Abstract
Patient: Female, 42
Final Diagnosis: Breast cancer with leptomeningeal metastasis
Symptoms: Headache
Medication: Etoposide
Clinical Procedure: Intrathecal chemotherapy
Specialty: Oncology
Objective:
Unusual setting of medical care
Background:
Leptomeningeal metastasis (LM) is recently on the rise as one of important clinical issues in the management of metastatic breast cancer (MBC). Clinical research on salvage intrathecal chemotherapy after failure of first-line treatment for MBC patients with LM has rarely been reported.
Case Report:
We report the case of a breast cancer patient with LM who showed durable response to salvage intrathecal etoposide subsequent to failure of methotrexate. Etoposide 1 mg was injected through an Ommaya reservoir every week. Corticosteroid was used for a prophylaxis of chemical arachnoiditis. The treatment was successful palliation of LM for 33 weeks without significant adverse effects. Time to neurologic progression was estimated to be about 230 days for the treatment and overall survival was 301 days from the diagnosis of LM.
Conclusions:
Intrathecal etoposide can be considered as an additional treatment option for LM in breast cancer. Further large clinical studies are necessary to investigate the effectiveness and safety of the treatment.
MeSH Keywords: Breast Neoplasms; Etoposide; Injections, Intraventricular; Meningeal Carcinomatosis
Background
Leptomeningeal metastasis (LM) is a form of central nervous system (CNS) metastasis characterized by dissemination and infiltration of malignant cells through the pia mater and arachnoid membrane, associated with multifocal neurologic deficits and dismal prognosis [1]. Recent advances in systemic chemotherapy and targeted agents have markedly improved clinical outcome of MBC patients. However, prolonged survival has led to a relative increase in the risk of progression to CNS, where the blood-brain barrier (BBB) impedes the efficient permeation of effective systemic agents. Prognosis of LM from breast cancer has not improved and median overall survival from the diagnosis of LM still remains less than 3 to 4 months [2–4]. Due to poor penetration of systemically administered chemotherapy through the BBB, intrathecal administration has been regarded as one of the most efficient ways to deliver effective therapeutic agent into the subarachnoidal space and is widely used as an important component of LM treatments. Despite combined multimodality treatments, including radiotherapy and intrathecal and systemic chemotherapy, most MBC patients with LM have neurologic progression within a few weeks. Some patients who maintain good performance status need subsequent treatment to prevent further neurologic deficits and to prolong survival. Unfortunately, clinical research on salvage therapy after failure of first-line treatment for MBC patients with LM has rarely been reported in the literature. Here, we report a case of breast cancer with LM in a patient who showed durable response to salvage intrathecal etoposide subsequent to failure of MTx.
Case Report
A 42-year-old premenopausal woman diagnosed with breast cancer underwent breast-conserving surgery and axillary lymph node dissection. Pathological examination demonstrated the tumor was invasive ductal carcinoma with stage IIB (T2N1M0), Nottingham histologic score of 8, estrogen receptor negative, progesterone receptor negative, and human epidermal growth factor receptor type 2 negative. She was treated by a combination of doxorubicin and cyclophosphamide followed by docetaxel as adjuvant chemotherapy.
She complained of left-sided headache and nausea during the third week of adjuvant radiotherapy. The symptoms aggravated over the next 2 weeks and she was hospitalized for further evaluation in June 2013. Brain magnetic resonance imaging (MRI) revealed a 2.2×2 cm enhancing mass in the left temporal lobe, multifocal meningeal enhancements in both cerebellar folia, and enhancing lesion along the ependymal lining of the lateral ventricles (Figure 1A). A lumbar puncture was done and the cerebrospinal fluid (CSF) examination showed normal opening pressure, elevated protein level (91 mg/dL), normal glycorrhachia, and the presence of malignant cells. She was diagnosed with brain metastasis with LM. There was no evidence of other systemic extra-cranial recurrence on imaging study.
Figure 1.
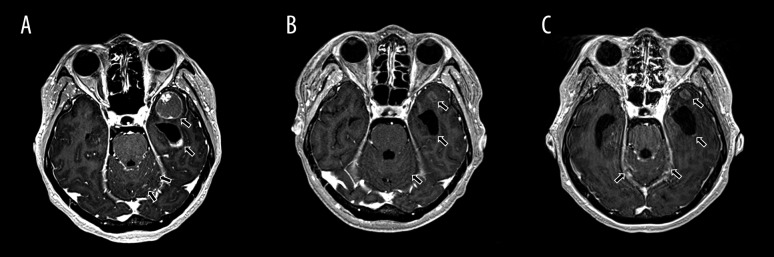
(A) Brain magnetic resonance imaging (MRI) at the diagnosis of leptomeningeal metastasis revealed 2.2×2 cm sized heterogeneous enhancing mass in left temporal lobe, multifocal meningeal enhancement in both cerebellar folia, and enhancing lesions along the ependymal lining of the lateral ventricles. (B) Brain MRI performed after intrathecal etoposide for 8 weeks and Gamma knife radiosurgery demonstrated remarkable decreased leptomeningeal and ependymal enhancing lesions, and temporal lobe mass lesion. (C) Brain MRI at the time of neurological progression subsequent to intrathecal etoposide for 33 weeks demonstrated newly developed meningeal enhancement in both cerebellar folia, and a new enhancing lesion along the ependymal lining of the lateral ventricles.
Gamma knife radiosurgery with a radiation dose of 18 Gy was performed for the temporal lobe mass lesion. Intrathecal administration of 15 mg MTx was done bi-weekly via an Ommaya reservoir with corticosteroid (10 mg of dexamethasone). Despite 2 weeks of treatments, she developed progressive neurological deteriorations, including worsening headache, diplopia, and lower leg weakness. The follow-up CSF analysis showed high opening pressure of 240 mmH2O and persistent malignant cells. Treatment failure was highly suspected. There was no specific recommended salvage treatment subsequent to LM progression to first-line therapy in the literature.
After explaining the experimental nature of salvage treatment, written informed consent was obtained both from the patient and her family. Intrathecal etoposide was administered as salvage therapy based on prior clinical trials employing intrathecal etoposide against LM [5,6]. We modified the dosing scheme for intrathecal etoposide to a once-a-week administration schedule instead of consecutive 5 days, with 2 to 5 weeks of rest. For intrathecal administration, a 5-ml vial containing etoposide 100 mg (E.P.S®, Boryung Pharm Co., Ltd, Seoul, Korea) was diluted with normal saline, yielding a final concentration of 0.2 mg per ml. The final solution of 5 ml containing etoposide 1 mg was injected over a 2-min period after draining of 5 ml of CSF from the reservoir for discarding. The treatment was given weekly through an Ommaya reservoir. Corticosteroid (10 mg of dexamethasone) was used for a prophylaxis of chemical arachnoiditis.
After the second injection of etoposide, the patient showed neurological improvements and CSF protein level decreased. CSF malignant cells were undetectable after the fifth injection. She recovered to be ambulatory with Eastern Cooperative Oncology Group Performance Status of 2, and was discharged after the eighth treatment. Follow-up brain MRI demonstrated remarkable decreased extent of leptomeningeal enhancements, disappearance of enhancing lesion along the ependymal lining of the lateral ventricles, and decreased temporal lobe mass (Figure 1B).
The weekly etoposide injection was maintained on an outpatient basis and no adverse event was observed during the treatment period. The patient received intrathecal etoposide for 33 weeks until she developed progressively worsening headache and ataxia. Follow-up brain MRI demonstrated newly-developed leptomeningeal enhancement in cerebellar folia (Figure 1C). High opening pressure of 240 mmH2O, increased protein (144 mg/dL), and reappearance of malignant cells were noted in CSF analysis, indicating LM progression. Despite whole-brain radiotherapy and intrathecal cytarabine, her neurological deteriorations progressed and she died due to the disease.
Discussion
As the number of MBC patients diagnosed with LM has been growing, LM is recently on the rise as one of important clinical issues in the management of MBC. However, development of new treatment options is lacking and treatment protocol is still poorly standardized. Conventional treatment options for LM include intrathecal chemotherapy, systemic chemotherapy, and radiotherapy. Due to its advantage to deliver effective chemotherapeutic agents into CNS, intrathecal chemotherapy has been the mainstay of treatment for LM in MBC patients. However, a limited number of therapeutic agents are available for intrathecal injection. Methotrexate (MTx), cytarabine, liposomal cytarabine, and thiotepa are commonly-used agents in newly-diagnosed LM cases. Despite intrathecal chemotherapy and other combined treatments, most of the treated patients develop treatment failure accompanied by neurologic deterioration within a few weeks and salvage therapy is required to prevent worsening neurologic deficit and to prolong survival. Selected patients who respond favorably to multimodal treatments and intrathecal chemotherapy [7–11] might especially benefit from salvage treatment in terms of time to neurologic progression and overall survival. However, research on salvage intrathecal chemotherapy for LM in breast cancer has been insufficient for the recent clinical demand and very few studies reported data to evaluate its efficacy in the literature. Le Rhun et al. and Comte et al. presented retrospective analysis of the outcome of second-line intrathecal thiotepa for MBC patients with LM after failure of Depocyte and MTx, respectively [12,13]. They demonstrated the median survival following intra-CSF thiotepa was 4 to 5 months. No chemotherapeutic agents other than thiotepa have been investigated as salvage intrathecal regimen for MBC patients after failure of first-line treatment, and further research is warranted to develop other salvage treatment options.
Two pilot trials evaluated the feasibility of intraventricularly administered etoposide in patients with relapsed metastatic brain tumors, and the preliminary data suggested that intraventricular etoposide was well tolerated without significant adverse effects [5,6]. Because a specific recommended salvage regimen was not available after failure of first-line treatment, we utilized etoposide after failure of MTx in this case, based upon the above results. Instead of the previous 5 consecutive days of intrathecal etoposide, we devised a weekly administration scheme. A single injection per week is more simple and convenient in clinical practice than 5 days of injection and 2 to 4 weeks of rest, which was the previously evaluated dosing schedule for intrathecal etoposide [5,6].
This patient had a durable response to the modified intrathecal administration of etoposide and she received the treatment for 33 weeks; time to neurologic progression was estimated to be about 230 days for the treatment and overall survival was 301 days from the diagnosis of LM. Jaeckle et al. reported that median time to neurologic progression was 49 days and median survival was 88 days in 53 breast cancer patients with LM who were treated with intrathecal Depocyte [14]. Rudnicka et al. demonstrated median survival of 67 breast cancer patients with LM was 16 weeks despite multimodality treatments including intrathecal chemotherapy [2]. If we consider the overall poor outcome of MBC patients with LM, the current case is of significant clinical interest by presenting favorable response to salvage intrathecal chemotherapy for a relatively long period of about 230 days despite failure of first-line MTx. Importantly, there was no significant adverse effect related to the salvage treatment in this case. Previous studies on intrathecal etoposide demonstrated favorable toxicity profile related to its administration [5,6], suggesting it could be an alternative option for elderly or frail MBC patients with LM, in whom adverse events during the treatment are of great concern.
Conclusions
To the best of our knowledge, this is the first published report of salvage intrathecal etoposide after failure of MTx, leading to prolonged treatment response for LM of breast cancer. Intrathecal etoposide can be considered as an additional treatment option for LM in breast cancer. Further large clinical studies are needed to investigate the effectiveness and safety of the treatment.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References:
- 1.Leal T, Chang JE, Mehta M, et al. Leptomeningeal metastasis: challenges in diagnosis and treatment. Curr Cancer Ther Rev. 2011;7(4):319–27. doi: 10.2174/157339411797642597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis – the role of multimodality treatment. J Neurooncol. 2007;84(1):57–62. doi: 10.1007/s11060-007-9340-4. [DOI] [PubMed] [Google Scholar]
- 3.Niwińska A, Rudnicka H, Murawska M. Breast cancer leptomeningeal metastasis: the results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin Breast Cancer. 2015;15(1):66–72. doi: 10.1016/j.clbc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Rhun EL, Taillibert S, Zairi F, et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol. 2013;113(1):83–92. doi: 10.1007/s11060-013-1092-8. [DOI] [PubMed] [Google Scholar]
- 5.Fleischhack G, Reif S, Hasan C, et al. Feasibility of intraventricular administration of etoposide in patients with metastatic brain tumours. Br J Cancer. 2001;84(11):1453–59. doi: 10.1054/bjoc.2001.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slavc I, Schuller E, Falger J, et al. Feasibility of long-term intraventricular therapy with mafosfamide (n=26) and etoposide (n=11): experience in 26 children with disseminated malignant brain tumors. J Neurooncol. 2003;64(3):239–47. doi: 10.1023/a:1025633704071. [DOI] [PubMed] [Google Scholar]
- 7.Le Rhun E, Taillibert S, Zairi F, et al. Prolonged survival of patients with breast cancer-related leptomeningeal metastases. Anticancer Res. 2013;33(5):2057–63. [PubMed] [Google Scholar]
- 8.Peroukides S, Onyenadum A, Starakis I, et al. Prolonged survival of neoplastic meningitis from breast cancer with letrozole and intrathecal methotrexate: a case report. J Neurooncol. 2011;101(3):509–11. doi: 10.1007/s11060-010-0268-8. [DOI] [PubMed] [Google Scholar]
- 9.Vincent A, Lesser G, Brown D, et al. Prolonged regression of metastatic leptomeningeal breast cancer that has failed conventional therapy: A case report and review of the literature. J Breast Cancer. 2013;16(1):122–26. doi: 10.4048/jbc.2013.16.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira M, Braga S, Passos-Coelho JL, et al. Complete response in HER2+ leptomeningeal carcinomatosis from breast cancer with intrathecal trastuzumab. Breast Cancer Res Treat. 2011;127(3):841–44. doi: 10.1007/s10549-011-1417-2. [DOI] [PubMed] [Google Scholar]
- 11.Santa-Maria CA, Cimino-Mathews A, Moseley KF, et al. Complete radiologic response and long-term survival with use of systemic high-dose methotrexate for breast cancer–associated leptomeningeal disease. Clin Breast Cancer. 2012;12(6):445–49. doi: 10.1016/j.clbc.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Rhun E, Taillibert S, Devos P, et al. Salvage intracerebrospinal fluid thiotepa in breast cancer-related leptomeningeal metastases: a retrospective case series. Anticancer Drugs. 2013;24(10):1093–97. doi: 10.1097/CAD.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 13.Comte A, Jdid W, Guilhaume MN, et al. Survival of breast cancer patients with meningeal carcinomatosis treated by intrathecal thiotepa. J Neurooncol. 2013;115(3):445–52. doi: 10.1007/s11060-013-1244-x. [DOI] [PubMed] [Google Scholar]
- 14.Jaeckle KA, Phuphanich S, van den Bent MJ, et al. Intrathecal treatment of neoplastic meningitis due to breast cancer with a slow-release formulation of cytarabine. Br J Cancer. 2001;84(2):157–63. doi: 10.1054/bjoc.2000.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]


