Abstract
In the current studies we generated novel capsid-optimized AAV serotype 6 (AAV6) vectors expressing a tumor-associated antigen, and assessed their ability to activate a protective T-cell response in an animal model. First, we showed that specific mutations in the AAV6 capsid increase the transduction efficiency of these vectors in mouse bone marrow-derived dendritic cells in vitro for approximately 5-fold compared with the wild-type (WT) AAV6 vectors. Next, we evaluated the ability of the mutant AAV6 vectors to initiate specific T-cell clone proliferation in vivo. Our data indicate that the intramuscular administration of AAV6-S663V+T492V vectors expressing ovalbumin (OVA) led to a strong activation (approximately 9%) of specific T-cells in peripheral blood compared to AAV6-WT treated animals (less than 1%). These OVA-specific T-cells have a superior killing ability against mouse prostate cancer cell line RM1 stably expressing the OVA antigen when propagated in vitro. Finally, we evaluated the ability of capsid-optimized AAV6-S663V+T492V vectors to initiate a protective anti-cancer immune response in vivo. Our results document the suppression of subcutaneous tumor growth in animals immunized with AAV6-S663V+T492V vectors expressing prostatic acid phosphatase (PAP) for approximately four weeks in comparison to one week and two weeks for the negative controls, AAV6-EGFP and AAV6-WT-PAP treated mice, respectively.
These studies suggest that successful inhibition of tumor growth in an animal model would set the stage for potential clinical application of the capsid-optimized AAV6-S663V+T492V vectors.
Keywords: Adeno-associated virus vectors, gene expression, dendritic cells, vaccine, prostate cancer, prostatic acid phosphatase
INTRODUCTION
Although a naturally occurring anti-tumor immune response is detectable in patients, this response fails to control tumor growth. The possibility of stimulating a specific anti-tumor immune response via genetically-modified dendritic cells (DCs) in both ex vivo and in vivo protocols, has been proven in a number of clinical trials (1–4). However, current methods for therapeutic antigen delivery, control of expression, proper mobilization of antigen presenting cells and, thus presentation of epitopes to effector cells, are not sufficient (3, 5). The common methods of antigen delivery include naked or lipid encapsulated DNA/RNA, peptides or recombinant proteins. The safety of these methods of delivery comes with a price of poor immunogenicity. In contrast, viral vectors are often highly immunogenic, and they also carry the risk of pathogenesis. For example, the major advantages of using adenovirus (Ad) vectors as a vaccine platform include their ability to infect a broad range of hosts and to induce high levels of transgene expression. However, infection with Ad vectors up-regulate co-stimulatory molecules accompanied by increase in proinflammatory cytokine and chemokine production by DCs. This early stimulation of DCs can contribute to more a effective presentation of virus-derived epitopes rather than epitopes from recombinant antigens. In contrast, vaccinia virus-based vectors suppress maturation on antigen presenting cells and, thus impart the ability of DCs to properly stimulate specific T-cell clone proliferation (5–9). The need for an optimal medium is motivating efforts in the development of recombinant viral vectors with a good balance of immunogenicity and safety. Vectors based on adeno-associated virus (AAV) have recently attracted attention particularly because of its superior transduction efficiency in broad cell types and lack of pathogenicity (10–13). AAV vector-based antigen delivery to different subsets of DCs has been utilized successfully (14–19). These vectors have also been used for both passive and active immunization strategies (20–26).
We have previously reported that the efficacy of wild-type (WT) AAV vectors can be significantly enhanced by substituting critical serine (S) and threonine (T) residues on their capsids to valine (V). These residues were identified by analysis of the AAV capsid crystal structure and they can be recognized and phosphorylated by common serine/threonine cellular kinases such as JNK and p38 MAPK (14). Several different amino acids were tested and (V) was chosen because of the similarity of its structure with both (S) and (T), and lack of recognition by kinases. Thus, these modifications can prevent kinase-mediated phosphorylation of the AAV capsid, subsequent ubiquitination and proteasome-mediated degradation of the vectors (14, 27–29). These studies have led to the development of a number of AAV serotype 2 (AAV2) and serotype 6 (AAV6) vectors with high activity in human monocyte-derived dendritic cells (moDCs) (14, 15, 18).
In the present studies, we explored the possibility of using capsid-optimized AAV6 vectors for active immunization against prostate cancer in vivo. Subcutaneously injected mouse prostate carcinoma cell line RM1 (from Ras+Myc transformed C57BL/6 mouse) stably expressing firefly luciferase (Fluc) for the visual presentation of tumor size was used as an animal model. We have documented the following observations: (i) Site-directed mutagenesis of the surface-exposed (S) and (T) residues on the AAV6 capsid to (V) significantly improves transduction efficiency of S663V+T492V mutant compared with the AAV6-WT vectors in mouse bone morrow-derived DCs; (ii) Intramuscular injection of AAV6-S663V+T492V vectors expressing ovalbumin (OVA) leads to specific OVA-CD8+ cell proliferation with a higher number in peripheral blood two weeks after injection; (iii) Specific CD8+ cells generated by AAV6-S663V+T492V vectors can be expanded in vitro and show an increased killing ability when compared with cells generated by AAV6-WT vectors; (iv) Vaccination with AAV6-S663V+T492V vectors encoding the prostatic acid phosphatase (PAP) gene leads to a significant delay in prostate cancer progression and extends life span in a mouse model. These observations suggest that vaccination with capsid-modified AAV6 vectors against cancer is feasible, which supports the potential utility of these vectors as a useful platform for vaccine studies.
RESULTS
Site-directed mutagenesis of surface-exposed serine (S) and threonine (T) residues on AAV6 capsid improves vector-mediated transgene expression
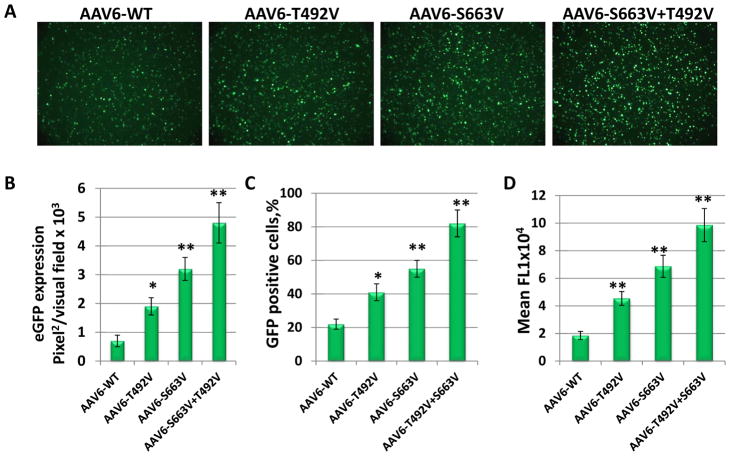
The AAV6 capsid contains 17 serine (S) and 15 threonine (T) surface-exposed residues in the viral protein 3 (VP3) common regions. We previously showed that mutations of the single critical serine at position 663 and threonine at position 492 to valine (V) increased the transduction efficiency of the AAV6 vectors in human moDCs. Moreover, a combination of these mutations on the same viral capsid (S663V+T492V) further improved the transduction efficiency (15). In the current studies, we wished to evaluate whether a similar approach could be used to increase the activity of AAV6 vectors in mouse bone morrow-derived DCs. These results generated as total area of fluorescence/per visual field (Fig. 1 A, B) indicate that the AAV6-T492V-EGFP and AAV6-S663V-EGFP mutants transduced mouse DCs 2-fold and 3-fold more efficiently than their WT counterpart. Similar to previous observations in human DCs, a combination of two single mutations had an additive effect, since transduction efficiency of double-mutant AAV6-S663V+T492V-EGFP was increased to ~5-fold compared with AAV6-WT. These results were confirmed by flow cytometry analysis and suggest that both, number of infected cells and fluorescence intensity (expression level), were increased by AAV6-S663V+T492V over AAV6-WT for ~4-fold and ~5-fold correspondingly (Fig. 1 C, D). Our data suggest that the capsid-optimized AAV6 vectors can be used to achieve a high level of expression in mouse antigen presenting cells.
Fig. 1. Analysis of EGFP expression after transduction of mouse bone morrow-derived DCs with AAV6 capsid mutants.
Surface-exposed serines (S) at position 663 and threonine (T) at position 492 were substituted with valine (V) and the mutant vectors here evaluated for their efficiency to mediate transgene expression. (a) EGFP expression analysis at 48 hrs post-infection at an MOI of 2x104 vgs/cell. (b) Quantitation of transduction efficiency of each of the mutant AAV6 vectors. Flow cytometry analysis of number of EGFP positive cells (c) and mean fluorescence intensity (d). *P<0.05,**P<0.01 vs. AAV6-WT.
Capsid-optimized AAV6 vectors can stimulate specific T-cell clone proliferation in vivo
In the current studies, we evaluated the possibility of using capsid-optimized AAV6 vectors to express antigens in mouse antigen presenting cells and to activate a specific T-cell proliferation in vivo. Ovalbumin (OVA), commonly used as an immunogen, delivered by capsid-optimized AAV6-S663V+T492V or WT vectors was used to evaluate the immune responses. C57BL/6 mice were injected intramuscularly (i.m.) at various time points post viral injection. Numbers of OVA-specific CD8+ cells in peripheral blood were analyzed by staining them with MHC Class I Murine Tetramer. C57BL/6-Tg mice with a transgenic T-cell receptor designed to recognize ovalbumin epitope, were used as positive controls for OVA-CD8+ cells. The data shown in Fig. 2 A, B suggest that the administration of AAV6-S663V+T492V vectors expressing OVA led to a robust activation (approximately 9%) of specific T-cells compared to the AAV6-WT-OVA treated animals (less than 1%). The maximum of amplitude of the OVA-CD8+ cell number was observed two weeks after i.m. injection. Three weeks after injection, number of OVA specific T-cells was below the detectable level (Fig. 2C). These data indicate that capsid-optimized AAV6 vectors have the potential of being used as an immune modulator more efficiently than the AAV6-WT vectors.
Fig. 2. Phenotypic analysis of specific CD8+ cells induced by AAV6 vectors expressing OVA.
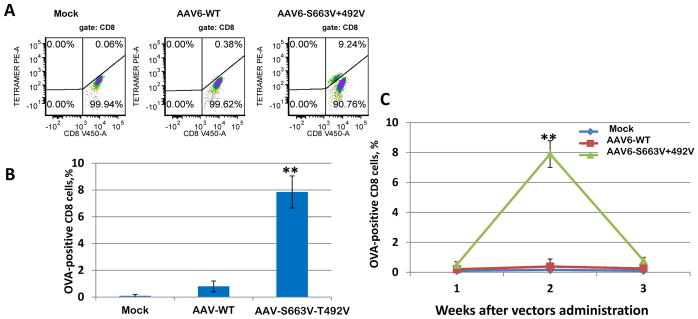
C57BL/6 mice (n = 3 per group) were i.m injected with AAV6-WT-OVA, AAV6-S663V+T492V-OVA and AAV6-EGFP. OVA-CD8+ cells were analyzed weekly in peripheral blood. (a), Representative examples of OVA-CD8+ cells induced by different vectors at 2 weeks after injection; (b) Quantitation of the number of OVA-CD8+ cells by mutant AAV6 vectors; (c) Time course of OVA-CD8+ over 1, 2 and 3 weeks after vector administration, *P<0.05, **P<0.01 vs. AAV6-WT.
Capsid-optimized AAV6 vectors stimulate specific T-cell clone with a high killing ability
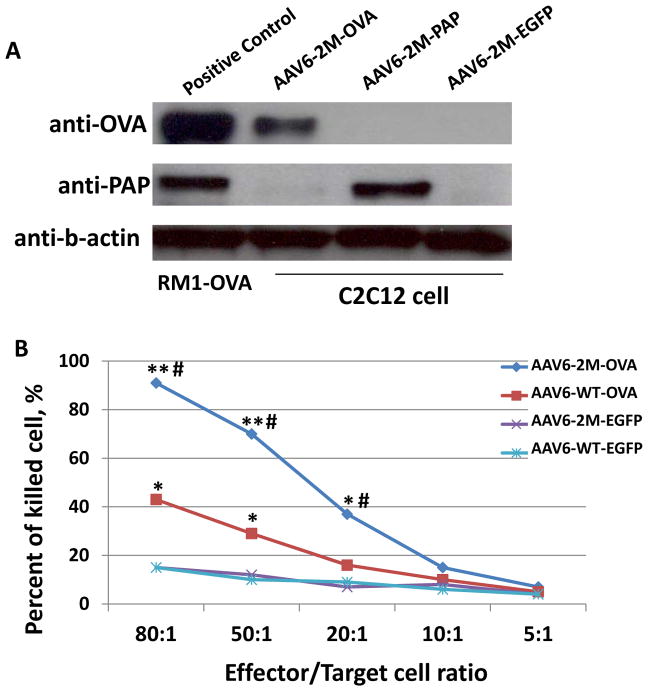
Since the capsid-optimized AAV6 vector-mediated specific T-cells clone proliferation was significantly improved when compared with the AAV6-WT vectors, we next evaluated the cytotoxic ability of these T-cells against cancer cells in vitro. First, we evaluated the expression level of PAP and OVA proteins following infection of capsid-optimized AAV6 vectors in a murine myoblast cell line, C2C12, and compared them with the naturally occurring expression of PAP and stable expression of OVA in RM1 and RM1-OVA cells (Fig. 3a). AAV6 vectors expressing EGFP were used to rule out possible stimulation of expression during infection. These results indicate a high level of expression of proteins with AAV6 mutant vectors. Then, OVA-CD8+ cells were generated from splenocytes from mice i.m. injected with AAV6-WT-OVA and AAV-S663V+T492V-OVA vectors, as described in Methods. AAV6-WT-EGFP and AAV6-S663V+T492V-EGFP vectors were used as appropriate controls. Mouse prostate cancer cells, RM1, stably expressing OVA, were used as a specific target for a two-color fluorescence assay of cell-mediated cytotoxicity to generate a killing curve using reduced effector to target cell ratio, as described in Methods. Result of these experiments, shown in Fig. 3, suggest that i.m. injection of capsid-optimized AAV6 vectors can effectively stimulate specific T-cell clone proliferation and with a ~4-fold higher killing activity compared with non-specific control and ~2-fold higher than AAV6-WT vectors. The use of AAV6-WT-EGFP or AAV6-S663V+T492V-EGFP vectors for non-specific control T-cells rules out the possible auto-reactivity or possible non-specific response of stimulated cytotoxicity. Since immunization strategies that generate potent effector responses are essential for effective immunotherapy, our results support the efficacy of capsid-optimized AAV6-based vectors for vaccination studies.
Fig. 3. Analysis of OVA-specific cytotoxic T-lymphocytes (CTLs) killing activity on RM1-OVA cells.
(a) Western blot analysis of the expression level of OVA (top blot) and PAP (middle blot) in murine myoblasts after delivery with AAV6 vectors. 2M is two mutations (S662V+T492V). Mouse prostate cancer cells and RM1-OVA served as positive control (first band in each blot). AAV-EGFP was used as negative control to eliminate the possibility of non-specific stimulation of gene expression. (b) CTLs were generated from mice splenocytes after i.m. injection of AAV6-S662V+T492V and AAV6-WT vectors encoding OVA. AAV2-S662V+T492V-EGFP and AAV6-WT vectors were used to generate non-specific CTLs. A killing curve was generated with a decreasing number of effector cells and specific target cell lysis was determined by FACS analysis of live/dead cell ratios. *P<0.005 between the same capsid & a different gene, and #P<0.005 between a different capsid & the same gene, considered as significant.
Capsid-optimized AAV6 vectors suppress tumor growth and extend survival in vivo
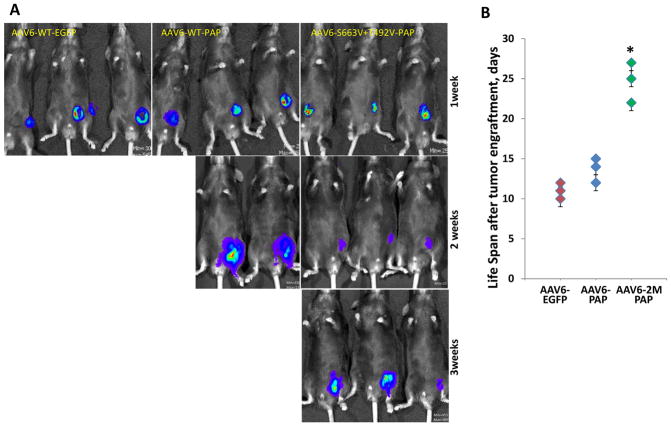
Finally, we assessed the ability of capsid-optimized AAV6 vectors to suppress tumor growth in an animal model. Prostatic acid phosphatase (PAP), a gene upregulated in both human and mouse prostate cancer, was used as a specific target in vivo. Three groups of three C57BL/6 mice (n=3) were i.m. injected with either the AAV6-S663V+T492V-PAP, AAV6-WT-PAP or AAV6-WT-EGFP vectors. Two weeks post viral injection, when the number of specific T-cells reached maximum amplitude, as was evaluated previously, mice were challenged with the prostate cancer cell line, RM1, stably expressing the firefly luciferase (RM1-FLuc), by subcutaneous injection. Tumor growth was then measured weekly by bioluminescence imaging (Fig. 4). Mice injected with AAV6-WT vectors containing EGFP were sacrificed one week after cancer cell challenge according to body score condition recommended by the Institutional Animal Care and Use Committee (IACUC). However, mice injected with AAV6-WT-PAP survived over two weeks. A significant suppression of tumor growth was observed in the group of animals injected with the capsid-optimized AAV6-S663V+T492V vectors expressing PAP. The life span of two mice was extended for about three weeks, and for the third mouse, up to four weeks (Fig. 4B). Our results clearly indicate the ability of the mutant AAV6-S663V+T492V-PAP vectors to suppress tumor growth for a longer duration of time compared with the AAV6-WT vectors, containing either a cancer specific (PAP) or the reporter (EGFP) gene.
Fig. 4. In vivo imaging of tumor growth progression evaluated by activity of luciferase stably expressed in murine prostate cancer cells, RM1.
C57BL/6 mice were injected i.m. with 5x10e10 vgs/animal of the most efficient mutant AAV6 vectors carrying the prostatic acid phosphatase gene. Live images were taken weekly to analyze differences in luciferase activity for visual representation of the tumor size. The visual output represents the number of photons emitted/second/cm2 as a false color image where the maximum is red and the minimum is blue (a) and relative signal intensity (b). The life span of each animal challenged with cancer cells, *P<0.005 was considered as significant.
DISCUSSION
Recent developments of preclinical and clinical gene therapy protocols have demonstrated favorable safety profile and long-term gene expression by AAV vectors (11, 30, 31). However, generally low immunogenicity of commonly used serotypes of AAV can likely explain the lack of interest to employ these vectors for immunization studies (32, 33). Currently, more than a hundred of AAV variants have been isolated from human and non-human tissues, but only a few have been used as recombinant vectors. Selection of these AAV-based vectors was determined mostly by specific tissue-tropism and transduction efficiency. However, several studies have documented significant differences in immune response induced by AAV depending on the capsid composition and the route of vector administration (32, 34–37). Thus, the natural plasticity of AAV warrants discovering or developing variants capable of stimulating stronger immune responses (28, 29, 38, 39). For example, a recently identified AAV/rh32.33 serotype, has a unique capsid structure that elicits cellular immune response toward vector encoded antigens (40). An even greater possibility to identify a superior AAV vector for the manipulation of host immune response was offered by bioengineering of the viral capsid using recombinant libraries or by inserting an immunogenic peptide on the capsid surface (41–43). We also recently showed that naturally occurring serotypes of AAV vectors are not optimal for the transduction of a number of cell types, including DCs, and that the efficacy of these vectors can be significantly enhanced by mutation of critical surface-exposed serine and threonine residues on their capsids. Our previously published data indicate that such optimization of AAV2 and AAV6 leads to high activity of these vectors in human monocyte-derived dendritic cells (moDCs) (14, 15). Importantly, this increase in infectivity is associated with advanced nuclear translocation rather than improved viral entry into cells. Another significant advantage of the employment of such capsid-modified AAV vectors for targeting DCs is that escape from the proteasomal pathway provides less material for potential presentation, and prevents induction of immune responses against vector-derived epitopes. Indeed, it was shown that such engineered AAV vectors minimize in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T-cells (44). Also, considering the relatively short period of time for cytopathic changes in DCs transduced by AAV vectors, early onset of antigen expression is critical for appropriate presentation, and consequentially, T-cell activation.
The time of T-cell activation and the potency and longevity of responses are crucial factors in evaluation of the possible therapeutic outcomes. Thus, we also evaluated whether an increased transduction efficiency of DCs by the capsid-optimized AAV6-S663V+T492V vectors correlated with superior priming of T-cells. Our results suggest that modification of the AAV6 capsid is beneficial in terms of producing a robust antigen-specific peripheral T-cell response. Moreover, vaccination via intramuscular injection of the capsid-optimized AAV6-S663V+T492V vectors encoding a specific antigen (PAP) induces a protective immune response which leads to significant delay in prostate cancer progression and extends survival in mice.
Though the results of our experiments are promising, it is necessary to induce a more efficient immune response. One such possibility is the use of a novel AAV serotype with immunogenic properties. Another possibility of improving effectiveness of the immune response is the use of adjuvants, such as unmethylated CpG oligodinucleotide, granulocyte-macrophage colony-stimulating factor (GM-CSF) or interleukin 12 (Il–12)(45). We previously described transient activation of toll-like receptors 9 (TLR9) followed by AAV infection (46). Thus, a selective TLR9 agonist also can be used along with AAV administration to enhance immune response (47).
The current studies provide a better understanding of the possibility of using the capsid-optimized AAV vectors for immunomodulation in general, and inducing a protective anti-cancer immune response in particular.
MATERIALS AND METHODS
Cells
The mouse prostate carcinoma cell line RM1 and RM1 stably expressing OVA protein (RM1-OVA) (generous gift from Dr. Xiang-Yang Wang, Department of Human and Molecular Genetics, Virginia Commonwealth University, Richmond, VA) were maintained as monolayer cultures in DMEM (Invitrogen) supplemented with 10% FBS (Sigma) and antibiotics (Lonza). The cell line was derived from a heterogeneous primary tumor in the prostate of a Ras-and-Myc transformed C57BL/6 mouse and genetically modified for stable OVA expression under the control of the strong CMV promoter. We also modified a RM1 cell line to stably express a firefly luciferase (FLuc) driven by a CMV promoter for the monitoring of progression or reduction of the tumor, using previously described methods (48). We showed that RM1-FLuc cells are tumorigenic when grafted subcutaneously into syngeneic C57BL/6 hosts. Mouse conventional DCs were differentiated from bone morrow derived CD34+ cells in the presence of mrGM-CSF (2000U/ml) and mrIL-4 (1000U/ml) for 7 days. Briefly, marrow from 6 wk old C57BL/6 male mice was harvested by flushing with 1 ml PBS/bone. Cells were pelleted by centrifugation and contamination with red blood cells was cleaned with ACK lysis buffer at room temp for 5 min. Cells were purified with magnetic MicroBeads labeled with CD117 antibodies by loading suspension into a MACS Column which was placed in the magnetic field of a MACS Separator (Miltenyi Boitec). Prior to rAAV6 transduction, cells were characterized for co-stimulatory molecules expression to ensure that they met the typical phenotype of dendritic cells (DCs) (CD11c-RPE, Invitrogen).
Production of recombinant AAV vectors
Site-directed mutagenesis was performed with plasmid pACGr2c6 as described previously (14, 15). Recombinant AAV vectors containing the EGFP, OVA and PAP genes driven by the chicken β-actin promoter were generated in HEK293 cells transfected with Polyethylenimine (PEI, linear, MW 25,000, PolySciences, Inc.). Vectors were purified by iodixanol (Sigma) gradient centrifugation and ion exchange column chromatography (HiTrap Sp Hp 5 ml, GE Healthcare). Virus was then concentrated and the buffer exchanged in three cycles to lactated Ringer’s using centrifugal spin concentrators (Apollo, 150-kDa cut-off, 20-ml capacity, CLP). DNase I-resistant AAV particle titers were determined by RT-PCR with the following primer-pair, specific for the CBA promoter: forward 5′-TCCCATAGTAACGCCAATAGG-3′, reverse 5′-CTTGGCATATGATACACTTGATG-3′ and SYBR Green PCR Master Mix (Invitrogen) (14, 15, 49).
Recombinant AAV Vector Transduction Assays In Vitro
Bone morrow-derived mouse DCs, were transduced with AAV6 vectors with 20,000 vgs/cell or and incubated for 48 hrs. Transgene expression was assessed as the total area of green fluorescence (pixel2) per visual field (mean ± SD) as described previously (14, 15, 27). Analysis of variance was used to compare test results and the control, which were determined to be statistically significant.
Antibodies
Western blotting was performed as described previously (14, 15) with following antibodies: anti-PAP (Fitzgerald, 1:1000); anti-OVA (Thermo Scientific Pierce, 1:1000) and b-actin (CellSignaling, 1:2000). Antibodies for FACS analysis were obtained as follow Anti-Human/Mouse CD45R (B220)-APC-A, CD19-APC-Cy7-A and CD8-V450-A (BD Biosciences); MHC Class I Murine Tetramer (Beckman Coulter).
Specific cytotoxic T-lymphocytes generation and cytotoxicity assay
Ten weeks old C57BL/6 male mice were injected i.m. with AAV6-WT-OVA, AAV-S663V+T492V-OVA and AAV6-WT-GFP. Spleens were harvested 2 weeks after and OVA-CD8+ cells were expanded in vitro in RPMI-1640 medium, supplemented with predominant for C57BL/6 mice OVA-derived SIINFEKL peptide (10ug/ml) (AnaSpec), rmIL-15 (10ng/ml) and rmIL-21(25ng/ml). Fresh supplements were added every 2 days. Stimulated T-cells were used for a killing assay against mouse prostate cell line RM1 stably expressing OVA. A killing curve was generated and specific cell lysis was determined by FACS analysis of live/dead cell. The target cells were pre-stained with 3,3-dioctadecyloxacarbocyanine (DiOC18(3)), a green fluorescent membrane stain, 1x105 target RM1-OVA cells were co-cultured overnight with different ratios of CTLs (80:1, 50:1, 20:1, 10:1, 5:1). Membrane-permeable nucleic acid counter-stain, propidium iodide, was added to label the cells with compromised plasma membranes. Percentages of killed, double-stain positive cells were analyzed by flow cytometry (14, 15, 50).
Evaluation of the tumor growth by in vivo bioluminescence imaging
C57BL/6 male mice (Jackson Laboratory, Bar Harbor, ME) were used for animal studies. Ten-week-old C57BL/6 male mice were injected intramuscularly with 5x10e10 vgs/animal of AAV6-WT-PAP, AAV6-S663V+T492V-PAP and AAV6-WT-GFP. Two weeks later RM1-FLuc cancer cells were injected subcutaneously. Luciferase activity was analyzed every week after injection using a Xenogen IVIS Lumina System (Caliper Life Sciences). Briefly, mice were anesthetized with 2% isoflurane and injected intraperitoneally with luciferin substrate (Beetle luciferin, Caliper Life Sciences) at a dose of 150 ug/g of body weight. Mice were placed in a light-tight chamber and images were collected at 5 minutes after the substrate injection. Images were analyzed by the Living Image 3.2 software (Caliper Life Sciences) to determine relative signal intensity(27). All animal experiments were approved by the University of Florida Institutional Animal Care and Use Committee. All procedures were done in accordance with the principles of the National Research Council’s Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize suffering of the animals challenged with cancer cells. Animals were monitored daily and humanely euthanized when tumor reached 0.5 cm in diameter.
Statistical analysis
All results are presented as mean ± S.D. Differences between groups were identified using a grouped-unpaired two-tailed distribution of Student’s T-test. P-values <0.05 were considered statistically significant.
Acknowledgments
We thank Dr. Arun Srivastava for a critical reading of this manuscript. We also thank Dr. Xiang-Yang Wang, Department of Human and Molecular Genetics, Virginia Commonwealth University, Richmond, VA, for providing the modified mouse prostate cancer cell line, RM1-OVA. This research was supported in part by Children’s Miracle Network (GA and CL).
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(9521319):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10(5):475–80. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 3.Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. J Immunol. 2011;186(21248270):1325–31. doi: 10.4049/jimmunol.0902539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234(20193020):199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoos A, Britten CM, Huber C, O’Donnell-Tormey J. A methodological framework to enhance the clinical success of cancer immunotherapy. Nat Biotechnol. 2011;29(21997622):867–70. doi: 10.1038/nbt.2000. [DOI] [PubMed] [Google Scholar]
- 6.Humbert JM, Halary F. Viral and non-viral methods to genetically modify dendritic cells. Curr Gene Ther. 2012;12(2):127–36. doi: 10.2174/156652312800099580. [DOI] [PubMed] [Google Scholar]
- 7.Tacken PJ, de Vries IJM, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7(17853902):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 8.Ueno H, Klechevsky E, Schmitt N, Ni L, Flamar A-L, Zurawski S, et al. Targeting human dendritic cell subsets for improved vaccines. Semin Immunol. 2011;23(21277223):21–7. doi: 10.1016/j.smim.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18(6):546–56. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21(18854481):583–93. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.High KA, Aubourg P. rAAV human trial experience. Methods Mol Biol. 2011;807:429–57. doi: 10.1007/978-1-61779-370-7_18. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava A. Adeno-associated virus-mediated gene transfer. J Cell Biochem. 2008;105(18500727):17–24. doi: 10.1002/jcb.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling CQ, Wang LN, Wang Y, Zhang YH, Yin ZF, Wang M, et al. The roles of traditional Chinese medicine in gene therapy. J Integr Med. 2014;12(2):67–75. doi: 10.1016/S2095-4964(14)60019-4. [DOI] [PubMed] [Google Scholar]
- 14.Aslanidi GV, Rivers AE, Ortiz L, Govindasamy L, Ling C, Jayandharan GR, et al. High-efficiency transduction of human monocyte-derived dendritic cells by capsid-modified recombinant AAV2 vectors. Vaccine. 2012;30(22497875):3908–17. doi: 10.1016/j.vaccine.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandya J, Ortiz L, Ling C, Rivers AE, Aslanidi G. Rationally designed capsid and transgene cassette of AAV6 vectors for dendritic cell-based cancer immunotherapy. Immunol Cell Biol. 2013 doi: 10.1038/icb.2013.74. [DOI] [PubMed] [Google Scholar]
- 16.Ponnazhagan S, Mahendra G, Curiel DT, Shaw DR. Adeno-associated virus type 2-mediated transduction of human monocyte-derived dendritic cells: implications for ex vivo immunotherapy. J Virol. 2001;75(11533211):9493–501. doi: 10.1128/JVI.75.19.9493-9501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin O, Kim SJ, Lee WI, Kim JY, Lee H. Effective transduction by self-complementary adeno-associated viruses of human dendritic cells with no alteration of their natural characteristics. J Gene Med. 2008;10(18452239):762–9. doi: 10.1002/jgm.1204. [DOI] [PubMed] [Google Scholar]
- 18.Ussher JE, Taylor JA. Optimized transduction of human monocyte-derived dendritic cells by recombinant adeno-associated virus serotype 6. Hum Gene Ther. 2010;21(20578847):1675–86. doi: 10.1089/hum.2010.087. [DOI] [PubMed] [Google Scholar]
- 19.Veron P, Allo V, Riviere C, Bernard J, Douar A-M, Masurier C. Major subsets of human dendritic cells are efficiently transduced by self-complementary adeno-associated virus vectors 1 and 2. J Virol. 2007;81(17314166):5385–94. doi: 10.1128/JVI.02516-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balazs AB, Bloom JD, Hong CM, Rao DS, Baltimore D. Broad protection against influenza infection by vectored immunoprophylaxis in mice. Nat Biotechnol. 2013;31(7):647–52. doi: 10.1038/nbt.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deal C, Balazs AB, Espinosa DA, Zavala F, Baltimore D, Ketner G. Vectored antibody gene delivery protects against Plasmodium falciparum sporozoite challenge in mice. Proc Natl Acad Sci U S A. 2014;111(34):12528–32. doi: 10.1073/pnas.1407362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisold S, Schmidt J, Ryschich E, Gock M, Klar E, von Knebel Doeberitz M, et al. Induction of an antitumoral immune response by wild-type adeno-associated virus type 2 in an in vivo model of pancreatic carcinoma. Pancreas. 2007;35(17575547):63–72. doi: 10.1097/mpa.0b013e31804b4941. [DOI] [PubMed] [Google Scholar]
- 23.Mahadevan M, Liu Y, You C, Luo R, You H, Mehta JL, et al. Generation of robust cytotoxic T lymphocytes against prostate specific antigen by transduction of dendritic cells using protein and recombinant adeno-associated virus. Cancer Immunol Immunother. 2007;56(17356843):1615–24. doi: 10.1007/s00262-007-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieto K, Salvetti A. AAV Vectors Vaccines Against Infectious Diseases. Front Immunol. 2014;5:5. doi: 10.3389/fimmu.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieto K, Stahl-Hennig C, Leuchs B, Muller M, Gissmann L, Kleinschmidt JA. Intranasal vaccination with AAV5 and 9 vectors against human papillomavirus type 16 in rhesus macaques. Hum Gene Ther. 2012;23(7):733–41. doi: 10.1089/hum.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y, Pilgrim P, Zhou W, Gagliano N, Frezza EE, Jenkins M, et al. rAAV/Her-2/neu loading of dendritic cells for a potent cellular-mediated MHC class I restricted immune response against ovarian cancer. Viral Immunol. 2008;21(19115932):435–42. doi: 10.1089/vim.2008.0029. [DOI] [PubMed] [Google Scholar]
- 27.Aslanidi GV, Rivers AE, Ortiz L, Song L, Ling C, Govindasamy L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One. 2013;8(3):e59142. doi: 10.1371/journal.pone.0059142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381(18834608):194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105(22):7827–32. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20(22273577):699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gene Ther. 2012;22(10):1239–47. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7(5):316–24. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 33.Mueller C, Chulay JD, Trapnell BC, Humphries M, Carey B, Sandhaus RA, et al. Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. J Clin Invest. 2013;123(12):5310–8. doi: 10.1172/JCI70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J, Calcedo R, Vandenberghe LH, Bell P, Somanathan S, Wilson JM. A new genetic vaccine platform based on an adeno-associated virus isolated from a rhesus macaque. J Virol. 2009;83(24):12738–50. doi: 10.1128/JVI.01441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin J, Zhi Y, Mays L, Wilson JM. Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J Virol. 2007;81(21):11840–9. doi: 10.1128/JVI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieto K, Kern A, Leuchs B, Gissmann L, Muller M, Kleinschmidt JA. Combined prophylactic and therapeutic intranasal vaccination against human papillomavirus type-16 using different adeno-associated virus serotype vectors. Antivir Ther. 2009;14(8):1125–37. doi: 10.3851/IMP1469. [DOI] [PubMed] [Google Scholar]
- 37.Vandenberghe LH, Wilson JM. AAV as an immunogen. Curr Gene Ther. 2007;7(17979679):325–33. doi: 10.2174/156652307782151416. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Diprimio N, Bowles DE, Hirsch ML, Monahan PE, Asokan A, et al. Single amino acid modification of adeno-associated virus capsid changes transduction and humoral immune profiles. J Virol. 2012;86(15):7752–9. doi: 10.1128/JVI.00675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T, et al. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J Virol. 2000;74(10954565):8635–47. doi: 10.1128/jvi.74.18.8635-8647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mays LE, Vandenberghe LH, Xiao R, Bell P, Nam H-J, Agbandje-McKenna M, et al. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J Immunol. 2009;182(19414756):6051–60. doi: 10.4049/jimmunol.0803965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene Ther. 2012;19(6):694–700. doi: 10.1038/gt.2012.20. [DOI] [PubMed] [Google Scholar]
- 42.Vandenberghe LH, Wilson JM, Gao G. Tailoring the AAV vector capsid for gene therapy. Gene Ther. 2009;16(3):311–9. doi: 10.1038/gt.2008.170. [DOI] [PubMed] [Google Scholar]
- 43.Nieto K, Weghofer M, Sehr P, Ritter M, Sedlmeier S, Karanam B, et al. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS One. 2012;7(6):e39741. doi: 10.1371/journal.pone.0039741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martino AT, Basner-Tschakarjan E, Markusic DM, Finn JD, Hinderer C, Zhou S, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121(12):2224–33. doi: 10.1182/blood-2012-10-460733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119(15):3383–93. doi: 10.1182/blood-2011-11-370130. [DOI] [PubMed] [Google Scholar]
- 46.Jayandharan GR, Aslanidi G, Martino AT, Jahn SC, Perrin GQ, Herzog RW, et al. Activation of the NF-kappaB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy. Proc Natl Acad Sci U S A. 2011;108(9):3743–8. doi: 10.1073/pnas.1012753108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Triozzi PL, Aldrich W, Ponnazhagan S. Inhibition and promotion of tumor growth with adeno-associated virus carcinoembryonic antigen vaccine and Toll-like receptor agonists. Cancer Gene Ther. 2011;18(12):850–8. doi: 10.1038/cgt.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang YH, Wang Y, Yusufali AH, Ashby F, Zhang D, Yin ZF, et al. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med. 2014;12(6):483–94. doi: 10.1016/s2095-4964(14)60057-1. [DOI] [PubMed] [Google Scholar]
- 49.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28(12413414):158–67. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 50.Mattis AE, Bernhardt G, Lipp M, Forster R. Analyzing cytotoxic T lymphocyte activity: a simple and reliable flow cytometry-based assay. J Immunol Methods. 1997;204(9212830):135–42. doi: 10.1016/s0022-1759(97)00047-1. [DOI] [PubMed] [Google Scholar]