Abstract
Inflammation can cause damage and even death. What controls this primitive and potentially lethal innate immune response to injury and infection? Molecular and neurophysiological studies during the past decade have revealed a pivotal answer: immunity is coordinated by neural circuits that operate reflexively The afferent arc of the reflex consists of nerves that sense injury and infection. This activates efferent neural circuits, including the cholinergic anti-inflammatory pathway that modulate immune responses and the progression of inflammatory diseases. It might be possible to develop therapeutics that target neural networks for the treatment of inflammatory disorders.
The discovery ~10 years ago that pro-inflammatory responses are controlled by evolutionarily ancient neural circuits steered together the fields of immunology and neuroscience. The principle established is that the immune system can no longer be regarded as almost entirely autonomous. The ‘inflammatory reflex’ is one example of how action potentials originating in neurons continuously shape immunity1,2. Neurotransmitters propagate essential information that regulates the magnitude of the host response to infection or injury. The lymphoid organs of the immune system are innervated by cholinergic, catecholaminergic (Box 1), peptidergic and other neurons; this is a topic that has been extensively reviewed elsewhere and is beyond the scope of this Review3–7. Another large body of work had established that neurotransmitters can interact with immune cells and alter their function4,8. In this Review, I discuss how integrated neural networks influence innate immune responses, with particular focus on the cholinergic anti-inflammatory pathway. This well-established neural circuit terminates excessive pro-inflammatory cytokine responses, thereby preventing immune-mediated damage. Tonic neural activity in this pathway is essential for health because when it is impaired the consequences include unrestrained cytokine responses that damage tissue or kill the host. In addition, enhancing the activity of this immune-mediated neural circuit confers protection against damage by inhibiting cytokine release during infection, autoimmunity, shock and other inflammatory syndromes.
Box 1 | Catecholamines modulate immune cell function.
Work by early investigators in neuroimmunology established that immune cells express neurotransmitter receptors and that ligand–receptor interactions modulate cellular functions8. Since then, a host of immunoregulatory activities has been assigned to all known neurotransmitters. For example, catecholamines have been implicated in many aspects of innate and adaptive immune responses, including the capacity to modulate the efficiency of antigen presentation by dendritic cells, the clonal expansion of lymphocytes, the migration and trafficking of cells, the suppression of cellular immune responses and the enhancement of humoral immune responses4,6,91. The net effect of these signals depends on whether the α- or β-adrenergic receptors are activated in the responding cells. Studies of these mechanisms have used pharmacological techniques to selectively activate or antagonize the signalling pathways that are triggered by these receptors or to create experimental lesions in selective nerves, followed by assessing the effects on immunological endpoints.
Using these techniques, intriguing results have been obtained for autoimmune diabetes and collagen-induced arthritis, supporting the idea that disease progression is influenced by loss of neural inhibitory activity92,93. For instance, in the non-obese diabetic mouse model, neurons surrounding the insulin-producing β-cells are destroyed before β-cells, so it is possible that loss of tonic inhibitory neural signals contributes to the subsequent immune-mediated destruction of the β-cells92. In DBA mice subjected to collagen-induced arthritis, there is a depletion of neurons in the spleen, which is pronounced before the onset of clinical signs of disease93. Furthermore, recent evidence indicates that stimulating the cholinergic anti-inflammatory pathway in these mice reverses arthritis94,95. Given the role of splenic neuronal fibres in transmitting inhibitory signals from the cholinergic anti-inflammatory pathway to the spleen, the observed time course of loss of splenic neuronal function before the development of arthritis is strikingly consistent with the possibility that loss of activity in the cholinergic anti-inflammatory pathway directly contributes to disease pathogenesis. Recent advances in integrative neurophysiology and immunology reveal that neural networks function reflexively to modulate immune responses.
Advances in delineating the neurophysiology, functional anatomy and molecular mechanisms of the cholinergic anti-inflammatory pathway rendered it a pre-eminent model circuit to understand the neural control of immunity. This laid the foundational methodology for studying and identifying discrete neural circuits that reflexively influence innate and adaptive immune responses. The idea that neural circuits provide functional control over immune responses was surprising to many who had previously considered the immune system to be essentially autonomous. We have now learnt how to study the behaviour of the immune system as the contextualized output of a distributed system, coordinated by neural circuits and regulated by neural principles that maintain physiological homeostasis in other organ systems.
As is the case for all complex organ systems, it is nearly impossible to predict immune responses with any degree of certainty without knowing the state of the neural controllers. Consider, for example, that an isolated heart can, if properly treated, continue to beat on a laboratory bench long after it has been deprived of its nerve supply. But this experimental design will not enable us to fully understand why the heart of a motor vehicle driver begins racing minutes after a nearmiss collision with another car. By analogy, monocyte and lymphocyte cultures can be stimulated to manifest a range of cellular responses and immunologically relevant functions in vitro, lacking any input from the nervous system. But this isolated system cannot provide a meaningful understanding of how complex immunological responses develop or are coordinated in the intact organism. A denervated or isolated system possesses neither the full range of physiological potential nor integration of diverse responses, both of which are indispensable for maintaining health during conditions of stress in real life.
The evolution of integrative neural circuits that control the behaviour of organ systems has facilitated the fine level of homeostatic control that is necessary for optimized survival and species adaptation to a wide range of environmental stresses and threats. We now know that these principles of basic neural circuitry apply to the control of immunity. In this Review, I describe the status of this field and address the question of how this new knowledge can be applied for therapeutic benefit.
Reflex circuits in physiological responses
Physiological systems in mammals are regulated by the autonomic nervous system. Functioning largely without voluntary control, and sometimes referred to as the visceral nervous system, it is composed of two separate divisions, the sympathetic and parasympathetic nervous systems. The stimulation of the sympathetic nervous system mediates physiological responses, termed ‘fight or flight’, that are characterized by increased heart rate and blood pressure, mobilization of energy stores and heightened arousal. epinephrine and noradrenaline, the principal sympathetic neurotransmitters, mediate cellular responses by interacting with G protein-coupled adrenergic receptors (termed α1, α2, β1, β2 and β3 adrenergic receptors). The stimulation of the parasympathetic nervous system tends to produce effects that oppose sympathetic actions, such as decreased heart rate and cardiac contractility and enhanced digestive functions. Acetylcholine, the principal parasympathetic neurotransmitter, interacts with the G protein-coupled muscarinic acetylcholine receptors m1–m5 and nicotinic ligand-gated ion channels (termed neuronal type I–III and muscle type IV)9–11.
The sympathetic and parasympathetic nervous systems are distinguished by unique anatomical features that are related to the distribution of the nerve cell bodies and patterns of organ innervation. Parasympathetic neuronal cell bodies reside in the brainstem medulla and sacral portions of the spinal cord, whereas sympathetic neuronal cell bodies reside in the thoracic and lumbar portion of the spinal cord. Sympathetic and parasympathetic primary cell bodies or presynaptic neurons project myelinated axons through cranial nerves or spinal nerves that form synapses on secondary or postsynaptic neurons, which are located in ganglia. These postsynaptic neurons in turn project unmyelinated axons to the cells that are targeted in the innervated organ. The location of these ganglia relative to the target organ distinguishes the two divisions: parasympathetic ganglia tend be close to or inside the innervated organ, whereas sympathetic ganglia are located close to the spinal cord.
The isolated activation of sympathetic and parasym-pathetic outflow can produce functionally opposing results, but it is important to note that the net physiological output of an intact system cannot always be predicted by simply summing the effects mediated by the two divisions. In cardiac physiology, for example, the direct stimulation of the sympathetic nerves to the heart produces the predicted net increase in cardiac output. Also, as expected, the isolated stimulation of the parasympathetic vagus nerve to the heart produces an opposing effect, which results in decreased cardiac output. However, co-stimulation of sympathetic and parasympathetic nerves to the heart in an intact organism produces a significant increase in cardiac output, a net effect that exceeds the effect observed when the sympathetic nerve is stimulated alone. This occurs because parasympathetic stimulation slows the heart rate, which improves the efficiency by which the heart is filled with blood12. This illustrates that the observed net behaviour of an organ system is the result of the composite activity of many neural circuits, which can comprise sympathetic, parasympathetic and other neural paths working together to regulate the integrated output of the system.
The inflammatory reflex
A reflex is the involuntary response to a stimulus. Autonomic reflex arcs control the actions of inner organs (for example, the heart) and somatic reflex arcs control muscles. The anatomical and functional basis of the reflex resides in the reflex arc, a neural pathway consisting of sensory neurons that are interconnected with motor neurons. Input or stimulation of the afferent arc activates output in the efferent or motor arc. Reflexes provide homeostasis to complex systems, as illustrated, for example, by the baroreflex, which controls blood pressure (Box 2). The inflammatory reflex arc consists of an afferent sensory neural arc that detects the molecular products of injury, infection and inflammation, and an efferent motor neural arc that transmits signals to modulate immune responses.
Box 2 | Blood pressure set point in cardiovascular physiology.
In cardiovascular physiology, the baroreflex is the reflex action unit that regulates the homeostasis of blood pressure. Specialized neurons, the arterial baroreceptors, are stretch-sensitive mechanoreceptors that are located in the aortic arch, the carotid body and elsewhere. They respond to the amount of stretch or tension in the arterial wall, such that the magnitude of the stretch produces a graded neural response that is proportionate to the stimulus. The more the arterial wall is stretched, as occurs with increases in blood pressure, the more frequently these neurons fire action potentials. The action potential output of the aortic baroreceptors is projected along sensory fibres travelling in the vagus nerve to the brain stem and terminating in the nucleus of the solitary tract (nucleus tractus solitarius). From there the signals are passed along polysynaptic projections to the rostral ventrolateral medulla, which regulates the output of preganglionic neurons in the sympathetic nervous system96. The signals are also relayed to the cell bodies of the efferent parasympathetic vagus nerve, which are located in the medullary nucleus ambiguus. It is not known precisely how these neural circuit centres process incoming neural information and integrate it with input from other centres (for example, respiratory centres).
The result of the integrated action in this circuit is that increased baroreceptor firing rate that is caused by increased blood pressure leads to inhibition of sympathetic output and a compensatory decrease in blood pressure. Alternatively, when blood pressure falls there is a corresponding decrease in baroreceptor firing rate that is relayed through the integrating brain stem centres and culminates in a compensatory increase in blood pressure. A fundamental principle illustrated by the baroreceptor reflex is that it responds acutely to changes in blood pressure that deviate from a blood pressure set point. Chronic or persistent deviations in blood pressure away from the normal blood pressure set point causes the baroreflex to adapt to a new level. Once reset, the baroreflex subsequently maintains blood pressure around the new set point. Advances in understanding the inflammatory reflex have enabled us to apply these fundamental principles of neurophysiological homeostasis to immunology.
The afferent arc
Early investigators studying the host response to infection and injury determined that brain neurons express specific receptors that respond to intracerebral cytokines to mediate physiological and behavioural changes8,13,14. For example, intracerebral administration of the pro-inflammatory cytokines interleukin-1 (IL-1) and tumour necrosis factor (TNF) lead to fever, anorexia, behavioural depression, decreased social and exploratory activity, activation of the hypothalamic– pituitary–adrenal (HPA) stress response and somnolence, a collection of symptoms referred to as sickness syndrome15,16. Substantial progress has been made in understanding the mechanism that underlies these responses. Neurons express the type I IL-1 receptor (termed IL1-R1), which is the principle receptor for mediating cellular responses to IL-1. Following binding of IL-1 to IL-1RI, the receptor forms a heterodimer with the IL-1R accessory protein. This complex associates with myeloid differentiation primary response protein 88 (myD88; a cytoplasmic Toll–IL-1R (TIR) domain-containing adaptor protein) and recruits signalling proteins that activate mitogen-activated protein kinases (MAPKs) and nuclear factor-κB (NF-κB)17. NF-κB activation in neurons has been implicated in controlling the transcription of genes, the products of which are involved in inflammation, injury and ischaemia and processes underlying memory formation and neuronal plasticity18. Addition of IL-1 to slice preparations of rat paraventricular nucleus (a neuronal nucleus in the hypothalamus) also stimulates neuronal depolarization through the activation of non-selective cationic conductance that depends on cyclooxygenase 2 (REF. 19). Together, these and other studies established the paradigm that the presence of IL-1 in the brain during injury or infection activates neural circuits that mediate the local and systemic pathophysiological behaviour of sickness syndrome.
These important concepts about the central nervous system became widely known among immunologists studying the physiological response to infection and injury, but few considered the possibility that peripheral nerves could directly function in detecting inflammation in the body. During the course of studying fever responses mediated by IL-1 administered into the abdomen of rodents, Watkins and colleagues20 discovered that peripheral neurons indeed sense the presence of inflammation in tissue. Specifically, they observed that the vagus nerve was required to transmit signals from the abdomen to the brain for fever to occur; cutting the subdiaphragmatic vagus nerve prevented the onset of fever in animals that were given quantities of IL-1 which were shown to induce fever in animals with intact vagus nerves21. Co-administration of IL-1 with the selective, competitive antagonist IL-1RA (IL-1R antagonist) significantly inhibited the development of fever induced by administration of intra-abdominal IL-1 (REF. 22). The inescapable conclusion was that the vagus nerve detected the presence of IL-1 in tissues and that this information was relayed to the hypothalamus, which in turn stimulated neural networks that initiated the onset of fever and sickness syndrome. Severing of the vagus nerve (vagotomy) rendered the animals physiologically unaware that pyrogens were present, such that they seemed numb or anaesthetized to the pyrogenic action of IL-1. So, how could this sensing mechanism operate?
In cardiovascular and metabolic physiology, oxygen, glucose and other metabolites in the extracellular milieu are sensed by specialized cells. When levels deviate from the normal range, these specialized cells activate the afferent arc of homeostatic autonomic reflexes, which then trigger compensatory reflex circuits. In the case of oxygen, the specialized cells that detect oxygen levels and activate the afferent arc of the autonomic reflex are glomus cells. During hypoxia, for example, glomus cells depolarize and release dopamine and noradrenaline. These neurotransmitters cause depolarization of nearby sensory fibres travelling in the vagus nerve, which propagate to the brain stem, where a motor arc is activated to mediate compensatory responses to increase oxygen delivery23,24. Watkins and colleagues22,25 reasoned that the molecular basis of the IL-1-dependent afferent arc to the brain might be mediated by the binding of IL-1 to glomus cells. They observed that biotinylated IL-1RA selectively bound to glomus cells located in anatomically discrete clusters that are adjacent to the vagus nerve. Although not yet proven, one suggestion is that IL-1 binding to glomus cells leads to cell depolarization and neurotransmitter release, which activates afferent neural pathways to the brain. Together with evidence that afferent vagus nerve firing rates increase following the administration of IL-1 (REF. 26) and that IL-1 activates neuronal depolarization19, these results indicate that IL-1 is sensed in peripheral tissues by the glomus cell-mediated activation of an afferent sensory arc in the vagus nerve.
IL-1 might prove to have a pivotal role in activating the sensory arc of the inflammatory reflex, but it is also possible that other endogenous and exogenous factors that are associated with inflammation can activate the inflammatory reflex. molecular products of infection activate macrophages, monocytes, dendritic cells and other early responding cells through pathogen-associated molecular patterns (PAMPs) — including endotoxins, enterotoxins, lipopeptides, glycopeptides and nucleic acids (FIG. 1) — that interact with Toll-like receptors (TLRs), which transduce intracellular signals, ultimately leading to the activation of NF-κB and the increased release of pro-inflammatory cytokines27. endogenous molecular products that are released from damaged cells during sterile injury or ischaemia even in the absence of pathogens include cytokines, high-mobility group box 1 protein (HMGB1), heat shock proteins, hyaluronan fragments, ATP, uric acid, heparin sulphate and other damage-associated molecular patterns (DAMPs)28. These molecules can activate TLRs, mannose receptors and nucleotide-binding oligomerization domain (NOD)-like receptors27–29. The net effect of exposure to either PAMPs or DAMPs is the initiation of intracellular signal transduction pathways that induce the increased expression of pro-inflammatory cytokines, including IL-1, which activates afferent signals in the vagus nerve. Therefore, the nervous system is alerted to the presence of infection, sterile injury or ischaemia through the activation of the afferent arc of the inflammatory reflex. As explained below, this activation is a pivotal step in governing the net output of innate immunity.
Figure 1. Neural circuitry of the inflammatory reflex.
The inflammatory reflex controls innate immune responses by a mechanism that targets the regulatory transcription factor nuclear factor-κB (NF-κB). Exogenous and endogenous molecular products of infection and injury interact with receptors that are expressed by cells of the innate immune system, including Toll-like receptors (TLRs) and NLRs (nucleotide-binding domain, leucine-rich-repeat-containing family, such as NALPs (NACHT-, LRR- and PYD-domain containing proteins) and nucleotide-binding oligomerization domain (NOD)-like receptors). Ligand–receptor interactions activate innate immune responses and induce the secretion of pro-inflammatory cytokines. These molecules also activate afferent sensory neurons, which constitute the sensory arc of the inflammatory reflex. Axons travelling in the vagus nerve relay this information as action potentials to the brain stem. This in turn activates the efferent arc, which is known as the cholinergic anti-inflammatory pathway. This inhibits innate immune responses in the spleen through inhibitory signals that arise in the brain stem, traverse the vagus nerve and signal through nicotinic acetylcholine receptor subunit α7 (α7nAChR), which is expressed by cytokine-producing immune cells. This leads to the suppression of NF-κB activation and the inhibition of innate immune responses. Note that the initiation of the inflammatory reflex by many possible ligands through key receptors is a crucial point of innate immune control. So, information from many stimulating molecules is processed by a smaller number of pattern recognition receptors that transduce signalling information to a small number of transcription factors, including NF-κB, that regulate innate immune responses. Maximal control is thereby derived from a circuit in which the inflammatory reflex targets this restricted point of information processing. dsRNA, double-stranded RNA; HMGB1, high-mobility group box 1 protein; IκB, inhibitor of NF-κB; IL-1α, interleukin 1α.
The efferent arc
The efferent arc of the inflammatory reflex is termed the cholinergic anti-inflammatory pathway. Action potentials transmitted to the periphery by the vagus nerve culminate in the release of the neurotransmitter acetylcholine, which interacts with innate immune cells that express the nicotinic acetylcholine receptor subunit α7 (α7nAChR) (FIG. 1). The functional requirement for these molecular components was established by studies of mice deficient in Chrna7 (the gene encoding α7nAChR) because these animals produce significantly more cytokines during endotoxaemia than wild-type controls30. The absence of one molecular signal transduction component of the cholinergic anti-inflammatory pathway results in exaggerated innate immune responses, indicating that α7nAChR has a tonic inhibitory role in modulating innate immune responses. This may be considered analogous to the well known tonic inhibitory influence of the vagus nerve on heart rate; when the vagus nerve input to the heart is lost, heart rate increases. moreover, exaggerated responses to PAMPs and DAMPs also occur following vagotomy, leading to significantly increased cytokine production and amplification of tissue damage in infection, colitis, haemorrhagic shock and pancreatitis31–35. Thus, the net output of the innate immune response to infection and injury is governed by the activity of the efferent arc of the inflammatory reflex.
Direct electrical stimulation of the cholinergic anti-inflammatory pathway with electrodes that generate action potentials in the vagus nerve significantly inhibits cytokine production by innate immune cells in the spleen, liver, gastrointestinal tract, heart and other tissues that are innervated by the vagus nerve2,36–38. Stimulation of the vagus nerve significantly downregulates the production of TNF, IL-1, IL-6 and IL-8, but does not alter the production of the anti-inflammatory cytokines IL-10 and transforming growth factor-β (TGFβ). By restraining the output of potentially toxic mediators that are produced by innate immune cells, the cholinergic anti-inflammatory pathway protects the organism from organ damage and death during endotoxaemia, sepsis, haemorrhagic shock, colitis, arthritis, ileus, pancreatitis and other syndromes of excessive cytokine release (TABLE 1).
Table 1.
Experimental systems for defining the inflammatory reflex
| Experimental model | Activation method | Result | Refs |
|---|---|---|---|
| Endotoxin-induced shock | Direct electical stimulation of cervical vagus nerve | Inhibition of serum TNF secretion and attenuation of shock | 2 |
| Sepsis induced by lethal peritonitis | Transcutaneous stimulation of cervical vagus nerve | Inhibition of serum HMGB1 secretion and improved survival | 97 |
| Pancreatitis | Administration of the α7nAChR agonist GTS-21 | Decreased pancreatitis severity | 33 |
| Vagotomy and administration of an α7nAChR antagonist | Increased pancreatitis severity | 33 | |
| Peripheral inflammation in subcutaneous tissue | Direct electical stimulation of cervical vagus nerve | Inhibition of neutrophil recruitment to the wound | 37 |
| Haemorrhagic shock | Direct electrical stimulation of cervical vagus nerve | Decreased hypotension severity, inhibition of TNF secretion, prolonged survival and downregulateion of NF-κB activity | 35 |
| Ischaemia-reperfusion injury secondary to suprarenal aortic clamping | Direct electrical stimulation of cervical vagus nerve | Inhibition of cytokine production in spleen, liver and heart, and attenuation of shock and tissue damage | 36 |
| Collagen-induced arthritis | Surgical stimulation of the cervical vagus nerve | Decreased arthritis severity | 95 |
| Administration of the α7nAChR agonist AR-R17779 | Inhibition of TNF secretion and decreased arthritis severity | 94 | |
| Vagotomy | Increased arthritis severity | 94 | |
| Ileus | Vagotomy | Increased ilieus severity | 41 |
| Administration of the α7nAChR agonist AR-R17779 | Attenuation of post-operative ileus and amelioration of intestinal inflammation | 41 | |
| Myocardial infarction | Direct electrical stimulation of cervical vagus nerve | Inhibition of cardiac myoglobin release during reperfusion injury | 98 |
| Colitis | Administration of α7nAChR antagonists and vagotomy | Increased colitis severity | 28,31,32 |
| Administration of α7nAChR agonists | Decreased colitis severity | 28,99,100 |
α7nAChR; nicotinic acetylcholine receptor subunit α7; HMBG1, high-mobility group box 1 protein; NF-κB, nuclear factor κB; TNF, tumour necrosis factor.
Molecular mechanism of the efferent arc
α7nAchR, a member of the AchR family, is widely expressed by cells of the nervous system. The members of this family form homopentameric and heteropentameric receptors in neurons, which function as ligand-gated ion channels that mediate fast signal transmission at synapses. α7nAChR is encoded by CHRNA7 on chromosome 15q14 and is the product of ten exons yielding a mature protein of ~ 50 kDa. An alternative α7 transcript in human cells is DUPα7, which is a hybrid of duplicated exons 5–10 combined with four other exons. ligation of α7nAChR in neurons stabilizes the receptor into one of three functional states: the resting state, the open channel state or the desensitized state. The open channel state does not require second messengers, at least not in neurons, and leads to membrane depolarization, activation of other voltage-gated ion channels and generation of action potentials. Ligand binding stabilizes the open channel state to enable the entry of sodium and calcium into the channel for a short time, on the order of 1 millisecond. Phosphorylation of the cytoplasmic domain of the neuronal receptor by SRC-family protein kinases modulates the conformational status of the receptor structure and the activity of the channel39. α7nAChR can physically interact with phosphoinositide 3-kinase, FYN and Janus kinase 2 (JAK2)40,41. Increases in intracellular calcium mediated by α7nAChR signalling induce protein kinase A-dependent activation of extracellular signal-regulated kinase 1 (ERK1) and ERK2 (REF. 42).
α7nAchR signalling in immune cells
Although α7nAchR signalling mechanisms have been extensively studied in neurons, much less is known about its actions in immune cells. α7nAChR is widely expressed by cells of the immune system and has been identified in monocytes, macrophages, B cells, T cells and dendritic cells. unlike neurons, however, molecular mechanistic evidence indicates that signal transduction by α7nAchR in immune cells does not require ion channel activity or membrane depolarization (FIG. 2). ligation of α7nAChR using nonselective agonists (such as nicotine) and highly selective agonists (such as choline or the experimental therapeutic GTS-21) activates pleiotropic intracellular signal transduction cascades in monocytes and macrophages that downregulate the nuclear translocation of NF-κB and the expression of TLR4 and suppress the transcription of pro-inflammatory cytokines2,30,37,43–47. In monocytes and macrophages, α7nAChR signal transduction inhibits the phosphorylation of inhibitor of NF-κB (IκB), a proximal step that regulates the activation of NF-κB48. α7nAChR ligation also recruits JAK2 to form a heterodimeric complex that initiates signal transduction mediated by signal transducer and activator of transcription 3 (STAT3), a pathway that negatively regulates NF-κB binding to DNA and can also increase the activity of suppressor of cytokine signalling 3 (SOCS3) as part of the anti-inflammatory response41. In agreement with the in vivo observations using vagus nerve stimulation, the downregulation of cytokine expression by α7nAChR signalling in activated cytokine-producing immune cells in vitro is selective because the production of anti-inflammatory cytokines, including IL-10 and TGFβ, is not suppressed2,44,45.
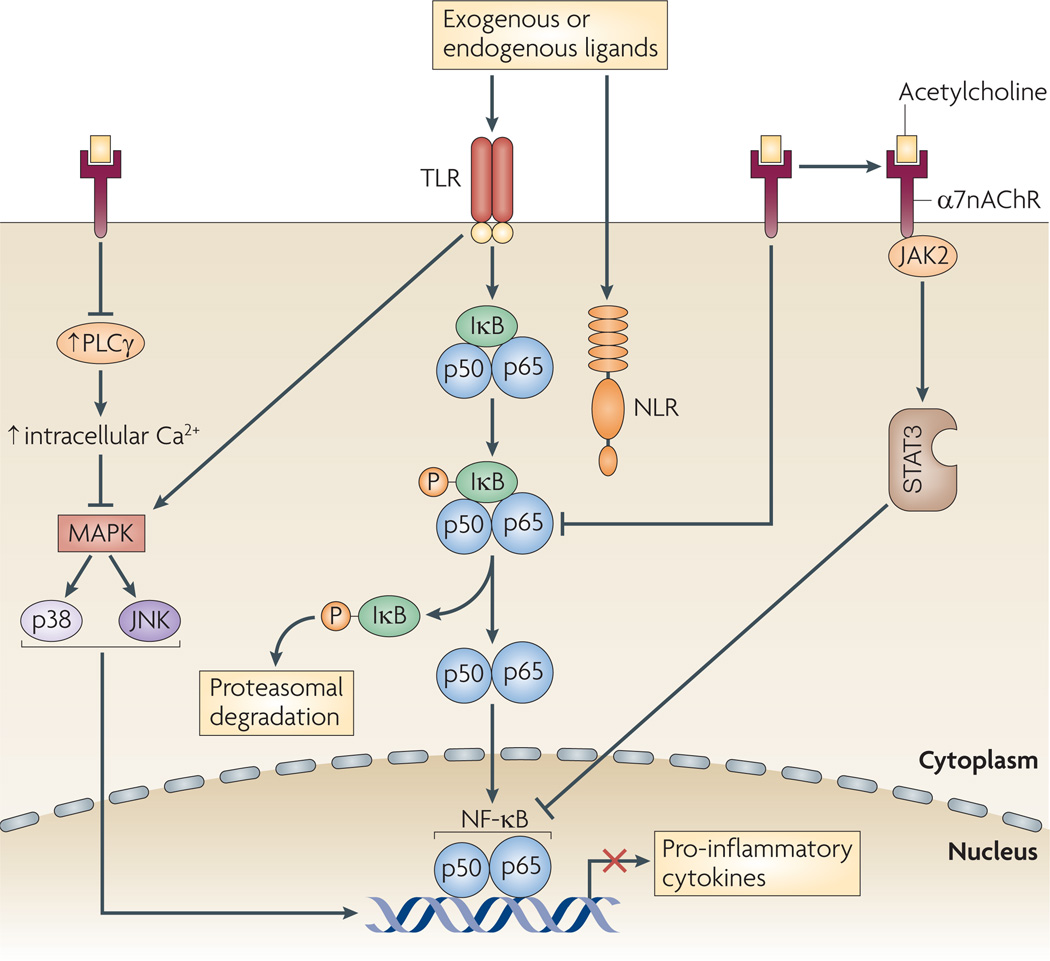
Figure 2. Mechanism of function of the efferent arc.
In the cholinergic anti-inflammatory pathway, acetylcholine binding to nicotinic acetylcholine receptor subunit α7 (α7nAChR) leads to the inhibition of the phosphorylation of inhibitor of NF-κB (I κB), the downregulation of the activation of mitogen-activated protein kinases (MAPKs), inhibition of the release of intracellular Ca2+ stores and the formation of a heterodimeric protein complex with Janus kinase 1 (JAK2), which activates signal transducer and activator of transcription 2 (STAT3). Together, these signalling cascades lead to inhibition of pro-inflammatory cytokine release. JNK, JUN N-terminal kinase; NF-κB, nuclear factor κB; NLR, nucleotide-binding domain, leucine-rich-repeat-containing family; PLCγ, phospholipase Cγ; TLR, Toll-like receptor.
In microglial cells, α7nAChR signalling inhibits endotoxin-induced TNF release by a mechanism that involves decreased phosphorylation of the MAPKs p38 (also known as MAPK14) and p44 (also known as MAPK3)49,50. Activation of these signalling cascades leads to transient increases in intracellular calcium levels; these increases are independent of extracellular calcium and instead depend on calcium ions provided by the activity of phospholipase Cγ and inositol-1,4,5-trisphosphate (InsP3)49,50. Similar mechanisms operate in T cells, in which activation of α7nAChR induces the release of calcium ions from intracellular stores by a mechanism that requires the activation of protein tyro-sine kinases51. Activation of α7nAChR in T cells also leads to the formation of a heterodimeric protein complex with CD3ζ51. Activation of these mechanisms has been implicated in decreasing the expression of adhesion molecules, cytokine production and lymphocyte proliferation52. Notably, in contrast to how α7nAChR functions in neurons, there is no evidence showing that it regulates responses in immune cells as a ligand-gated ion channel on the plasma membrane.
Anatomy of the cholinergic anti-inflammatory pathway
The functional anatomy of the cholinergic anti-inflammatory pathway has been mapped by electrically stimulating the proximal vagus nerve during endotoxaemia and injury and comparing the effect on serum TNF levels with that of animals that had resections of the vagus nerve at distal locations. The results indicate that action potentials transmitted in the vagus nerve traverse the subdiaphragmatic vagus nerve, the coeliac ganglion and the splenic nerve38,46. The functional neural signals in the spleen converge on the red pulp and marginal zone macrophages to suppress immune activation through a molecular mechanism that requires signal transduction through α7nAChR30,46. Vagus nerve fibres terminate in the coeliac ganglion, which is the origin of nerve cell bodies, the axons of which make up the splenic nerve53,54. Vagus nerve fibres form synapses on these cell bodies in the coeliac ganglion55,56, and the nerve fibres originating there control innate immune responses in the spleen.
Knowledge of the cholinergic anti-inflammatory pathway necessitated revising long-standing attempts to separate the neural circuitry that controls immune responses into discrete sympathetic or parasympathetic components. Indeed, understanding the neural circuit described above has led to the revision of the previous anatomical dogma that the splenic nerve is a purely sympathetic nerve. The separation of the neural circuitry controlling immune responses into discrete sympathetic or parasympathetic components is at best artificial and imprecise, and at worst incorrect. For example, the cholinergic anti-inflammatory pathway is functionally neither parasympathetic nor sympathetic (FIG. 3) because classic parasympathetic signals in the periphery are transduced by the vagus nerve and by muscarinic, not α7nAChR, receptors. The splenic nerve, a catecholaminergic nerve component of the cholinergic anti-inflammatory pathway, can be controlled by either presynaptic neurons that originate in the spinal cord or presynaptic neurons transmitting in the vagus nerve (FIG. 3).
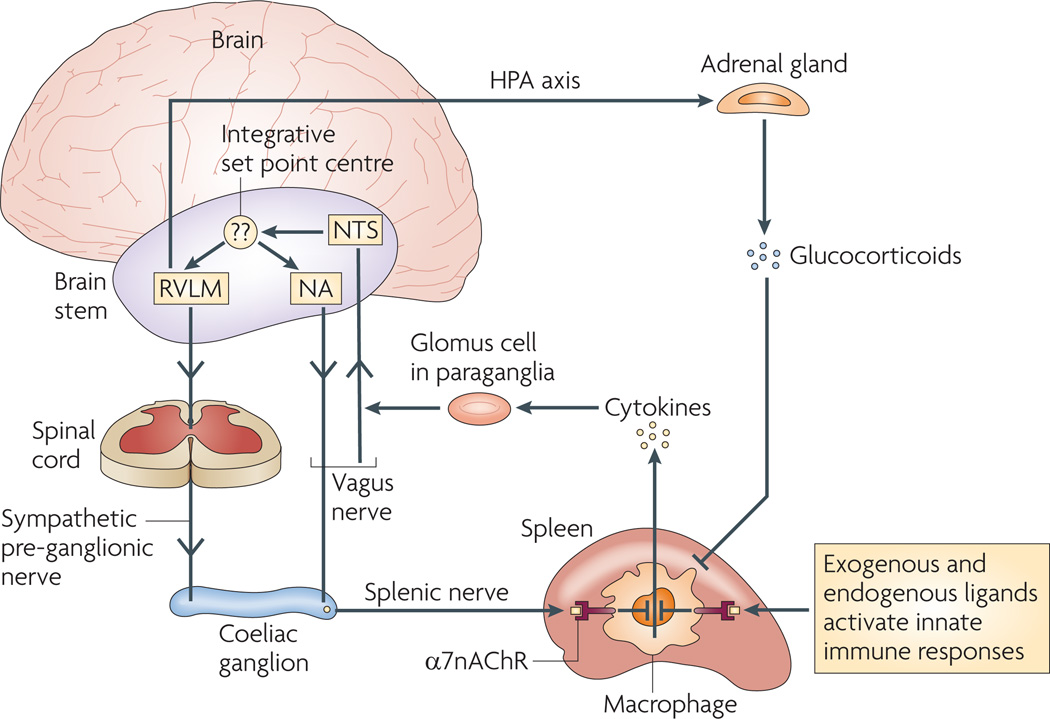
Figure 3. Functional anatomy of the inflammatory reflex.
Afferent (sensory) neural signals to the brain stem are relayed by the vagus nerve to the nucleus of the solitary tract (nucleus tractus solitarius; NTS). Polysynaptic relays then connect to the outflow centres of the autonomic nervous system, the rostral ventrolateral medullary (RVLM) sympathoexcitatory neurons and the vagal motor neurons in the nucleus ambiguus (NA) and the dorsal vagal motor nucleus. Outflow arrives at the coeliac ganglion from either the vagus nerve or the preganglionic efferent nerves, which originate in the sympathetic trunk. Stimulating the vagus nerve suppresses innate immune responses and downregulates pro-inflammatory cytokine release in the spleen through a mechanism that depends on nicotinic acetylcholine receptor subunit α7 (α7nAChR). Note that, following the activation of the inflammatory reflex by sensory input to the brainstem, the signals are also relayed to the nuclei controlling the function of the hypothalamic–pituitary-–adrenal (HPA) axis, which increases glucocorticoid hormone release by the adrenal gland. This provides an important connection between the neural networks that can acutely provide compensatory signals to adjust immune responses, and the humoral anti-inflammatory mechanisms that can more chronically modulate innate and adaptive immune responses. The identity and mechanism of function of the set point centre is unknown. It establishes the magnitude of the set function of immune output around which compensatory reflex responses maintain homeostasis.
We can now move on to consider the next question. How can there be a requirement for α7nAChR-dependent signalling to control cytokine production in the spleen if the splenic neurons release catecholamines, not acetylcholine? It has been known for decades that the spleen is a rich source of acetylcholine (indeed, this molecule was originally identified in spleen tissue) and that electrical stimulation of the splenic nerve mediates the release of large amounts of acetylcholine into splenic tissue and into the effluent splenic vein57–59. Despite exhaustive efforts by many groups, including ours, earlier searches failed to find cholinergic nerve fibres in the spleen, even though it was well established that α7nAChR is an integral component of the cholinergic anti-inflammatory pathway30,43,44. Indeed, before the discovery of the cholinergic anti-inflammatory pathway, most work on neuro–immune signalling to lymphoid organs and the spleen focused almost exclusively on the roles of catecholamines and peptides. one explanation as to why no cholinergic nerve fibres could be found in the spleen despite the finding that acetylcholine is produced there is that acetylcholine could be produced by other, non-neuronal cell sources. Potential candidates include endothelial cells and lymphocytes such as splenic T cells, which are richly innervated by the catecholaminergic axons travelling in the splenic nerve60,61. The hypothesis that is currently being explored is that vagus nerve signals activate splenic nerve endings to release noradrenaline in the white pulp of the spleen close to B and T cells, which are known to produce acetylcholine46. ongoing studies are expected to clarify the neural, cellular and molecular pathways in this mechanism, with important implications for understanding how the cholinergic anti-inflammatory pathway controls innate immunity. This possibility renders plausible the hypothesis that in addition to controlling innate immunity, the cholinergic anti-inflammatory pathway also modulates the development of adaptive immune responses by regulating the activity of splenic lymphocytes.
Reflex control of immune homeostasis
The data reviewed here and elsewhere have moved the field of neuroim-munology from a series of in vitro interaction assays, pharmacological studies and limited in vivo studies to the more important field of integrated neurophysiology and immunology. The inflammatory reflex is a prototypical neural mechanism that influences the magnitude of innate immune responses and maintains homeostasis. As the list of neural circuits that control immune responses will undoubtedly grow, it is useful to consider the next question. How does the activity of neural circuits in basal conditions control innate immune responses? During resting conditions, the inflammatory reflex contributes to establishing the set point for the magnitude of the innate immune response to the molecular products of infection, injury or ischaemia. The activity of vagus neural output maintains homeostasis by limiting pro-inflammatory responses within the healthy, protective and non-toxic range (FIG. 4). However, when neural activity is absent or diminished, the set point increases, resulting in exaggerated pro-inflammatory responses and tissue damage under conditions of milder stimulation. Numerous factors can experimentally or clinically impair the cholinergic anti-inflammatory pathway, each resulting in exaggerated innate immune responses. examples include experimental animals that are deficient in α7nAChR or have decreased activity in the vagus nerve outflow as a result of vagotomy, which significantly increases the magnitude of the pro-inflammatory cytokine response and tissue damage that occur during infection, endotoxaemia, colitis, haemorrhagic shock and pancreatitis30–35. There is abundant clinical evidence that patients with inflammatory diseases have functionally increased set points at rest, producing higher levels of pro-inflammatory mediators and functionally decreased instantaneous heart rate variability, an index of decreased vagus nerve activity. Healthy subjects have decreased set points occurring in association with increased indices of vagus nerve activity and diminished pro-inflammatory cytokine responses. exercise, controlled breathing, fish oil consumption and relaxation therapies have been implicated in increasing vagus nerve activity and suppressing pro-inflammatory cytokine release62–68. A recent study in 183 healthy subjects revealed that increased vagus nerve activity measured during paced respiration significantly decreased the production of TNF and IL-6 in whole blood cultures activated by the addition of endotoxin64. Similar results were obtained in a recent study of 61 sedentary adults who undertook a 12-week exercise training programme69. Aerobic exercise was associated with significantly decreased TNF production in endotoxin-activated whole blood cultures compared with blood drawn before exercise 69. These and other clinical results indicate that the immune system can no longer be regarded as autonomous. like other complex organ systems, it is modulated by reflexes that adjust function around set points that can be increased or decreased in a particular individual. This has proved useful for understanding the risk of developing inflammatory diseases.
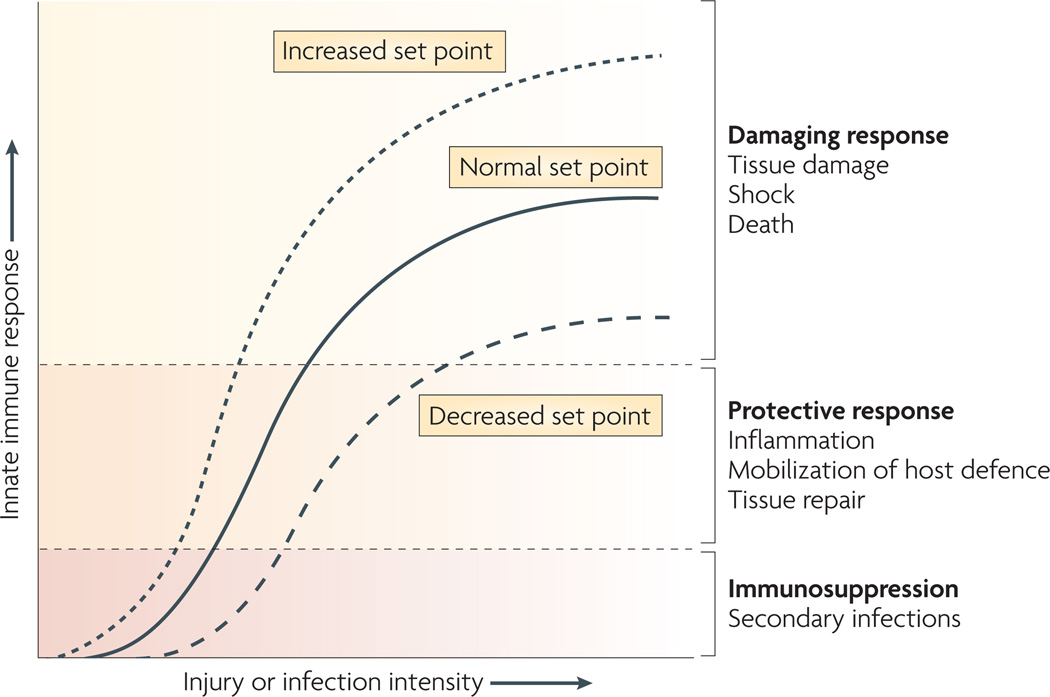
Figure 4. The set point of the immune system.
The set point function of the immune response is defined by the magnitude of innate immune responses relative to the infection or injury stimulus. Increasing the set point or shifting the curve to the left increases the chance that tissue damage will occur from the response to infection or injury. Decreasing the set point or shifting the curve to the right reduces the probability that tissue damage will occur. The inflammatory reflex is the neural circuit that provides acute compensatory input to adjust the magnitude of the immune response relative to the set point. Note that chronic changes in other systems (for example, the hypothalamic–pituitary–adrenal axis) can increase or decrease the set point. In this example, depletion of glucocorticoids following adrenalectomy would significantly increase the set point.
Therapeutic implications
The treatment of inflammatory diseases, including rheumatoid arthritis and inflammatory bowel disease, has been greatly improved by the clinical use of selective agents that modulate the activity of TNF, IL-1 and IL-6. In some cases, however, these agents can be immunosuppressive, resulting in potentially serious secondary infections. This raises an important question: will activation of the inflammatory reflex cause immunosuppression? evidence from animal studies (reviewed above) indicates that targeted therapies that increase the activity of the inflammatory reflex normalize innate immune responses without abolishing them or causing immunosuppression. electrical or pharmacological stimulation of the inflammatory reflex adjusts the set point into the protective range instead of completely inhibiting innate immune responses. electrical stimulation of the vagus nerve or administration of α7nAChR agonists reduces the magnitude of pro-inflammatory cytokine production by 50–75% but does not eliminate cytokine activity2,36,38,44,45. Activation of this pathway has not been observed to cause immunosuppression because maximal suppression reduces pro-inflammatory cytokine levels from the toxic to the healthy range (FIG. 4). This concept has been studied as a potential treatment in a range of inflammatory disease models, including infection, ischaemia-reperfusion and injury (TABLE 1).
Whether it will be possible to safely develop therapeutic approaches for human diseases awaits the results of clinical testing, but this possibility raises the next question. What is known about the activity of inflammatory diseases in humans with decreased vagus nerve activity, and can determining the basal levels of vagus nerve activity act as a surrogate marker for measuring the activity of the inflammatory reflex in resting conditions? A complete discussion of the clinical studies addressing this question is beyond the scope of this Review, but data indicate that vagus nerve activity in basal conditions provides inhibitory input that modulates innate immune responses and that depressed vagus nerve activity is associated with exaggerated pro-inflammatory responses and increased morbidity and mortality63,70–72. Vagus nerve activity can be measured in humans with research techniques based on extrapolating indices of vagus nerve activity from measurements of instantaneous heart rate variability (the time duration between individual heart beats). Vagus nerve activity in resting conditions provides an inhibitory influence on instantaneous heart rate. Accordingly, time domain and frequency domain analysis of instantaneous heart rate variability data provide several extremely useful indices of vagus nerve activity70,71. The relationship between decreased vagus nerve activity and increased pro-inflammatory responses has been studied in the context of several inflammatory diseases, including rheumatoid arthritis, systemic lupus erythematosus, sepsis, inflammatory bowel disease and sarcoidosis73–80.
One of these studies analysed heart rate variability in 42 patients with rheumatoid arthritis and 44 matched controls76. Analysis revealed that the high-frequency values, which correspond to vagus nerve activity, were significantly decreased in patients with rheumatoid arthritis compared with healthy controls76. In a separate study of patients with sepsis, heart rate variability on admission to the hospital was significantly reduced in those patients that went on to develop organ failure compared with those that had an uneventful hospital stay75. The mortality rate in subjects with decreased vagus nerve activity on admission was 63.6% compared with 0% in the other group (P <0.0001)75. Decreased heart rate variability has also been significantly associated with the risk of atherosclerosis, an observation that is particularly interesting when considering the pathogenic role of inflammation in this disease62,81. Analysis of 102 healthy middle-aged men and women revealed that indices of decreased vagus nerve activity were significantly associated with increased plasma IL-6 concentrations. The authors concluded that “systemic low-grade inflammatory activity is associated with a decrease in heart rate variability” (REF. 62). Another study examined 611 healthy adults and observed a significant inverse association between vagus nerve activity indices and plasma C-reactive protein (CRP) levels70. They concluded that this effect was due to decreased activity in the cholinergic anti-inflammatory pathway, with potentially important implications for the inflammatory basis of cardiovascular disease70. In our collaborative studies with R. Sloan82 we measured heart rate variability, CRP levels and IL-6 in 757 young adult subjects. Indices of vagus nerve activity were significantly and inversely related to IL-6 and CRP levels, which is consistent with the hypothesis that the cholinergic anti-inflammatory pathway exerts a tonic inhibitory influence on innate immune responses. So, these and other clinical data indicate that loss of the inhibitory influence of the inflammatory reflex essentially unleashes innate immunity, resulting in exacerbation of damage to the organism.
Future directions
Mechanisms
There are important mechanistic implications that stem from understanding the principles of reflex action that control immune responses through discrete neurotransmitter receptors. As noted above, the future will undoubtedly reveal other reflex circuits, in addition to the inflammatory reflex and the cholinergic anti-inflammatory pathway, that maintain homeostasis in the innate and adaptive immune system. extensive in vitro pharmacological and in vivo studies implicate a role for other neurotransmitters and neural pathways that can stimulate pro-inflammatory cytokine release and enhance inflammation. For example, the inter action of substance P with neurokinin 1 receptors, and the interaction of noradrenaline with α-adrenergic receptors, stimulates pro-inflammatory cytokine release83,84 By contrast, activation of 5-hydroxytryptamine receptors or β-adrenergic receptors inhibits pro-inflammatory cytokine release and suppresses inflammation85,86. Clearly, as occurs in other complex organ systems, neural pathways using these and other neurotransmitters function either antagonistically or synergistically to modulate and fine-tune the function of the immune system. looking ahead, we will continue to learn about how these neural pathways intersect, and this knowledge will be advanced through our understanding of the functional role of the isolated receptors and neuro-transmitters in integrated neurophysiological circuits that control the entire range of cellular immune responses. The greatest advances are not expected to arise from restricted studies that focus on an isolated nerve, neurotransmitter or signal transduction pathway, but from studies that reveal the neurophysiological basis for modulating immunity. The organization of the nervous system in the body provides the anatomical basis for integrated neural circuits to be linked together in information webs that modulate immune homeostasis.
Such mechanistic advances will enable us to understand how the immune set point is influenced by descending neural circuits that originate in the higher brain to control the activity of the inflammatory reflex. We have begun to explore one such neural brain network that is regulated by muscarinic receptors. The administration of intracerebral McN-A 343, a selective muscarinic acetylcholine receptor M1 receptor agonist, significantly increases the firing rate of action potentials in the vagus nerve and inhibits innate immune responses in the periphery87,88. The anatomy of this and other putative descending controlling neural circuits awaits precise elucidation. undoubtedly, these pathways, and the associated brain nucleii that influence immune homeostasis, will be revealed and mapped in the foreseeable future. The end product will be a brain map, so named an ‘immunological homonculus’, of sensory and motor components corresponding to neuroanatomical and functional neurophysiological pathways that modulate the innate immune response to PAMPs and DAMPs and the adaptive immune response to pathogens and invaders3.
Clinical studies
Physiological health is characterized by organized variability in organ functions, which is controlled by neural networks that provide compensatory outputs to maintain homeostasis around set points in an organ system. Disease is characterized by imbalances in reflex action that are associated with loss of this physiological flexibility. Future studies are likely to reveal methods to determine the status of the immune set point in patients. The inhibitory role of the cholinergic anti-inflammatory pathway on innate immune function can be thought of as analogous to the inhibitory role of the vagus nerve on resting heart rate. Future studies will probably clarify the relationship between decreased vagus nerve activity to the heart, decreased vagus nerve activity to the immune system and excessive inflammation70. There is abundant epidemiological evidence for an association between resting heart rate and risk of morbidity and mortality from diabetes, sepsis, cardiovascular disease, arthritis, Alzheimer’s disease and other syndromes3,71,72. In addition, there is a compelling need to measure the set point of inflammatory reflex activity to predict the risk of inflammatory disease. Consider that in a typical intensive care unit there may be dozens of patients with indwelling catheters in the urinary bladder, trachea and vascular system. many of these sites will be infected with bacteria, but the magnitude of the innate immune responses in these patients will vary considerably, spanning from having no observable inflammation to the release of life-threatening pro-inflammatory cytokines that mediate sepsis, shock and tissue injury. Future devices may make it possible to measure the activity of neural networks that control the immune system. like modern day electrocardiograms or electroencephalograms, such instruments may be used to assess a patient’s immune set point to better guide therapy.
Therapeutics
It should also be possible in the future to develop pacemaker-like devices to decrease the set point of the inflammatory reflex. Prototype vagus nerve stimulation devices have already been successfully used in animals to prevent tissue damage associated with dysregulated innate immune responses1,3. Vagus nerve stimulators are currently in clinical use and have been safely implanted in tens of thousands of patients as a treatment for seizure disorders that are refractory to medication89,90. Based on the data reviewed here and elsewhere, future clinical trials should assess the effects of pharmacological targeting of α7nAChR in the periphery and of muscarinic acetylcholine receptors in the brain, and of devices that directly stimulate or inhibit nerves to modulate the progression of inflammatory diseases.
Acknowledgements
The author thanks S. Chavan, M. Rosas-Ballina, V. Pavlov, B. Volpe, P. Huerta, B. Diamond and S. Warren for helpful discussions. Work in the author’s laboratory was supported in part by the National Institute of General Medical Sciences, one of the National Institutes of Health.
Glossary
- Sickness syndrome
The physiological and behavioural responses to the molecular products of infection or injury that can be triggered by the accumulation of pro-inflammatory cytokines in the brain. These include (but are not limited to) fever, acute phase responses, anorexia, weight loss, increased sleep, decreased social interaction, exploration, decreased sexual activity and altered hypothalamic, pituitary and autonomic output.
- Pyrogen
An agent that is derived from either a pathogen or the host and that can cause fever in vivo. examples of pyrogens include bacterial endotoxin and cytokines, such as tumour necrosis factor, interleukin-1 (IL-1) and IL-6.
- Glomus cell
A type of epithelial cell located in the carotid body or in clusters (termed paraganglia) adjacent to the vagus and other autonomic nerves. A glomus cell can be activated by chemical changes in the cellular milieu to release dopamine or other neurotransmitters close to nerves.
- Pathogen-associated molecular pattern
A microbial motif that can stimulate innate immune responses; for example, bacterial endotoxins, peptidoglycan, flagellin, double-stranded RNA and unmethylated CpG-containing DNA.
- Toll-like receptor
A membrane receptor that is expressed by innate immune cells and that interacts with molecular products of infection or injury to initiate cytokine release and trigger inflammation.
- Damage-associated molecular pattern
A molecule that is produced by the host and that can stimulate innate immune responses; for example, high-mobility group box protein, uric acid, heat shock proteins, heparin sulphate, hyaluronan fragments, ATP and DNA.
- Nucleotide-binding oligiomerization domain (NOD)-like receptor
An intracellular pattern recognition receptor belonging to a family comprising 20 members that recognize endogenous or exogenous molecular products of infection or injury, several of which activate caspases that activate cytokine release and nuclear factor-κB signalling.
- Sepsis
A systemic inflammatory syndrome that occurs after infection or injury and is characterized by a constellation of symptoms, including, but not limited to, alterations in body temperature, white blood cell count, heart rate, respiratory rate and organ function.
- Catecholamines
A family of catechol-containing molecules, including adrenaline, noradrenaline and dopamine, which are produced by chromaffin cells of the adrenal medulla and are released as neurotransmitters from postganglionic fibres in the sympathetic nervous system.
- Set point
The magnitude of a function in physiology and metabolism (for example, mean arterial blood pressure or body temperature) targeted as the output of homeostasis, such that deviations away from this target initiate compensatory reflexes to adjust the output back to that target.
Footnotes
Competing interests statements
The author declares competing financial interests: see web version for details
DATABASES
Entrez gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene.
FURTHER INFORMATION
The feinstein Institute homepage: http://www.feinsteininstitute.org
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 2.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J. Clin. Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman L. Elaborate interactions between the immune and nervous systems. Nature Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 5.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nature Rev. Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellinger DL, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.del Rey A, Besedovsky HO. Sympathetic nervous system–immune interactions in autoimmune lymphoproliferative diseases. Neuroimmunomodulation. 2008;15:29–36. doi: 10.1159/000135621. [DOI] [PubMed] [Google Scholar]
- 8.Blalock JE, Smith EM. Conceptual development of the immune system as a sixth sense. Brain Behav. Immun. 2007;21:23–33. doi: 10.1016/j.bbi.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Koppen CJ, Kaiser B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacol. Ther. 2003;98:197–220. doi: 10.1016/s0163-7258(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 11.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nature Rev. Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi K, Terui N, Kollai M, Brooks CM. Functional significance of coactivation of vagal and sympathetic cardiac nerves. Proc. Natl Acad. Sci. USA. 1982;79:2116–2120. doi: 10.1073/pnas.79.6.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello CA, et al. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J. Exp. Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracey KJ, et al. Metabolic effects of cachectin/tumor necrosis factor are modified by site of production Cachectin/tumor necrosis factor-secreting tumor in skeletal muscle induces chronic cachexia, while implantation in brain induces predominantly acute anorexia. J. Clin. Invest. 1990;86:2014–2024. doi: 10.1172/JCI114937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J. Intern. Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 16.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis CN, et al. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc. Natl Acad. Sci. USA. 2006;103:2953–2958. doi: 10.1073/pnas.0510802103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattson MP. NF-κB in the survival and plasticity of neurons. Neurochem. Res. 2005;30:883–893. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- 19.Ferri CC, Yuill EA, Ferguson AV. Interleukin-1 β depolarizes magnocellular neurons in the paraventricular nucleus of the hypothalamus through prostaglandin-mediated activation of a non selective cationic conductance. Regul. Pept. 2005;129:63–71. doi: 10.1016/j.regpep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Watkins LR, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 21.Hansen MK, O’Connor KA, Goehler LE, Watkins LR, Maier S. F The contribution of the vagus nerve in interleukin-1 β-induced fever is dependent on dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R929–R934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- 22.Goehler LE, et al. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res. Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang HY, Fitzgerald RS. Muscarinic modulation of hypoxia-induced release of catecholamines from the cat carotid body. Brain Res. 2002;927:122–137. doi: 10.1016/s0006-8993(01)03334-0. [DOI] [PubMed] [Google Scholar]
- 24.Neubauer JA, Sunderram J. Oxygen-sensing neurons in the central nervous system. J. Appl. Physiol. 2004;96:367–374. doi: 10.1152/japplphysiol.00831.2003. [DOI] [PubMed] [Google Scholar]
- 25.Goehler LE, et al. Interleukin-1 beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J. Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niijima A. The afferent discharges from sensors for interleukin 1 β in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 28.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 29.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 31.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J. Clin. Invest. 2008;118:2209–2218. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 33.van Westerloo DJ, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 34.van Westerloo DJ, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J. Infect. Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 35.Guarini S, et al. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 36.Bernik TR, et al. Pharmacological stimulation of the cholinergic anti-inflammatory pathway. J. Exp. Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saeed RW, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huston JM, et al. Splenectomy inactivates the cholinergic anti-inflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charpantier E, et al. α7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases. J. Neurosci. 2005;25:9836–9849. doi: 10.1523/JNEUROSCI.3497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kihara T, et al. α7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A β -amyloid-induced neurotoxicity. J. Biol. Chem. 2001;276:13541–13546. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 41.de Jonge WJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2–STAT3 signaling pathway. Nature Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 42.Dajas-Bailador FA, Soliakov L, Wonnacott S. Nicotine activates the extracellular signal-regulated kinase 1/2 via the α7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J. Neurochem. 2002;80:520–530. doi: 10.1046/j.0022-3042.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nature Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 44.Parrish WR, et al. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlov VA, et al. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 2007;35:1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 46.Rosas-Ballina M, et al. Splenic nerve is required for cholinergic anti-inflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamano R, et al. Stimulation of α7 nicotinic acetylcholine receptor inhibits CD 14 and the Toll-like receptor 4 expression in human monocytes. Shock. 2006;26:358–364. doi: 10.1097/01.shk.0000228168.86845.60. [DOI] [PubMed] [Google Scholar]
- 48.Yoshikawa H, et al. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity through nicotinic acetylcholine receptor α7. Clin. Exp. Immunol. 2006;146:116–123. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki T, et al. Microglial α7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J. Neurosci. Res. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- 50.Shytle RD, et al. Cholinergic modulation of microglial activation by α7 nicotinic receptors. J. Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 51.Razani-Boroujerdi S, et al. T cells express α7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J. Immunol. 2007;179:2889–2898. doi: 10.4049/jimmunol.179.5.2889. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi HK, et al. The immunosuppressive effects of nicotine during human mixed lymphocyte reaction. Eur. J. Pharmacol. 2007;559:69–74. doi: 10.1016/j.ejphar.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J. Auton. Nerv. Syst. 1993;42:153–169. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 54.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc. Res. Tech. 1996;35:80–86. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 55.Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav. Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 56.Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav. Immun. 1989;3:291–311. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 57.Brandon KW, Rand MJ. Acetylcholine and the sympathetic innervation of the spleen. J. Physiol. 1961;157:18–32. doi: 10.1113/jphysiol.1961.sp006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leaders FE, Dayrit C. The cholinergic component in the sympathetic innervation to the spleen. J. Pharmacol. Exp. Ther. 1965;147:145–152. [PubMed] [Google Scholar]
- 59.Bulloch K, Damavandy T, Badamchian M. Characterization of choline O-acetyltransferase (ChAT) in the BALB/C mouse spleen. Int. J. Neurosci. 1994;76:141–149. doi: 10.3109/00207459408985999. [DOI] [PubMed] [Google Scholar]
- 60.Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- 61.Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003;74:675–696. doi: 10.1016/j.lfs.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 62.von Kanel R, Nelesen RA, Mills PJ, Ziegler MG, Dimsdale JE. Relationship between heart rate variability, interleukin-6, and soluble tissue factor in healthy subjects. Brain Behav. Immun. 2008;22:461–468. doi: 10.1016/j.bbi.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology. 2008;33:1305–1312. doi: 10.1016/j.psyneuen.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsland AL, et al. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom. Med. 2007;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- 65.Holguin F, et al. Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest. 2005;127:1102–1107. doi: 10.1378/chest.127.4.1102. [DOI] [PubMed] [Google Scholar]
- 66.Lanza GA, et al. Relation of heart rate variability to serum levels of C-reactive protein in patients with unstable angina pectoris. Am. J. Cardiol. 2006;97:1702–1706. doi: 10.1016/j.amjcard.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 67.Jae SY, et al. Effects of lifestyle modifications on C-reactive protein: contribution of weight loss and improved aerobic capacity. Metabolism. 2006;55:825–831. doi: 10.1016/j.metabol.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 68.Goldhammer E, et al. Exercise training modulates cytokines activity in coronary heart disease patients. Int. J. Cardiol. 2005;100:93–99. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 69.Sloan RP, et al. Aerobic exercise attenuates inducible TNF production in humans. J. Appl. Physiol. 2007;103:1007–1011. doi: 10.1152/japplphysiol.00147.2007. [DOI] [PubMed] [Google Scholar]
- 70.Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J. Intern. Med. 2008;265:439–447. doi: 10.1111/j.1365-2796.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 71.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 72.Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann. NY Acad. Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 73.Stein KS, McFarlane IC, Goldberg N, Ginzler EM. Heart rate variability in patients with systemic lupus erythematosus. Lupus. 1996;5:44–48. doi: 10.1177/096120339600500109. [DOI] [PubMed] [Google Scholar]
- 74.Barnaby D, et al. Heart rate variability in emergency department patients with sepsis. Acad. Emerg. Med. 2002;9:661–670. doi: 10.1111/j.1553-2712.2002.tb02143.x. [DOI] [PubMed] [Google Scholar]
- 75.Pontet J, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J. Crit. Care. 2003;18:156–163. doi: 10.1016/j.jcrc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Evrengul H, et al. Heart rate variability in patients with rheumatoid arthritis. Rheumatol. Int. 2004;24:198–202. doi: 10.1007/s00296-003-0357-5. [DOI] [PubMed] [Google Scholar]
- 77.Laversuch CJ, et al. Reduction in heart rate variability in patients with systemic lupus erythematosus. J. Rheumatol. 1997;24:1540–1544. [PubMed] [Google Scholar]
- 78.Lindgren S, Stewenius J, Sjolund K, Lilja B, Sundkvist G. Autonomic vagal nerve dysfunction in patients with ulcerative colitis. Scand. J. Gastroenterol. 1993;28:638–642. doi: 10.3109/00365529309096103. [DOI] [PubMed] [Google Scholar]
- 79.Uslu N, et al. Heart rate variability in patients with systemic sarcoidosis. Ann. Noninvasive Electrocardiol. 2006;11:38–42. doi: 10.1111/j.1542-474X.2006.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biswas AK, Scott WA, Sommerauer JF, Luckett PM. Heart rate variability after acute traumatic brain injury in children. Crit. Care Med. 2000;28:3907–3912. doi: 10.1097/00003246-200012000-00030. [DOI] [PubMed] [Google Scholar]
- 81.Hanson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nature Rev. Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 82.Sloan RP, et al. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol. Med. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhatia M, Zhi L, Zhang H, Ng SW, Moore PK. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L896–L904. doi: 10.1152/ajplung.00053.2006. [DOI] [PubMed] [Google Scholar]
- 84.Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J. Immunol. 1994;152:3024–3031. [PubMed] [Google Scholar]
- 85.Yu B, et al. Serotonin 5-hydroxytryptamine2A receptor activation suppresses tumor necrosis factor-α-induced inflammation with extraordinary potency. J. Pharmacol. Exp. Ther. 2008;327:316–323. doi: 10.1124/jpet.108.143461. [DOI] [PubMed] [Google Scholar]
- 86.Yoshimura T, et al. Inhibition of tumor necrosis factor-alpha and interleukin-1-beta production by beta-adrenoceptor agonists from lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Pharmacology. 1997;54:144–152. doi: 10.1159/000139481. [DOI] [PubMed] [Google Scholar]
- 87.Pavlov VA, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl Acad. Sci. USA. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pavlov VA, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Labiner DM, Ahern GL. Vagus nerve stimulation therapy in depression and epilepsy: therapeutic parameter settings. Acta Neurol. Scand. 2007;115:23–33. doi: 10.1111/j.1600-0404.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 90.DeGiorgio CM, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 91.Sanders VM, Straub RH. Norepinephrine, the β-adrenergic receptor, and immunity. Brain Behav. Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- 92.Saravia F, Homo-Delarche F. Is innervation an early target in autoimmune diabetes? Trends Immunol. 2003;24:574–579. doi: 10.1016/j.it.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 93.Straub RH, Rauch L, Fassold A, Lowin T, Pongratz G. Neuronally released sympathetic neurotransmitters stimulate splenic interferon-γ secretion from T cells in early type II collagen-induced arthritis. Arthritis Rheum. 2008;58:3450–3460. doi: 10.1002/art.24030. [DOI] [PubMed] [Google Scholar]
- 94.van Maanen MA, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 95.Zhang P, Han D, Tang T, Zhang X, Dai K. Inhibition of the development of collagen-induced arthritis in Wistar rats through vagus nerve suspension: a 3-month observation. Inflamm. Res. 2008;57:322–328. doi: 10.1007/s00011-008-8070-1. [DOI] [PubMed] [Google Scholar]
- 96.Dampney RA, et al. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin. Exp. Pharmacol. Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 97.Huston JM, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 2007;35:2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 98.Kawada T, et al. Vagal stimulation suppresses ischemia-induced myocardial interstitial myoglobin release. Life Sci. 2008;83:490–495. doi: 10.1016/j.lfs.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 99.Ghia JE, Blennerhassett P, El Sharkawy RT, Collins SM. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G711–G718. doi: 10.1152/ajpgi.00240.2007. [DOI] [PubMed] [Google Scholar]
- 100.Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G560–G567. doi: 10.1152/ajpgi.00098.2007. [DOI] [PubMed] [Google Scholar]