Abstract
Objective
High salt intake is known to be the most pivotal environmental factor in the pathogenesis of hypertension. However, the association of high sodium intake with central hemodynamics in hypertensive subjects has not been well defined. Here, we determined the association of estimated 24-hour urine sodium and potassium excretion estimated from a spot urine analysis with parameters of central pulse wave analysis in 515 hypertensive subjects.
Methods
Fasting spot urine samples were obtained in the early morning after the first void, and estimated 24-hour urine sodium and potassium excretion were estimated from measurement of urine sodium, potassium, and creatinine. Central hemodynamics and arterial stiffness parameters were assessed via pulse wave analysis of the radial artery.
Results
The estimated 24-hour sodium and potassium excretion values were 150 ± 40 and 49 ± 10 mEq, respectively. There was a step-wise decrease in pulse pressure amplification with increasing estimated 24-hour urine sodium excretion. Multiple linear regression analyses revealed that both estimated 24-hour urine sodium excretion and sodium/potassium ratio were independently associated with increases in central pulse pressure, augmented aortic pressure, augmentation index and were inversely associated with pulse pressure amplification.
Conclusion
The estimated 24-hour urinary sodium excretion is independently associated with central hemodynamics. This may provide the basis for prospective interventional studies of epidemiologic scale to determine the potential beneficial effects of reduced salt consumption on central hemodynamics.
Keywords: Hypertension, sodium, pulse pressure amplification, central pulse pressure
Introduction
Although brachial blood pressure (BP) has been used clinically as a surrogate marker of a patient’s usual BP for 100 years, central arterial pressure more closely reflects the pressure load imposed on the heart and the cerebral circulation[1]. Recent studies have demonstrated that central BP is independently, and more closely, associated with adverse cardiovascular outcomes than brachial BP[2-5]. Differences between central and peripheral BP are due to peripheral pulse wave amplification, which is itself a marker of cardiovascular risk[6]. Factors that cause augmentation of central systolic BP, such as increased pulse wave velocity due to arterial stiffness, or increased magnitude of reflected waves due to peripheral vascular remodeling, may act to decrease pulse pressure amplification (PPA)[6].
High salt intake has been shown to be the most pivotal environmental factor in the pathogenesis of hypertension[7]. An increase in dietary salt imposes an oxidative stress on the arterial wall, inducing pressure-independent changes in arterial structure and function[8]. Therefore, increased sodium intake may result in adverse remodeling of the central and peripheral arterial vasculature that underlies central pressure augmentation. The association of high sodium intake and central hemodynamics in hypertensive subjects, however, has not been defined. In this study, we sought to determine whether 24-hour urine sodium and potassium excretion, estimated from a spot urine analysis, are associated with parameters of central hemodynamics in 515 hypertensive subjects.
Materials and Methods
Study population
The study consisted of 515 hypertensive patients, diagnosed and treated at the Cheil General Hospital of Kwandong University, and evaluated prior to receiving prescriptions for antihypertensive medications. We recruited hypertensive subjects with a systolic BP greater than 140 mmHg and/or a diastolic BP greater than 90 mmHg after at least 5 minutes at rest in a sitting position, over at least two different visits, and prior to taking antihypertensive medication. Exclusion criteria included valvular heart disease, peripheral vascular disease, significant systemic disease, history of inflammatory disease and/or treatment with anti-inflammatory medications, clinically significant atrioventricular conduction disturbances, history of atrial fibrillation or other serious arrhythmias, severe hypertension (>210/130 mmHg), or serum creatinine levels greater than 1.4 mg/dL. The presence of valvular heart disease was ruled out by echocardiography. Peripheral vascular disease was diagnosed on the basis of overt symptoms such as claudication.
This study received prior approval from the Institutional Ethics Committee, and the procedures followed are in accordance with Institutional guidelines. All patients provided informed consent.
Spot urine sodium, potassium, and creatinine measurements
Fasting spot urine samples were obtained from study subjects in the early morning after the first void. Twenty-four hour urine sodium and potassium excretion were estimated from spot urine sodium (mEq/L), potassium (mEq/L) and creatinine (mg/dL) as described previously[9]. Briefly, the predicted 24-hour creatinine (PRCr) was calculated as (−2.04 × age) + [14.89 × weight (kg)] + [16.14 × height (cm)] - 2244.45; the estimated 24-hour sodium excretion (Na mEq/day) was calculated as 21.98 multiplied by XNa0.392; and the estimated 24-hour potassium excretion (K mEq/day) was calculated as 7.59 multiplied by XK0.431, where XNa (XK) = spot urine sodium (or potassium)/spot urine creatinine × PRCr.
BP measurements
After 5 minutes of rest, sitting brachial BP was measured three times at 2-minute intervals in the dominant arm with an OMRON 712C device, which has been validated by the British Hypertension Society[10]. The average of the last two measurements was used for statistical analyses.
Pulse wave analysis
Central hemodynamics and parameters of arterial stiffness were assessed in the sitting position after a minimum of 5 minutes of rest via pulse wave analysis of the radial artery using a commercially available radial artery tonometry device (SphygmoCor; AtCor Medical, Sydney, Australia). Peripheral pressure waveforms were recorded from the radial artery at the wrist by applanation tonometry using a high-fidelity micromanometer (Millar Instruments, Houston, TX, USA), as previously reported[11,12]. After 20 sequential waveforms had been acquired, a validated generalized transfer function was used to generate the corresponding central aortic pressures and pressure waveforms. Central systolic BP, diastolic BP, pulse pressure, augmentation pressure, forward wave amplitude, and augmentation index were derived from the pulse waveform analysis. Pulse pressure was calculated as the difference between systolic and diastolic pressure. Augmentation pressure is the difference between the second and first systolic peak pressures, and the augmentation index(AI) is defined as the ratio of augmentation pressure to aortic pulse pressure. In addition, given that the AI is influenced by heart rate, an augmentation index normalized for a heart rate of 75 beats/min (augmentation index at 75) was derived. PPA was calculated as the ratio of the peripheral to central pulse pressure. High quality recordings, defined as those with a within-device quality index greater than 90%, were derived from an algorithm that included average pulse height, pulse height variation, diastolic variation, and the maximum rate of rise of the peripheral waveform.
Statistical analysis
The results are expressed as mean ± standard deviation (SD). Discrete variables were compared using a chi-squared analysis, and continuous variables were analyzed using independent t-tests. Independent predictors of PPA, central pulse pressure, augmented aortic pressure, and AI were determined via a multiple linear regression analysis, using a standard, simultaneous regression method. Briefly, variables that were significant at the P < 0.10 level, based on a simple linear regression analysis, and/or those known to be significantly associated with augmentation index/central BP elevation, were entered into the multiple linear regression analysis. Because augmented aortic pressure was not normally distributed, it was log transformed for linear regression analysis. For comparison of pulse pressure amplification according to tertiles of 24 hour Na excretion, a one way ANOVA was performed with Bonferroni post hoc analysis. All statistical analyses were performed using SPSS v13.0 software (SPSS Inc., Chicago, IL, USA)
Results
Clinical characteristics
The baseline characteristics of the study population are shown in Table 1. The average age was 48.5 ± 11.0 years; 190 subjects were male and 325 were female. The average systolic/diastolic BP was 159.6 ± 15.8/98.5 ± 11.3 mmHg. Comparison of baseline clinical characteristics between genders demonstrated significant differences in age, smoking history, height, weight, body mass index, triglyceride, HDL cholesterol, and fasting blood sugar (Table 1). There were no significant gender differences in estimated 24-hour sodium or potassium excretion (Table 2). The estimated values for 24-hour sodium and potassium excretion were 150 ± 40 and 49 ± 10 mEq, respectively. Compared to men (Table 3), women had a significantly higher central augmented pressure, AI, AIHR75, central pulse pressure, central systolic BP and central diastolic BP, and a significantly lower PPA.
Table 1. Baseline clinical characteristics.
| Male (n = 190) | Female (n = 325) | P-value* | |
|---|---|---|---|
| Age (years) | 45.4 ± 12.4 | 50.3 ± 9.7 | < 0.001 |
| Office BP | |||
| SBP (mmHg) | 158.7 ± 17.1 | 160.1 ± 15.0 | 0.344 |
| DBP (mmHg) | 99.8 ± 12.7 | 97.8 ± 10.4 | 0.053 |
| Smoking (%) | 71 (37.4%) | 17 (5.2%) | < 0.001 |
| Height (cm) | 170.0 ± 6.5 | 155.7 ± 5.1 | < 0.001 |
| Weight (kg) | 75.1 ± 10.4 | 60.2 ± 8.7 | < 0.001 |
| BMI (kg/m2) | 25.9 ± 2.6 | 24.8 ± 3.3 | < 0.001 |
| T. chol (mg/dl) | 208.4 ± 34.2 | 211.8 ±3 4.4 | 0.287 |
| TG (mg/dl) | 167.2 ± 129.2 | 133.4 ± 78.3 | < 0.001 |
| HDL (mg/dl) | 51.9 ± 11.8 | 58.9 ± 15.1 | < 0.001 |
| LDL (mg/dl) | 124.0 ± 34.6 | 126.6 ± 32.8 | 0.394 |
| FBG (mg/dl) | 105.3 ± 25.9 | 100.5 ± 23.5 | 0.032 |
| hsCRP (mg/L) | 1.95 ± 3.08 | 1.63 ± 2.66 | 0.227 |
Values are presented as n (%) or mean ± SD.
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, Body Mass Index; T. chol, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FBG, fasting blood glucose; hsCRP, high-sensitivity C-reactive protein measured by high-sensitivity CRP test.
P-values < 0.05 are considered significant.
Table 2. Predicted 24-hour urine sodium and potassium excretion.
| Male (n = 190) | Female (n = 325) | P-value* | |
|---|---|---|---|
| SUCr (mg/dL) | 177 ± 99 | 123 ± 63 | < 0.001 |
| PRCr (mg/day) | 1525 ± 252 | 1061 ± 180 | < 0.001 |
| SUNa (mEq/L) | 139 ± 49 | 135 ± 49 | 0.355 |
| SUNa/SuCr × PRCr | 155 ± 105 (5–956) | 150 ± 41 (3–599) | 0.890 |
| PRNa (mEq/day) | 152 ± 39 (41–324) | 150 ± 41 (34–270) | 0.712 |
| SUK (mEq/L) | 82 ± 31 | 82 ± 42 | 0.977 |
| SUK/SUCr × PRCr | 81 ± 39 (22–384) | 78 ± 39 (13–417) | 0.521 |
| PRK (mEq/day) | 49 ± 9 (29–99) | 48 ± 9 (23–102) | 0.399 |
| PRNa/K | 2.83 ± 0.62 | 2.85 ± 0.70 | 0.732 |
SUCr, spot urine creatinine; SUNa, spot urine sodium; SUK, spot urine potassium; PRCr, predicted 24-hour urine creatinine value; PRNa, predicted 24-hour urine sodium value; PRK, predicted 24-hour urine potassium value; PRNa/K, predicted PRNa/K ratio.
Table 3. Pulse wave analysis.
| Male (n = 190) | Female (n = 325) | P-value* | |
|---|---|---|---|
| Central augmented pressure (mmHg) | 11.5 ± 8.0 | 19.0 ± 7.0 | < 0.001 |
| Forward wave amplitude (mmHg) | 32 ± 7.7 | 31 ± 7.5 | 0.201 |
| AI | 24.4 ± 13.3 | 37.3 ± 8.3 | < 0.001 |
| AI corrected for HR (AIHR75) | 23.6 ± 11.3 | 36.6 ± 6.9 | < 0.001 |
| CPP (mmHg) | 43.6 ± 12.5 | 50.1 ± 12.4 | < 0.001 |
| Central SBP (mmHg) | 144.8 ± 18.4 | 148.2 ± 16.7 | 0.033 |
| Central DBP (mmHg) | 101.2 ± 14.2 | 98.0 ± 11.8 | 0.007 |
| PPA | 1.35 ± 0.19 | 1.20 ± 0.09 | < 0.001 |
Values are presented as n (%) or mean ± SD.
AI, augmentation index; CPP, central pulse pressure; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; PPA, pulse pressure amplification.
Multiple linear regression
Partial correlations of 24-hour urine sodium and Na/K with indices of central hemodynamics after controlling for age, height, gender, smoking, mean peripheral BP, heart rate, fasting blood glucose(FBG) and total cholesterol indicated that estimated 24-hour Na/K had a slightly better correlation with PPA, central pulse pressure, augmented pressure and AI than did 24-hour urine sodium (Table 4).
Table 4. Partial correlation between 24-hour urine sodium and Na/K and parameters of pulse wave analysis.
| 24-hour urine Na | Correlation coefficient* | P-value*** |
|---|---|---|
| PPA * | −0.089 | 0.045 |
| CPP ** | 0.098 | 0.027 |
| AP ** | 0.130 | 0.004 |
| AI ** | 0.105 | 0.019 |
| 24 hour urine Na/K | Correlation coefficient* | P-value |
|---|---|---|
| PPA * | −0.099 | 0.027 |
| CPP ** | 0.110 | 0.014 |
| AP ** | 0.144 | 0.001 |
| AI ** | 0.121 | 0.007 |
PPA, pulse pressure amplification; CPP, central pulse pressure; AP, augmented pressure; AI, augmentation index.
Corrected for age, augmentation index, body mass index(BMI), gender, mean peripheral BP, heart rate, smoking, fasting blood glucose(FBG) and total cholesterol
Corrected for age, body mass index(BMI), gender, mean peripheral BP, heart rate, smoking, fasting blood glucose(FBG) and total cholesterol
P-values < 0.05 are considered significant.
Multiple linear regression analysis revealed that estimated 24-hour urine sodium excretion was independently associated with PPA, central pulse pressure, augmented aortic pressure and AI after controlling for age, BMI, gender, mean peripheral BP, smoking, heart rate, FBS and total cholesterol (Table 5). The association between PPA and sodium excretion was controlled for augmentation index in addition to age, height, gender, mean peripheral BP, smoking and heart rate. The estimated 24-hour urine Na/K was independently associated with PPA, central pulse pressure, augmented aortic pressure and AI (Table 5). Estimated 24-hour potassium excretion was not associated with parameters of pulse wave analysis in the multiple regression analysis (not shown in table). Also, estimated 24-hour sodium excretion was not associated with central mean BP when controlled for gender, age, BMI, smoking, total cholesterol, fasting blood sugar and heart rate(β= −0.019, P=0.666, not shown in table).
Table 5. Multiple linear regression analysis for associations between estimated 24-hour urine sodium excretion and Na/K with parameters of pulse wave analysis.
| Association between 24-hour sodium excretion with parameters of pulse wave analysis | |||
|---|---|---|---|
| Unstandardized | Standardized coefficient(β) | P-value * | |
|
| |||
| PPA (R2 = 0.818) a | −0.003±0.002 | −0.040 | 0.045 |
| CPP (R2 = 0.410) b | 0.509±0.230 | 0.079 | 0.027 |
| Log (AP) (R2 = 0.586) b | 0.026±0.009 | 0.088 | 0.004 |
| AI (R2 = 0.604) b | 0.423±0.179 | 0.069 | 0.019 |
|
| |||
| Unstandardized | Standardized coeffirient(β) | P-value * | |
| PPA (R2 = 0.819) a | −0.010±0.005 | −0.043 | 0.027 |
| CPP (R2 = 0.411) b | 1.622±0.656 | 0.086 | 0.014 |
| Log (AP) (R2 = 0.588) b | 0.081±0.025 | 0.095 | 0.001 |
| AI (R2 = 0.617) b | 1.397±0.511 | 0.078 | 0.007 |
PPA, pulse pressure amplification; CPP, central pulse pressure; AP, augmented pressure; AI, augmentation index.
P < 0.05 is considered significant
Controlled for age, augmentation index, BMI, mean peripheral blood pressure, heart rate, smoking, fasting blood sugar and total cholesterol
Controlled for age, BMI, mean peripheral blood pressure, heart rate, smoking, fasting blood sugar and total cholesterol
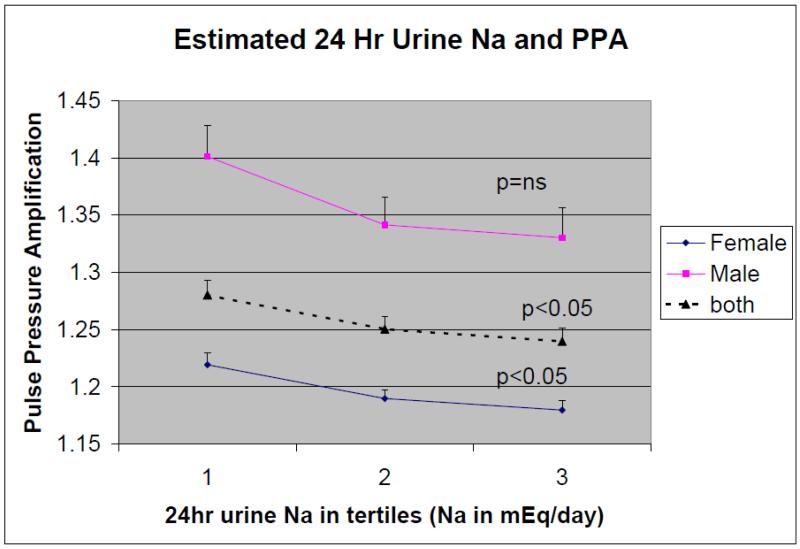
The estimated 24-hour urine sodium excretion for the entire cohort and by gender, stratified by tertiles and plotted against the PPA, is illustrated in Figure 1. A step-wise increase in estimated 24-hour urine sodium excretion in the entire cohort (P=0.036). The post hoc analysis demonstrated a tendency for the 2nd tertile (P=0.094) and 3rd tertile (P=0.064) to have significantly decreased PPA compared to the 1st tertile. In females, the decrease in PPA with increasing 24-hour urine sodium excretion is significant (P=0.038) with the post Hoc analysis demonstrating significant difference between the 3rd tertile and the 1st tertile (P=0.043). In males, although there was also a tendency for decrease in PPA with increasing 24-hour sodium excretion, but this did not reach statistical significance (P=0.093).
Figure 1. The association of estimated 24-hour urine sodium excretion, stratified into tertiles, with pulse pressure amplification in males and females.
Discussion
Recent results from the Conduit Artery Functional Endpoint study of the Anglo-Scandinavian Cardiac Outcomes Trial and the Strong Heart Study have highlighted the clinical importance of central BP in determining target organ damage and cardiovascular events[3,5]. In healthy young subjects, the stiffness of peripheral arteries is higher than that of central arteries. This difference in stiffness normally results in reflected pressure waves from the distal arterial tree, resulting in considerable amplification of pulse pressure between the aorta and brachial artery[13,14]. The key finding of the present study is that the estimated 24-hour excretion of urine sodium and Na/K are independently associated with PPA, central pulse pressure, augmented central pressure and AI in hypertensive subjects. To our knowledge, the present study is the first to demonstrate a significant association of urinary sodium excretion with central aortic pressure and PPA in a hypertensive study population although a study of the general African population by Redelinghuys et al. also demonstrated a significant association between urinary sodium excretion and indices of central BP, such as central pulse pressure, augmented pressure and central augmentation index[15].
Several factors are known to influence PPA. Unmodifiable factors, such as age, height and gender, may influence PPA[6]. Vascular properties, such as arterial stiffness, reflected pressure augmentation and peripheral resistance, also may influence PPA[16]. Heart rate has multiple effects on central arterial pressure: reduced heart rate augments central systolic BP, while an increase in heart rate is associated with an increase in central–peripheral pressure amplification that is dependent on the frequency of the transfer function[6,16-18]. Hemodynamic factors may influence PPA by modifying the frequency components of aortic pressure. The multiple regression analysis and a partial correlation analysis in this study showed that urine sodium is independently associated with PPA when corrected for both unmodifiable factors and modifiable hemodynamic factors that are known to influence PPA.
There are several possible mechanisms for the results observed in our study. First, increased sodium intake is associated with increased arterial stiffness. Both in vivo and clinical studies in humans have shown that increased salt consumption contributes to increased arterial stiffness independent of BP[19-21]. The resulting increase in pulse wave velocity and reduced reflected wave transit time increase the augmentation of central systolic BP. Dietary salt reduction can reduce arterial stiffness, as demonstrated in a study by He et al., in which a modest dietary salt reduction for 6 weeks resulted in a small, but significant, reduction in pulse wave velocity[22]. Second, increased sodium intake is associated with extracellular fluid volume expansion and the release of endogenous digitalis-like factors leading to an increase in intracellular sodium; these two factors may act in concert to induce vascular remodeling and increase peripheral vascular resistance[7]. The increase in peripheral vascular resistance may increase the magnitude of the reflected wave, resulting in an increased augmentation of central BP. Third, high sodium intake may be associated with up-regulation of renin–angiotensin–aldosterone activity[23,24]. The resulting increase in vascular inflammation and arterial wall remodeling in both peripheral and central arteries may collectively exert a deleterious effect on central hemodynamics.
Previous studies have demonstrated that the major determinants of pulse pressure are increased forward wave amplitude, early wave reflection (as determined by increased pulse wave velocity), and the magnitude of the reflected wave[25]. In the present study, 24-hour urine sodium and Na/K were not significantly associated with forward wave amplitude (P1)[Not in table]. These results demonstrate that an increase in sodium intake may increase PPA and central pulse pressure by increasing the magnitude of wave reflection, rather than by increasing forward wave amplitude. It is interesting to note that estimated 24-hour sodium excretion was not associated with central mean BP when controlled for gender, age, BMI, smoking, total cholesterol, fasting blood sugar and heart rate(β= −0.019, P=0.666, not shown in table). The results from this study suggest that increased salt intake is associated with increase in the pulsatile component of central BP, rather than the steady state central BP. Increased sodium intake is associated with adverse vascular remodeling and peripheral vascular rarefaction, which may act in concert to increase the magnitude of wave reflection and the pulsatile component of the central BP[7,26]. This finding may have clinical significance as a previous studies have shown that the pulsatile component of BP, rather than the steady state component, is associated with cardiovascular events[27,28].
In conclusion, we have found, in patients with untreated hypertension, that the estimated 24-hour urine sodium and urinary Na/K excretion are independently associated with an increase in central pulse pressure and a reduction in pulse pressure amplification (PPA). Prospective interventional studies, however, in patients with established hypertension are required to determine the effect of varying salt intake on central hemodynamics. More importantly, because an increase in dietary salt increases tissue renin-angiotensin-aldosterone activation that creates oxidative stress within the arterial wall that precedes hypertension, studies to determine the relationship of NaCl intake to arterial wall structure and function are of high priority with respect to global prevention of hypertension.
Study limitations
A limitation of this study is that the association of estimated 24-hour urine sodium with indices of central BP was its cross-sectional design. Prospective studies will be required to confirm the causal relation of salt intake to increased central BP, and prospective interventional studies are required to assess the potential direct beneficial effects of reduced salt consumption on central hemodynamics. Another limitation is the use of estimated 24 hour urine sodium excretion. Although 24-hour urine collection is the most accurate method for evaluating sodium intake, it is difficult to collect a complete and accurate 24-hour urinary sample. Therefore, we used a formula for estimating the 24-hour urinary sodium and potassium excretion rate that was developed based on a Japanese database as part of the INTERSALT study[9]. Because the formula for estimating 24-hour urine sodium excretion may be dependent on renal function, we excluded subjects with serum creatinine levels 1.4 mg/dL. According to a Report of the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension, “estimating sodium excretion from the Na/Cr ratio in spot urine is less reliable than directly measuring 24-hour urinary sodium, but it is a practical alternative suitable for general medical facilities”[29]. Although the formula for estimating sodium and potassium intake cannot supplant the 24-hour urine collection for estimating individual sodium intake, we believe it is useful for estimating sodium and potassium intake in a study population and for assessing the association of these factors with parameters measured by pulse wave analysis. Lastly, the association between estimated 24-hour sodium and indices of central hemodynamics are modest in this study. However, since there are numerous factors (aging, vascular stiffness, heart rate, renin angiotensin system, gender, peripheral vascular resistance) that can potentially influence the degree of central pulse pressure and pulse pressure amplification. A previous study by Redelinghuys et al showed a modest partial correlation coefficient between urinary sodium excretion and indices of central hemodynamics that was similar to the results from this study[15]. Therefore, we believe data such as this provides the basis for performing prospective interventional studies to determine the effect of varying salt intake on central hemodynamics.
Acknowledgements
This study was supported by a grant (2010-0020766) from the Happy Tech Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Republic of Korea, and in part by the Intramural Research Program of the NIH, National Institute on Aging. The authors would like to thank Ms. Eun Young Ju for her data management contribution.
Abbreviation and definition
- AI
augmentation index
- AP
augmented pressure
- BMI
Body Mass Index
- BP
blood pressure
- CPP
central pulse pressure
- DBP
diastolic blood pressure
- FBS
fasting blood sugar
- HDL
high-density lipoprotein
- hsCRP
high-sensitivity C-reactive protein measured by high-sensitivity CRP test
- HR
heart rate
- LDL
low-density lipoprotein
- P1
forward wave amplitude
- PPA
pulse pressure amplification
- PRCr
predicted 24-hour urine creatinine value
- PRNa
predicted 24-hour urine sodium value
- PRK
predicted 24-hour urine potassium value
- PRNa/K
predicted PRNa/K ratio
- SD
standard deviation
- SBP
systolic blood pressure
- SUCr
spot urine creatinine
- SUK
spot urine potassium
- SUNa
spot urine sodium
- T. chol
total cholesterol
- TG
triglyceride
References
- 1.O’Rourke MF, Adji A. Basis for use of central blood pressure measurement in office clinical practice. J Am Soc Hypertens. 2008;2:28–38. doi: 10.1016/j.jash.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, et al. High central pulse pressure is independently associated with adverse cardiovascular outcome. J Am Coll Cardiol. 2009;54:1730–1734. doi: 10.1016/j.jacc.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 4.Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol. 2008;51:2432–2439. doi: 10.1016/j.jacc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 5.The CAFÉ Investigators. on behalf of the Anglo-Scandinavian Cardiac Outcomes Trial(ASCOT) Investigators Differential impact of blood pressure-lowering drugs on cental aortic pressure and clinical outcomes. Principal results of the Conduit Artery Function Evaluation(CAFÉ) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 6.Benetos A, Thomas F, Joly L, Blacher J, Pannier B, Labat C, et al. Pulse pressure amplification: A mechanical biomarker of cardiovascular risk. J Am Coll Cardiol. 2010;55:1032–1037. doi: 10.1016/j.jacc.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 7.Adrugué HJ, Madias NE. Sodium and Potassium in the pathogenesis of Hypertension. N Engl J Med. 2007;356:1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 8.de Cavanagh EMV, Ferder LF, Ferder MD, Stella IY, Toblli JE, Inserra F. Vascular structure and oxidative stress in salt-loaded spontaneously hypertensive rats: Effects of Losartan and Atenolol. Am J Hypertens. 2010;23:1318–1325. doi: 10.1038/ajh.2010.167. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, Hashimoto T. A simple method to estimate populational 24h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. doi: 10.1038/sj.jhh.1001307. [DOI] [PubMed] [Google Scholar]
- 10.Graves JW. Blood Pressure Monit. 2005;10:103–107. doi: 10.1097/00126097-200504000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Weber T, O’Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–202. doi: 10.1038/ajh.2008.277. [DOI] [PubMed] [Google Scholar]
- 12.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 13.Latham RD, Westerhof N, Sipkema P, Rubal BJ, Reuderink P, Murgo J. Regional wave travel and reflections along the human aorta: a study with six simultaneous micromanometric pressures. Circulation. 1985;72:1257–1269. doi: 10.1161/01.cir.72.6.1257. [DOI] [PubMed] [Google Scholar]
- 14.Kroeker EJ, Wood EH. Comparison of simultaneously recorded central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res. 1955;3:623–632. doi: 10.1161/01.res.3.6.623. [DOI] [PubMed] [Google Scholar]
- 15.Redelinghuys M, Norton GR, Scott L, Maseko MJ, Brooksbank R, Majane OHI, et al. Relationship between urinary salt excretion and pulse pressure and central aortic hemodynamics independent of steady state pressure in the general population. Hypertension. 2010;56:584–90. doi: 10.1161/HYPERTENSIONAHA.110.156323. [DOI] [PubMed] [Google Scholar]
- 16.Papaioannou TG, Protogerou AD, Stefanadis C. What to anticipate from pulse pressure amplification. J Am Coll Cardiol. 2010;55:1038–1040. doi: 10.1016/j.jacc.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 17.Segers P, Mahieu D, Kips J, Rietzschel E, De Buyzere M, De De Bacquer D, et al. for the Asklepios Investigators Amplification of the pressure pulse in the upper limb in healthy, middle-aged men and women. Hypertension. 2009;54:414–420. doi: 10.1161/HYPERTENSIONAHA.109.133009. [DOI] [PubMed] [Google Scholar]
- 18.Papaioannou TG, Vlachopoulos CV, Alexopoulos NA, Dima I, Pietri PG, Protogerou AD, et al. The effect of heart rate on wave reflections may be determined by the level of aortic stiffness: clinical and technical implications. Am J Hypertens. 2008;21:334–340. doi: 10.1038/ajh.2007.52. [DOI] [PubMed] [Google Scholar]
- 19.Tobian L. Salt and hypertension: lessons learned from animal models that relate to human hypertension. Hypertension. 1991;17(Suppl I):152–158. doi: 10.1161/01.hyp.17.1_suppl.i52. [DOI] [PubMed] [Google Scholar]
- 20.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O’Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–210. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 21.Avolio AP, Clyde CM, Beard TC, Cooke HM, Ho KK, O’Rourke MF. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986;6:166–169. doi: 10.1161/01.atv.6.2.166. [DOI] [PubMed] [Google Scholar]
- 22.He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white,black and asian mild hypertensives. Hypertension. 2009;54:482–488. doi: 10.1161/HYPERTENSIONAHA.109.133223. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Lakatta EG. The salted artery and angiotensin II signaling: a deadly duo in arterial disease. J Hypertens. 2009;27:19–21. doi: 10.1097/HJH.0b013e32831d1fed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson ME, Bernberg E, Andersson IJ, Bie P, Skott O, Gan LM, Bergstrom G. High salted diet combined with elevated angiotensin II accelerates atherosclerosis in Apo E −/− mice. J Hypertens. 2008;27:41–47. doi: 10.1097/hjh.0b013e328318697b. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community based age, gene/environment susceptibility Reykjavik study. Hypertension. 2008;51:1123–1128. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He FJ, Marciniak M, Markandu ND, Antonios TF, MacGregor GA. Effect of modest salt reduction on skin capillary rarefaction in white, black, and Asian individuals with mild hypertension. Hypertension. 2010;56:253–259. doi: 10.1161/HYPERTENSIONAHA.110.155747. [DOI] [PubMed] [Google Scholar]
- 27.Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, Kloch-Badelek M, Wilinski J, Curylo AM, Dudek D. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51:848–855. doi: 10.1161/HYPERTENSIONAHA.107.101725. [DOI] [PubMed] [Google Scholar]
- 28.Buyck JF, Blacher J, Kesse-Guyot E, Castetbon K, Galan P, Safar ME, Hercberg S, Czernichow S. Differential associations of dietary sodium and potassium intake with blood pressure: a focus on pulse pressure. J Hypertens. 2009;27:1158–1164. doi: 10.1097/hjh.0b013e328329bc08. [DOI] [PubMed] [Google Scholar]
- 29.Kawano Y, Tsuchihashi T, Matsuura H, Ando K, Fujita T, Ueshima H. Report of the working group for dietary salt reduction of the Japanese Society of Hypertension: (2) Assessment of salt intake in the management of hypertension. Hypertens Res. 2007;30:887–893. doi: 10.1291/hypres.30.887. [DOI] [PubMed] [Google Scholar]