Abstract
High-mobility group box 1 (HMGB-1) has been reported as a “late” proinflammatory mediator in sepsis. In vitro data have shown that HMGB-1 can induce activation of intracellular signaling pathways via interaction with at least three pattern recognition receptors: Toll-like receptor (TLR) 2, TLR-4, and the receptor for advanced glycation end products (RAGE). The objective of this study was to investigate the role of these receptors in the in vivo response to HMGB-1. Therefore, we first performed a time-series experiment with wild-type (Wt) mice. High-mobility group box 1 induced time-dependent elevations of TNF-α, IL-6, monocyte chemoattractant protein 1, and thrombin-antithrombin complex levels in peritoneal lavage fluid and plasma. This inflammatory reaction was accompanied by a prominent and sustained rise in neutrophil counts in the peritoneal cavity. We next administered HMGB-1 to Wt, TLR-2−/−, TLR-4−/−, and RAGE−/− mice. All genotypes showed similar plasma levels of TNF-α, IL-6, IL-10, and thrombin-antithrombin complex at 2 h after intraperitoneal injection of HMGB-1. Compared with Wt mice, both TLR-4−/− and RAGE−/− mice displayed lower TNF-α and IL-6 concentrations and lower neutrophil numbers in their peritoneal lavage fluid. In contrast, TLR-2−/− mice showed increased levels of TNF-α and IL-6 in their peritoneal cavity relative to Wt mice. These data indicate that HMGB-1 induces release of cytokines, activation of coagulation, and neutrophil recruitment in vivo via a mechanism that at least in part depends on TLR-4 and RAGE.
Keywords: HMGB-1, sepsis, toll-like receptor 2, toll-like receptor 4, receptor for advanced glycation end products, host defense
INTRODUCTION
High-mobility group box 1 (HMGB-1) is a nuclear protein present in almost all eukaryotic cells, where it functions to stabilize nucleosome formation (1). High-mobility group box 1 is released from necrotic cells, as well as from macrophages, dendritic cells, and natural killer cells upon activation by infectious agents. This (extracellular) HMGB-1 has cytokine-like properties, and it has been implicated as a late mediator of sepsis (1). Unlike the prototypic proinflammatory cytokines TNF-α and IL-1β, HMGB-1 is secreted late after injection of LPS in mice, starting only after 8 h and remaining detectable up to 36 h thereafter (2). Similarly, experimental abdominal sepsis induced by cecal ligation and puncture was associated with a late (after 18 h) and sustained (more than 72 h) release of HMGB-1 in the circulation (3). Interestingly, dying of these mice paralleled the accumulation of systemic HMGB-1, and postponed treatment with an anti–HMGB-1 antibody protected against lethality caused by high-dose LPS administration or cecal ligation and puncture in mice (2, 3). Moreover, clinical observational studies have further implicated HMGB-1 as a late mediator of sepsis: patients with severe sepsis have elevated HMGB-1 concentrations in their circulation (2, 4, 5). In addition, we recently reported that patients with peritonitis have greater than 10-fold higher HMGB-1 concentrations in their abdominal fluid than in concurrently obtained plasma, suggesting that this mediator is released locally at the site of infection (5).
Understanding the nature of molecular signaling by HMGB-1 might be of value from a therapeutic point of view of those human diseases in which excessive amounts of HMGB-1 are released and where blockade of HMGB-1 may be beneficial. When present in extracellular fluid, HMGB-1 can activate various cell types to secrete proinflammatory cytokines (1). High-mobility group box 1 has been reported to transduce cellular signals in vitro by interacting with at least three receptors: Toll-like receptor 2 (TLR-2), TLR-4, and the receptor for advanced glycation end products (RAGE) (6–8). We here sought to determine the role of these pattern recognition receptors in the in vivo response to HMGB-1. For these studies, we chose to administer HMGB-1 at a dose previously shown to induce systemic toxicity (2, 9).
MATERIALS AND METHODS
Mice
Nine-week-old female C57Bl/6 wild-type (Wt) mice were purchased from Harlan Sprague Dawley Inc. Toll-like receptor 2–deficient (TLR-2−/−), TLR-4−/−, and RAGE−/− mice, backcrossed six times to a C57Bl/6 background, were generated as described previously (10, 11). The Institutional Animal Care and Use Committee of the Academic Medical Center, University of Amsterdam, approved all experiments.
Generation of CHO-psF-HMGB-1 cell line clone and production of HMGB-1 in Chinese hamster ovary cells
Methods for HMGB-1 isolation from mammalian Chinese hamster ovary (CHO) cells were described previously (7). Briefly, CHO cells were transfected with plasmid psF-HMGB-1 using the calcium phosphate method according to the manufacturer’s instructions (Gibco-BRL). The best HMGB-1–secreting cell line was adapted to grow in suspension by culturing in CHO-S-SFM II media supplemented with 2 mM glutamine, 300 µg/mL geneticin, and 1× penicillin/streptomycin. High-mobility group box 1 secretion was approximately 5 µg/mL in the medium. High-mobility group box 1 protein was isolated from conditioned medium by affinity purification using FLAG antibody (anti–FLAG 2 affinity gel) according to the manufacturer’s instructions (Sigma). The HMGB-1 used here was not treated with DNAse.
LPS content in HMGB-1
Contaminating LPS from HMGB-1 preparations was removed by either polymyxin B affinity column as per manufacturer’s instructions (Pierce) or phase separation using Triton X-114 (12). For Triton X-114 extraction, 1/20 volume of Triton X-114 was added to the HMGB-1 protein solution. After 10 min of gentle rotation at room temperature, the solution was centrifuged for 10 min (8,000g) at room temperature, and the top layer (containing HMGB-1 proteins) was carefully aspirated and saved. The LPS contamination of the HMGB-1 preparation was less than 3 pg/µg, as determined by the chromogenic Limulus amebocyte lysate assay according to the manufacturer’s instructions (Bio Whittaker, Walkersville, Md) (9). Addition of polymyxin B at 6 U/pg LPS did not inhibit HMGB-1 effects in in vitro experiments. Polyclonal anti–HMGB-1 antibodies did inhibit the HMGB-1 effects in vitro.
HMGB-1–induced inflammation in vivo
High-mobility group box 1 was generated and purified as described (7). High-mobility group box 1 was administered intraperitoneally at a dose of 500 µg in 650 µL phosphate buffered saline. For the time-series experiments, Wt mice (n = 4 at each time point) were killed before and 2, 4, 6, 10, and 24 h after HMGB-1 injection. For the experiment in which different mouse strains were compared, mice (n = 8 per group) were killed at 2 h after HMGB-1 administration. Two HMGB-1 batches were used: one for the time-series experiments and one for the experiment using different mouse strains; in these separate investigations, all mice were injected at the same time using the exact same HMGB-1 batch. Plasma, peritoneal lavage fluid (PLF), and tissue samples were harvested and processed exactly as described (13).
Assays
Cell counts and differentials were determined in PLF as described (13). TNF-α, IL-6, monocyte chemoattractant protein 1 (MCP-1), IL-10, interferon-γ (IFN-γ), and IL-12p70 were measured by cytometric bead array multiplex assay (BD Biosciences). Thrombin-antithrombin complexes (TATc) were measured by enzyme-linked immunosorbent assay (Enzygnost TAT Micro, Dade Behring). Levels of alanine aminotransferase, aspartate aminotransferase, creatinine, and urea were determined with commercially available kits (Sigma-Aldrich) using a Hitachi analyzer (Boehringer Mannheim). Histology was done as described (13).
Statistical analysis
All data are expressed as means ±SEM. Differences between groups were analyzed by Mann-Whitney U test. Overall differences within groups in time were analyzed using a Kruskal-Wallis test. Values of P < 0.05 were considered to represent statistical significance.
RESULTS
Time-dependent response to HMGB-1 in Wt mice
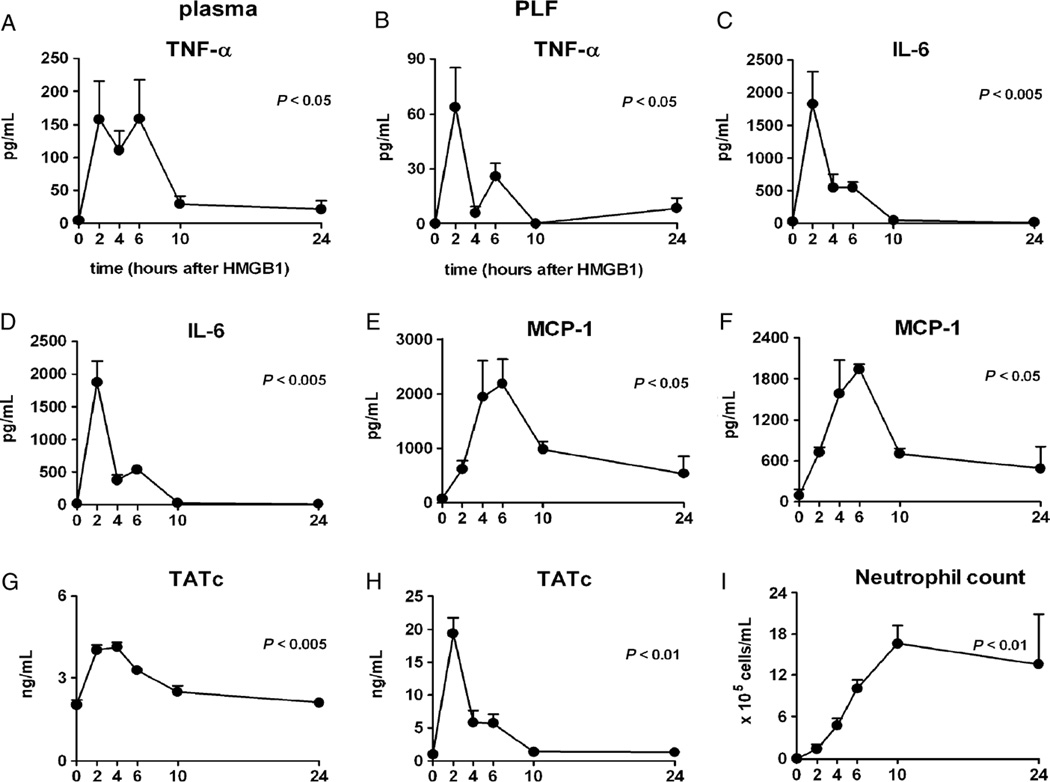
Intraperitoneal administration of HMGB-1 induced strong increases in the concentrations of TNF-α, IL-6, and MCP-1 in plasma (Fig. 1 A, C, and E) and PLF (Fig. 1 B, D, and F). The kinetics of HMGB-1–induced cytokine release varied, with TNF-α and IL-6 peaking early and MCP-1 showing a more delayed and sustained response. IL-10, IFN-γ, and IL-12p70 concentrations remained undetectable throughout. In addition, HMGB-1 administration elicited local and systemic activation of coagulation, as indicated by increases in plasma and PLF concentrations of TATc (Fig. 1, G and H). Intraperitoneal injection of HMGB-1 induced a prominent and sustained influx of neutrophils (Fig. 1I). High-mobility group box 1 administration did not cause organ damage, as reflected by unaltered plasma levels of alanine aminotransferase, aspartate aminotransferase, creatinine, and urea, and unremarkable histopathology of lungs, livers, spleens, and kidneys (data not shown). Based on these experiments, we decided to use the 2-h time point, at which most responses measured reached their maximum, for follow-up studies.
Fig. 1. High-mobility group box 1 induces inflammation in vivo.
Wild-type mice received an intraperitoneal injection of HMGB-1 (500 µg) at t = 0. Left panels show plasma levels, and right panels show concentrations/numbers in PLF. Data are expressed as means ± SEM (n = 4 mice per time point). P values indicate overall differences in time.
Role of TLR-2, TLR-4, and RAGE in HMGB-1–induced inflammation
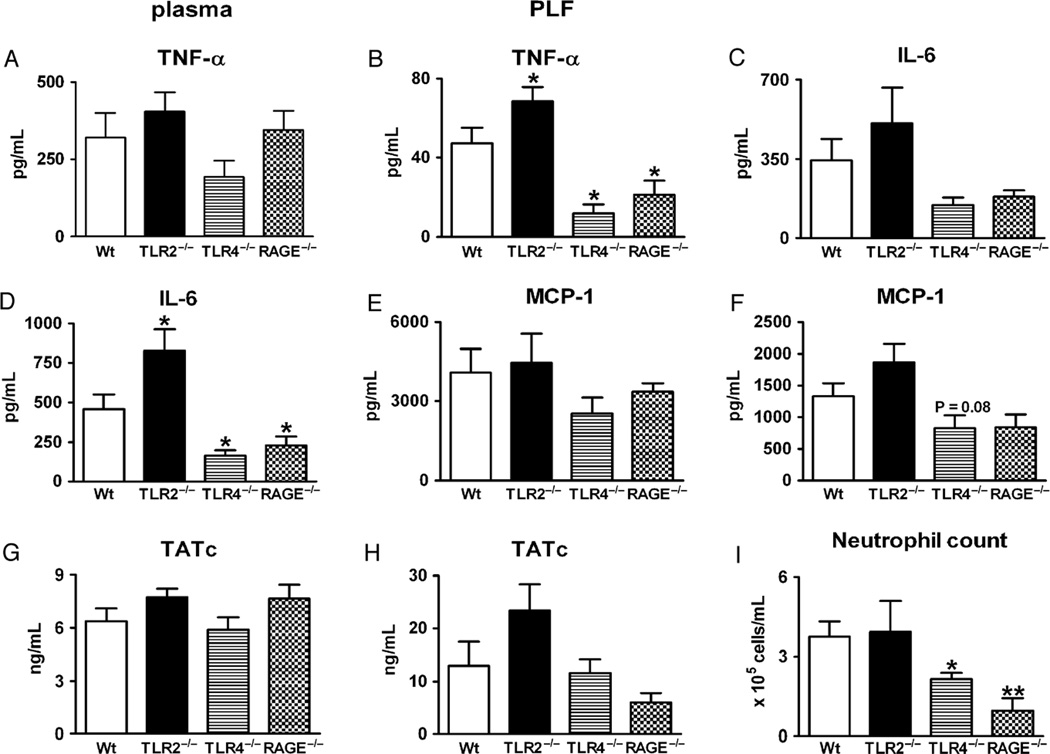
Wild-type, TLR-2−/−, TLR-4−/−, and RAGE−/− mice received an intraperitoneal injection with HMGB-1; 2 h later, they were killed for measurements of TNF-α, IL-6, MCP-1, and TATc in plasma (Fig. 2, left panel) and PLF (Fig. 2, right upper four panels). The plasma levels of these markers of inflammation and coagulation did not differ significantly between the knockout and Wt mice. Remarkably, relative to Wt mice, TLR-2−/− mice displayed an overall stronger responsiveness to HMGB-1 in the peritoneal cavity: TNF-α and IL-6 levels were higher in PLF (Fig. 2, B and D), whereas MCP-1 and TATc PLF concentrations tended to be higher in TLR-2−/− mice (Fig. 2, F and H). Interestingly, relative to Wt mice, both TLR-4−/− and RAGE−/− mice demonstrated lower TNF-α and IL-6 concentrations in their PLF (Fig. 2, B and D). In addition, MCP-1 tended to be lower in the TLR-4−/− mice (Fig. 2F), although the difference with Wt mice did not reach statistical significance. High-mobility group box 1–induced neutrophil influx in PLF was attenuated in TLR-4−/− and RAGE−/− mice and not altered in TLR-2−/− mice (Fig. 2I).
Fig. 2. High-mobility group box 1–induced inflammation is partially dependent on TLR-4 and RAGE.
Mice received an intraperitoneal injection of HMGB-1 (500 µg), and plasma and PLF were obtained 2 h later. Left panels show plasma levels, and right panels show concentrations/numbers in PLF. Baseline values of the measurements presented are given in Figure 1; there were no differences in these values between Wt and TLR-2−/−, TLR-4−/−, or RAGE−/− mice. Data are expressed as means ± SEM (n = 8 mice per group). *P < 0.05 vs. Wt mice. **P < 0.05 vs. Wt mice.
DISCUSSION
Although systemic injection of recombinant HMGB-1 induces a sepsis-like syndrome in mice (1), knowledge of the capacity of HMGB-1 to elicit systemic inflammatory responses relevant for sepsis is limited. Previous studies have documented that intrapulmonary delivery of HMGB-1 induces lung inflammation (14). We here show that intraperitoneal injection of HMGB-1 induces a systemic inflammatory and procoagulant response, as reflected by transient increases in the concentrations of TNF-α, IL-6, MCP-1, and TATc, and an influx of neutrophils into the peritoneal cavity. In addition, we demonstrate for the first time that TLR-4 and RAGE contribute to HMGB-1–induced inflammation in vivo.
Several studies have documented a role for TLR-2, TLR-4, and RAGE in HMGB-1–induced cell activation in vitro, although some discrepant results were reported. High-mobility group box 1 was shown to rapidly interact with TLR-4 exposed by RAW macrophage-like cells in one study (8), but not in another (7). Anti–TLR-4 treatment reduced HMGB-1–induced cytokine release by human whole blood and primary human macrophages, and murine TLR-4−/− macrophages were similarly unresponsive to HMGB-1 (7). In addition, HMGB-1 was reported to activate human embryonic kidney cells transfected with TLR-4 in one study (8), but not in another (7). Of note, recombinant HMGB-1 was previously reported to cause lethality in C3H/HeJ mice, which carry a loss-of-function mutation in their tlr4 gene (2). Although these data suggest that TLR-4 is not important for HMGB-1–induced lethality, they do not necessarily contradict our current findings. Indeed, we did not investigate mortality and detected a role for TLR-4 in some HMGB-1 responses (neutrophil recruitment, local release of TNF-α and IL-6) but not in all HMGB-1–induced effects (coagulation activation). In addition, a recent investigation has documented a clear role of TLR-4 in recombinant HMGB-1–induced effects during I/R injury, further supporting a role for TLR-4 in HMGB-1 signaling in vivo (15). Inhibition or genetic elimination of TLR-2 did not influence HMGB-1 responses by human whole blood or human or mouse macrophages (7, 16). However, HMGB-1 rapidly interacted with TLR-2 on RAW macrophage-like cells (8), and inhibition of TLR-2 expression in these cells by transfection with a dominant negative construct diminished HMGB-1–induced nuclear factor-κB activation (6). In addition, TLR-2–transfected human embryonic kidney cells responded to HMGB-1 (7, 8). With regard to the role of RAGE in HMGB-1–induced cell activation, RAGE−/− macrophages were found to release less TNF-α upon exposure to HMGB-1 (16); however, neither inhibition of RAGE expression by RAW macrophage-like cells (6) nor anti-RAGE treatment of human whole blood or human macrophages altered HMGB-1 effects (7). A very recent study has suggested that highly pure HMGB-1 does not have cytokine-inducing capacity; pure HMGB-1 could bind to RAGE and was able to activate immune cells after acquiring CpG DNA (17). Considering that HMGB-1 is rather “sticky,” it is conceivable that in the in vivo setting, HMGB-1 at least in part activates cells indirectly by first acquiring an immune-stimulating pathogen–associated or damage-associated molecular pattern.
It should be noted that earlier studies examining biological effects of HMGB-1 made use of at least three different HMGB-1 preparations, that is, HMGB-1 isolated from calf thymus, recombinant HMGB-1 derived from Escherichia coli, and HMGB-1 from transfected CHO cells (as used here). These different HMGB-1 proteins may be associated with different modifications and possible contaminants that may influence their ability to signal via TLRs and other receptors. As such, our current results should be interpreted with caution.
We recently reported that patients with peritonitis showed strongly elevated HMGB-1 concentrations in their abdominal fluid (5). Therefore, we here chose to administer HMGB-1 intraperitoneally to more closely mimic a possible clinical scenario of an (initially) localized infection. The fact that RAGE and TLR-4 deficiency exclusively or predominantly affected HMGB-1 responses in the peritoneal cavity may have been related to the route of administration and/or differences in cell types primarily activated by HMGB-1 in the abdominal cavity versus blood. In this respect, it is interesting to note that HMGB-1 activated neutrophils by a mechanism that did not rely on TLR-2 or TLR-4 (6). It remains to be established why TLR-2−/− mice responded more avidly to HMGB-1. One possible explanation could be that the absence of TLR-2, which presumably can be an HMGB-1–binding receptor (6–8), facilitates the interaction between HMGB-1 and other immune-activating receptors.
In conclusion, this study provides the first evidence that HMGB-1 elicits cytokine release, coagulation activation, and neutrophil recruitment in vivo by a mechanism that in part relies on TLR-4 and RAGE.
ACKNOWLEDGMENTS
The authors thank J. Daalhuisen and M.S. ten Brink for expert technical assistance.
This work has been performed at the Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
REFERENCES
- 1.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 As a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 3.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 5.van Zoelen MA, Laterre PF, van Veen SQ, van Till JW, Wittebole X, Bresser P, Tanck MW, Dugernier T, Ishizaka A, Boermeester MA, van der Poll T. Systemic and local high mobility group box 1 concentrations during severe infection. Crit Care Med. 2007;35:2799–2804. doi: 10.1097/01.CCM.0000287588.69000.97. [DOI] [PubMed] [Google Scholar]
- 6.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 7.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 8.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, et al. High mobility group box 1 protein interacts with multiple toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Wang H, Mason JM, Yu M, Ulloa L, Czura CJ, Tracey KJ, Yang H. Recombinant HMGB1 with cytokine-stimulating activity. J Immunol Methods. 2004;289:211–213. doi: 10.1016/j.jim.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 11.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aida J, Pabst MJ. Priming of neutrophils by lipopolysaccharide for enhanced release of superoxide. Requirement for plasma but not for tumor necrosis factor-alpha. J Immunol. 2000;145:3017–3025. [PubMed] [Google Scholar]
- 13.Renckens R, Roelofs JJ, ter Horst SA, van’t Veer C, Havik SR, Florquin S, Wagenaar GT, Meijers JC, van der Poll T. Absence of thrombin-activatable fibrinolysis inhibitor protects against sepsis-induced liver injury in mice. J Immunol. 2005;175:6764–6771. doi: 10.4049/jimmunol.175.10.6764. [DOI] [PubMed] [Google Scholar]
- 14.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 15.Izuishi K, Tsung A, Jeyabalan G, Critchlow ND, Li J, Tracey KJ, Demarco RA, Lotze MT, Fink MP, Geller DA, et al. Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol. 2006;176:7154–7158. doi: 10.4049/jimmunol.176.12.7154. [DOI] [PubMed] [Google Scholar]
- 16.Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 17.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]