidemiology Research and Information Center, U.S. Department of Veterans Affairs; NIEHS R01-ES015172. Additional funding support was provided by the Northwestern University Robert H. Lurie Comprehensive Cancer Center Rosenberg Research Fund. The funding institutions had no role in the study design, data collection or analysis, interpretation of findings, manuscript preparation, or decision to pursue peer-review publication.
3. Results
Characteristics of study participants were similar to previous reports (Zhu et al., 2011). Participants were male, with mean age of 72 at baseline (range 55–100), and mostly white (535/579 or 95.5%). BTL decreased over time among participants free of cancer at baseline, from mean BTL of 1.26 ± 0.48 units at baseline to 0.86 ± 0.25 units at the fourth visit (Table 1). See Supplemental Fig. 1 for a spaghetti plot of individual participants' BTL trajectory across the study. We found no significant associations between BTL and prevalent cancers (data available upon request).
Table 1.
Characteristics of study participants free of cancer at baseline by follow-up visit.
| First visit |
Second visit |
Third visit |
Fourth visit |
|
|---|---|---|---|---|
| Mean ± SD/n (%) | ||||
| N | 579 | 371 | 226 | 77 |
| Mean BTL (units) | 1.26 ± 0.48 | 1.24 ± 0.56 | 1.04 ± 0.45 | 0.86 ± 0.25 |
| Age (years) | 71.82 ± 6.80 | 74.20 ± 6.32 | 76.72 ± 6.23 | 77.23 ± 5.24 |
| BMI (kg/m2) | 28.26 ± 4.14 | 28.20 ± 4.26 | 27.82 ± 4.17 | 27.79 ± 4.07 |
| Pack-years of smoking | 20.87 ± 24.17 | 19.97 ± 24.19 | 18.41 ± 22.71 | 13.81 ± 18.58 |
| Race | ||||
| White | 553(95.5%) | 351(94.6%) | 211(93.4%) | 72(93.5%) |
| Non-White | 26(4.5%) | 20(5.4%) | 15(6.6%) | 5(6.5%) |
| Education (years) | ||||
| < 13 | 201(34.7%) | 126(34.0%) | 72(31.9%) | 25(32.5%) |
| 13–15 | 95(16.4%) | 59(16.0%) | 36(15.9%) | 14(18.2%) |
| > 15 | 283(48.9%) | 186(50.1%) | 118(52.2%) | 38(49.4%) |
| Smoking status | ||||
| Never | 159(27.5%) | 104(28.0%) | 73(32.3%) | 29(37.7%) |
| Current | 26(4.5%) | 15(4.0%) | 6(2.7%) | 2(2.6%) |
| Former | 394(68.1%) | 252(67.9%) | 147(65.0%) | 46(59.7%) |
| Average alcohol consumption | ||||
| 0–1 drinks/day | 475(82.0%) | 305(82.2%) | 191(84.5%) | 65(84.4%) |
| 2 + drinks/day | 104(18.0%) | 66(17.8%) | 35(15.5%) | 12(15.6%) |
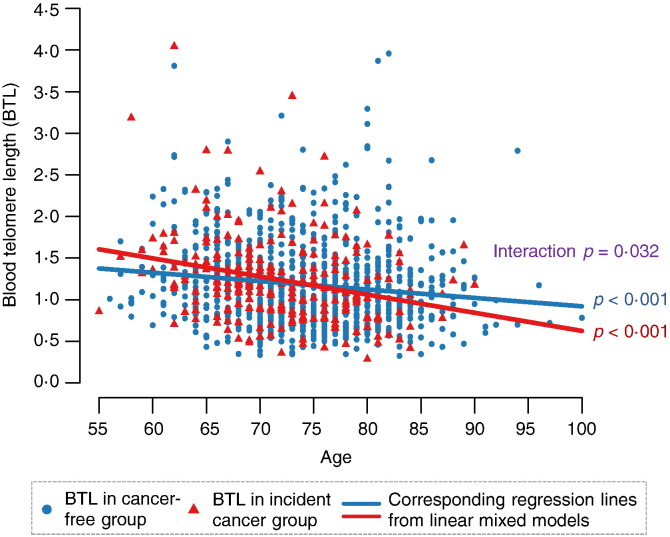
In cancer-free participants, those who were older (p = 0.010), better-educated (p = 0.007) and of white race (p = 0.017) tended to have shorter BTL (Table 2). Participants with an incident cancer diagnosis had accelerated BTL attrition as measured in pre-diagnostic blood draws (β = − 0.022 units/year, p < 0.001) compared to cancer-free participants (β = − 0.010 units/year, p < 0.001). The unadjusted mean between-group difference in BTL attrition rate was − 0.012 units/year (p = 0.032) (Fig. 1). These results were similar after adjusting for covariates, with BTL attrition rate of − 0.022 in participants with incident cancer, − 0.011 units/year in cancer-free participants, and a mean between-group difference of − 0.013 units/year (p = 0.017).
Table 2.
Cumulative mean BTL of participants free of cancer throughout the follow-up period.
| Characteristics at baseline | n | Cum. Mean | p |
|---|---|---|---|
| Total | 444 | 1.19⁎ | |
| Age, years⁎⁎ | |||
| < 69 | 143 | 1.19 | 0.010⁎⁎⁎ |
| 69–76 | 154 | 1.13 | |
| > 76 | 147 | 1.11 | |
| BMI, kg/m2⁎⁎ | |||
| < 26.3 | 147 | 1.26 | 0.14 |
| 26.3—29.5 | 147 | 1.25 | |
| > 29.5 | 150 | 1.21 | |
| Pack-years of smoking⁎⁎ | |||
| 0 | 113 | 1.27 | 0.64 |
| 0.1–30 | 185 | 1.24 | |
| > 30 | 134 | 1.24 | |
| Race | |||
| White | 423 | 1.16 | 0.017⁎⁎⁎ |
| Non-White | 21 | 1.33 | |
| Education, years | |||
| < 13 | 166 | 1.26 | 0.007⁎⁎⁎ |
| 13–15 | 72 | 1.29 | |
| > 15 | 206 | 1.18 | |
| Smoking status | |||
| Never | 113 | 1.27 | 0.23 |
| Current | 20 | 1.14 | |
| Former | 309 | 1.25 | |
| Average alcohol consumption | |||
| 0–1 drinks/day | 367 | 1.28 | 0.24 |
| 2 + drinks/day | 77 | 1.23 |
Unadjusted value.
p-values calculated based on continuous variables.
Statistically significant at p < 0.05.
Fig. 1.
BTL by age and cancer status (unadjusted). After adjusting for covariates, mean BTL attrition rate for participants with later cancer was β = − 0.022 units/year, p < 0.001; mean BTL attrition rate for cancer-free participants was β = − 0.011 units/year, p < 0.001; mean between-group difference was β = − 0.013 units/year p = 0.017. Regression model was given by the equation: BTL = β0 + β1 ∗ Age + β2 ∗ Cancer Status + β3 ∗ Age ∗ Cancer Status for each subject and time point (adjusted for other covariates).
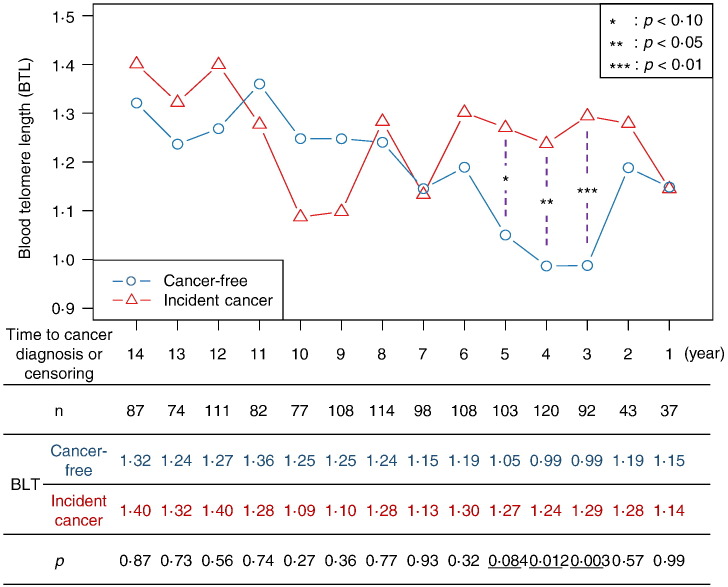
This trend in age-related BTL attrition reversed when it was examined relative to time to diagnosis/censoring. While age-adjusted attrition of BTL in participants who later developed cancer and those who did not was comparable 8–14 years pre-diagnosis, attrition rates in these two groups began to diverge starting seven years pre-diagnosis (ptrend < 0.001). This led to significantly longer mean BTL among participants with cancer compared to cancer-free participants at four (β = 0.25 units/year, p = 0.012) and three (β = 0.31 units/year, p = 0.003) years pre-diagnosis/censoring (Fig. 2).
Fig. 2.
Age-adjusted mean BTL by time to cancer diagnosis and cancer status. Participants free of cancer throughout the study follow-up period were censored either at date of death or last study visit. Regression model was given by the equation: BTL = β0 + β1 ∗ Years to Dx + β2 ∗ Cancer Status + β3 ∗ Age + β4 ∗ Years to Dx ∗ Cancer Status for each subject and time point (adjusted for other covariates).
Time-dependent BTL was positively associated with risk of developing prostate cancer (HR = 1.79, 95% CI: 1.14–2.80, p = 0.011) (Table 3). Based on the results in Fig. 2, we performed a stratified analysis by years pre-diagnosis/censoring. BTL measured within four years pre-diagnosis/censoring was positively associated with incidence of all cancers (OR = 3.27, 95% CI: 1.67–6.42; p = 0.0006), prostate cancer (OR = 6.87, 95% CI: 2.73–17.25; p = 0.0001), and other cancers (OR = 2.17, 95% CI: 1.02–4.59; p = 0.043).
Table 3.
BTL over time and risk of cancer.
| Full follow-up (1999–2012) |
Stratified by interval between BTL measurement and cancer diagnosis/censoring |
|||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 4 years |
4–8 years |
> 8 years |
||||||
| HR(95% CI) | p | OR(95% CI) | p | OR(95% CI) | p | OR(95% CI) | p | |
| Unadjusted results | ||||||||
| All cancer | 1.17(0.83–1.65) | 0.37 | 4.33(2.28–8.22) | 0.0001⁎ | 1.13(0.85–1.50) | 0.40 | 0.72(0.30–1.72) | 0.46 |
| Prostate cancer | 1.89(1.08–3.31) | 0.026⁎ | 6.44(2.79–14.88) | < 0.0001⁎ | 1.17(0.82–1.68) | 0.38 | 0.41(0.09–1.94) | 0.26 |
| Other cancer | 0.85(0.55–1.33) | 0.48 | 2.98(1.48–5.98) | 0.002⁎ | 1.08(0.78–1.50) | 0.64 | 0.95(0.34–2.61) | 0.92 |
| Multivariable-adjusted results | ||||||||
| All cancer | 1.17 (0.83–1.65) | 0.36 | 3.27 (1.67–6.42) | 0.0006⁎ | 1.03 (0.72–1.49) | 0.86 | 0.56 (0.23–1.39) | 0.21 |
| Prostate cancer | 1.89 (1.08–3.33) | 0.026⁎ | 6.87 (2.73–17.25) | 0.0001⁎ | 1.13 (0.72–1.79) | 0.59 | 0.37 (0.08–1.84) | 0.23 |
| Other cancer | 0.86 (0.55–1.35) | 0.51 | 2.17 (1.02–4.59) | 0.043⁎ | 1.03 (0.67–1.58) | 0.90 | 0.71 (0•24–2.06) | 0.52 |
Statistically significant at p < 0.05.
4. Discussion
After confirming the expected age-related BTL attrition, we found that cancer-free participants who were younger, nonwhite, and less educated tended to have longer mean BTL. Furthermore, age-related BTL attrition was accelerated among participants who ultimately developed cancer. Strikingly, this trend reversed when age-adjusted BTL was examined relative to time to diagnosis/censoring. We observed comparable age-adjusted BTLs 8–14 years pre-diagnosis between participants who ultimately developed cancer and those who did not, followed by a divergence of each group's mean age-adjusted BTL beginning seven years pre-diagnosis and culminating in significant higher BTL 3–4 years pre-diagnosis among cancer cases compared to cancer-free participants. Time-dependent BTL was positively associated with prostate cancer risk but not with risk of all cancers combined. However, BTL measured within four years of diagnosis was significantly associated with increased risk of all cancers combined and of prostate cancer.
Telomere length in prostate tissue decreases with cell division by ~ 100 base pairs per division due to incomplete replication (Meeker, 2006). Due to their high guanine content, telomeres are sensitive to damage from cumulative oxidative stress and chronic inflammation, resulting in telomeric single-strand breaks and loss of distal telomere fragments (Jennings et al., 2000, von Zglinicki, 2002). Thus, telomere attrition can serve as a marker of these major carcinogenic pathways. Conversely, promoting healthy behavior may serve to increase telomerase activity and telomere maintenance capacity, as shown in one pilot study (Ornish et al., 2008). In our study, we observed accelerated shortening of pre-diagnostic BTL in those who ultimately developed cancer as they age, suggesting that one mechanism by which cancer risk increases may be telomere shortening prior to cancer development. Alternatively, this acceleration in pre-diagnostic BTL attrition may be an early result of cancer development.
The divergence in age-adjusted BTL trajectory between participants who later developed cancer and those who did not several years pre-diagnosis is intriguing. This may reflect pre-diagnostic cancer, and explain previous inconsistent results in prospective studies of BTL and cancer. In particular, this suggests that the inconsistency may at least partially be caused by differences in sample collection time relative to cancer development and diagnosis. BTL maintenance is a complex process governed by a variety of telomere elongating and shortening processes, and BTL measurements are the result of balancing these processes. While telomere attrition can be accelerated by increased oxidative stress and inflammatory events (Jennings et al., 2000, von Zglinicki, 2002), telomere lengthening can occur when maintenance mechanisms are activated by critical BTL shortening, cancer development and treatment, etc. (Unryn et al., 2006) As cancer upsets this balance early in its development, the timing of BTL measurement relative to cancer's hijacking of telomere maintenance mechanisms may qualitatively affect the direction of any statistical associations calculated. In future studies of BTL and cancer risk, care should be taken to account for the timing of sample collection and BTL measurement relative to cancer development and diagnosis.
Although the role of BTL maintenance mechanisms in surrogate tissues like blood leukocytes remains largely unexplored in cancer patients, it is biologically plausible that critical BTL shortening (via age or increased oxidative stress or inflammation) may trigger telomere maintenance mechanisms to protect telomere integrity (Hodes et al., 2002). Our observation of decelerating age-adjusted BTL attrition in cancer cases as they approached diagnosis suggests that telomere-elongating mechanisms in blood leukocytes may also be activated by cancer initiation, leading to BTL elongation early during cancer development. In cancer tissues, this can occur via up-regulation of telomerase (Hackett and Greider, 2002, Shay et al., 2001) or other methods for lengthening telomeres after apoptosis would normally occur (Bryan et al., 1995). This finding confirms that cellular senescence induced by telomere shortening is a tumor suppressive process that must be overcome early in carcinogenesis (Giaimo and d'Adda di Fagagna, 2012), since ordinarily telomere length serves as an index of DNA repair potential and hematopoietic stem cell reserves (Aviv and Levy, 2012, Sidorov et al., 2009). Future studies to confirm these processes in blood leukocytes will facilitate the development of BTL (telomerase expression) (Lu et al., 2011) as a potential biomarker for early detection of cancer.
Telomere integrity is primarily maintained by telomerase, which catalyzes the synthesis of telomere repeats and adds telomere sequences onto chromosome ends (Hug and Lingner, 2006). Prior studies have reported that tumor and somatic cells with more frequent reproductive cycles, like leukocytes, have higher telomerase activity (Sampedro Camarena et al., 2007). Synthesis of telomere repeats by telomerase is believed necessary for the indefinite proliferation of tumor cells and growth, and telomerase activation significantly increases cellular lifespan and promotes carcinogenesis with multiple accelerated neoplasia (DePinho, 2000). This may explain our observed stabilization of age-adjusted BTL in cancer cases four years and less pre-diagnosis, and our finding elongated telomeres in prostate cancer patients. This result, coupled with our finding of accelerated age-related BTL shortening in cancer cases, suggests a complex and dynamic relationship between developing cancers and BTL. Biologically, this may be due to early carcinogenesis causing accelerated BTL shortening, followed by a hijacking of telomere elongation mechanisms by cancer at some point in its development.
In the present study, overall greater BTL measured 1–4 times over the 12-year follow-up was positively associated with risk of developing prostate cancer. Previous studies on the association between BTL and prostate cancer have been inconsistent (Meeker, 2006, Meeker et al., 2002, Mirabello et al., 2009, Hurwitz et al., 2014). The differences in results may be at least partially due to differences in study design and timing of blood sample collection. However, a recent and large prospective cohort study found a marginally significant reduction in the risk of developing prostate cancer associated with shorter BTL (Weischer et al., 2013). This is consistent with our findings regarding prostate cancer, though given our low number of prostate cancer cases the possibility of our finding being due to random chance (and/or our risk estimates being inflated) should not be ignored. However, other studies have also begun to examine the BTL–prostate cancer association by taking into account blood collection time relative to cancer diagnosis. Mirabello et al. reported in a prospective study that BTL measured in blood samples collected 3 or fewer years prior to diagnosis was not associated with prostate cancer risk (Mirabello et al., 2009). In a retrospective study, Hurwitz et al. showed that shorter BTL measured pre-diagnosis or within one year of cancer diagnosis was associated with increased risk of prostate cancer in the Hereditary Prostate Cancer (HPC) Families Project (Hurwitz et al., 2014). Our reported association between prostate cancer risk and BTL measured within four years of diagnosis adds to this contradiction, and suggests that future studies should examine prostate cancers in more detail with repeated measurements of BTL pre-diagnosis.
Time-dependent BTL was only associated with prostate cancer. However, our analyses stratified by timing of BTL measurement relative to cancer diagnosis found associations between both all incident cancers and prostate cancer, and longer BTL measured ≤ 4 years pre-diagnosis. This suggests (as above) that BTL attrition acts as a tumor suppressive process that must be overcome early in carcinogenesis. Interestingly, one longitudinal study of telomere length and cancer development (albeit a cancerous complication of lymphoma treatment) found a similar temporal pattern, with increasing BTL in cases before and immediately (100 days) after treatment, followed by accelerated telomere attrition in cancer cases compared to controls (Chakraborty et al., 2009). The complexity of the relationship between BTL attrition and cancer incidence found here is also similar to that reported in our previous study of BTL and air pollution, where short-term pollution exposure increased BTL but long-term exposure decreased it (Hou et al., 2012b). This underscores the complex “give and take” at the heart of telomere regulation, and reiterates the importance of timing BTL collection relative to diagnosis (and potentially exposure and treatment).
Our study's longitudinal nature enabled us to establish temporal associations between BTL and cancer risk using multiple measurements in relation to cancer incidence. However, our findings should be confirmed in future studies. Our prospective measurement of BTL helped avoid biases often encountered in cross-sectional studies. However, there were also limitations in our study. Our study participants were all male and mostly Caucasian, thus studies of females and non-Caucasians are warranted to confirm our findings more broadly. Our sample size limited our ability to analyze specific cancer subtypes other than prostate cancer. Thus, caution should be exercised in interpreting our results as different cancer subtypes have different biological mechanisms, and our low sample size increases the possibility of our findings being due to random chance and/or our measures of association being artificially high. Future, larger studies of multiple cancer subtypes are necessary to help confirm the value of BTL as a universal cancer biomarker.
In summary, BTL declined with age in both cancer-free participants and cancer cases but more rapidly among the latter. However, relative to approaching cancer diagnosis, age-adjusted BTL attrition decelerated in cancer cases, ultimately yielding significantly elongated BTL and suggesting that critical BTL shortening may contribute to cancer initiation which then, in turn, activates telomere maintenance mechanisms to compensate and further promote cancer. Thus, our results may help explain the inconsistent results of previous studies and provide more insight into using BTL as an early detection biomarker of cancer.
The following is the supplementary data related to this article.
Spaghetti plot of individual BTL trajectories.
Author Contributions
LH supervised the study and directed the analytic plan and manuscript production. BTJ completed manuscript preparation, assisted with data analysis. TG assisted with manuscript preparation and performed most of the data analysis for the study. LL provided biostatistical expertise for all data analysis. YZ assisted with data analysis. FJP assisted with manuscript preparation and developing the study design. SL assisted with data analysis. WZ assisted with data analysis and manuscript preparation. RB assisted with manuscript preparation and developing an analytic plan. DQ assisted with manuscript preparation and developing an analytic plan. PV supervised the Normative Aging Study cohort. MH conducted the telomere measurement and analysis. JS provided the initial data set for use in the study, consulted on the analytic plan, and assisted with manuscript preparation. AB supervised the telomere length analyses, and consulted on the study design.
Declaration of Interests
LL reports personal fees from Celladon, Outcome Research Solutions, and Zensun, all outside the submitted work.
JS reports grants from the NIH (R01ES015172 (PI: Schwartz), R01ES021733 (PI: Baccarelli), and P30ES00002 (PI: Baccarelli)) during the course of the study.
The other authors have no potential conflicts of interest to report.
References
- Aviv A., Levy D. Telomeres, atherosclerosis, and the hemothelium: the longer view. Annu. Rev. Med. 2012;63:293–301. doi: 10.1146/annurev-med-050311-104846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan T.M., Englezou A., Gupta J., Bacchetti S., Reddel R.R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14(17):4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S., Sun C.L., Francisco L. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J. Clin. Oncol. 2009;27(5):791–798. doi: 10.1200/JCO.2008.17.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePinho R.A. The age of cancer. Nature. 2000;408(6809):248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- Frias C., Pampalona J., Genesca A., Tusell L. Telomere dysfunction and genome instability. Front. Biosci. 2012;17:2181–2196. doi: 10.2741/4044. [DOI] [PubMed] [Google Scholar]
- Giaimo S., d'Adda di Fagagna F. Is cellular senescence an example of antagonistic pleiotropy? Aging Cell. 2012;11(3):378–383. doi: 10.1111/j.1474-9726.2012.00807.x. [DOI] [PubMed] [Google Scholar]
- Hackett J.A., Greider C.W. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene. 2002;21(4):619–626. doi: 10.1038/sj.onc.1205061. [DOI] [PubMed] [Google Scholar]
- Hodes R.J., Hathcock K.S., Weng N.P. Telomeres in T and B cells. Nat. Rev. Immunol. 2002;2(9):699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- Hou L., Savage S.A., Blaser M.J. Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiol. Biomarkers Prev. 2009;18(11):3103–3109. doi: 10.1158/1055-9965.EPI-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Zhang X., Gawron A.J., Liu J. Surrogate tissue telomere length and cancer risk: shorter or longer? Cancer Lett. 2012;319(2):130–135. doi: 10.1016/j.canlet.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Hou L., Wang S., Dou C. Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: a repeated-measure study. Environ. Int. 2012;48:71–77. doi: 10.1016/j.envint.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug N., Lingner J. Telomere length homeostasis. Chromosoma. 2006;115(6):413–425. doi: 10.1007/s00412-006-0067-3. [DOI] [PubMed] [Google Scholar]
- Hurwitz L.M., Heaphy C.M., Joshu C.E. Telomere length as a risk factor for hereditary prostate cancer. Prostate. 2014;74(4):359–364. doi: 10.1002/pros.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M., Williams R., Wang L., Vogl T., Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol. Cancer Res. 2011;9(2):133–148. doi: 10.1158/1541-7786.MCR-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings B.J., Ozanne S.E., Hales C.N. Nutrition, oxidative damage, telomere shortening, and cellular senescence: individual or connected agents of aging? Mol. Genet. Metab. 2000;71(1–2):32–42. doi: 10.1006/mgme.2000.3077. [DOI] [PubMed] [Google Scholar]
- Kong C.M., Lee X.W., Wang X. Telomere shortening in human diseases. FEBS J. 2013;280(14):3180–3193. doi: 10.1111/febs.12326. [DOI] [PubMed] [Google Scholar]
- Londono-Vallejo J.A. Telomere instability and cancer. Biochimie. 2008;90(1):73–82. doi: 10.1016/j.biochi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Lu L., Zhang C., Zhu G. Telomerase expression and telomere length in breast cancer and their associations with adjuvant treatment and disease outcome. Breast Cancer Res. 2011;13(3):R56. doi: 10.1186/bcr2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad V., Szostak J.W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57(4):633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- Ma H., Zhou Z., Wei S. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One. 2011;6(6):e20466. doi: 10.1371/journal.pone.0020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker A.K. Telomeres and telomerase in prostatic intraepithelial neoplasia and prostate cancer biology. Urol. Oncol. 2006;24(2):122–130. doi: 10.1016/j.urolonc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Meeker A.K., Hicks J.L., Platz E.A. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62(22):6405–6409. [PubMed] [Google Scholar]
- Mirabello L., Huang W.Y., Wong J.Y. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8(4):405–413. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornish D., Lin J., Daubenmier J. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- Sampedro Camarena F., Cano Serral G., Sampedro Santalo F. Telomerase and telomere dynamics in ageing and cancer: current status and future directions. Clin. Transl. Oncol. 2007;9(3):145–154. doi: 10.1007/s12094-007-0028-1. [DOI] [PubMed] [Google Scholar]
- Schnekenburger J., Schick V., Kruger B. The calcium binding protein S100A9 is essential for pancreatic leukocyte infiltration and induces disruption of cell–cell contacts. J. Cell. Physiol. 2008;216(2):558–567. doi: 10.1002/jcp.21433. [DOI] [PubMed] [Google Scholar]
- Shay J.W., Zou Y., Hiyama E., Wright W.E. Telomerase and cancer. Hum. Mol. Genet. 2001;10(7):677–685. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- Sidorov I., Kimura M., Yashin A., Aviv A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp. Hematol. 2009;37(4):514–524. doi: 10.1016/j.exphem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Unryn B.M., Hao D., Gluck S., Riabowol K.T. Acceleration of telomere loss by chemotherapy is greater in older patients with locally advanced head and neck cancer. Clin. Cancer Res. 2006;12(21):6345–6350. doi: 10.1158/1078-0432.CCR-06-0486. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Weischer M., Nordestgaard B.G., Cawthon R.M., Freiberg J.J., Tybjaerg-Hansen A., Bojesen S.E. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J. Natl. Cancer Inst. 2013;105(7):459–468. doi: 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- Willeit P., Willeit J., Mayr A. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Zhu Z.Z., Sparrow D., Hou L. Repetitive element hypomethylation in blood leukocyte DNA and cancer incidence, prevalence, and mortality in elderly individuals: the Normative Aging Study. Cancer Causes Control. 2011;22(3):437–447. doi: 10.1007/s10552-010-9715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spaghetti plot of individual BTL trajectories.