Abstract
Purpose
There has not been a recent comprehensive effort to examine existing studies on the resting metabolic rate (RMR) of adults to identify the effect of common population demographic and anthropometric characteristics. Thus, we reviewed the literature on RMR (kcal·kg−1·h−1) to determine the relationship of age, sex, and obesity status to RMR as compared with the commonly accepted value for the metabolic equivalent (MET; e.g., 1.0 kcal·kg−1·h−1).
Methods
Using several databases, scientific articles published from 1980 to 2011 were identified that measured RMR, and from those, others dating back to 1920 were identified. One hundred and ninety-seven studies were identified, resulting in 397 publication estimates of RMR that could represent a population subgroup. Inverse variance weighting technique was applied to compute means and 95% confidence intervals (CI).
Results
The mean value for RMR was 0.863 kcal·kg−1·h−1 (95% CI = 0.852–0.874), higher for men than women, decreasing with increasing age, and less in overweight than normal weight adults. Regardless of sex, adults with BMI ≥ 30 kg·m−2 had the lowest RMR (<0.741 kcal·kg−1·h−1).
Conclusions
No single value for RMR is appropriate for all adults. Adhering to the nearly universally accepted MET convention may lead to the overestimation of the RMR of approximately 10%for men and almost 15% for women and be as high as 20%–30% for some demographic and anthropometric combinations. These large errors raise questions about the longstanding adherence to the conventional MET value for RMR. Failure to recognize this discrepancy may result in important miscalculations of energy expended from interventions using physical activity for diabetes and other chronic disease prevention efforts.
Keywords: kilocalories, oxygen uptake, meta-analysis, body mass index, sexes, age
Resting metabolic rate (RMR), also called resting energy expenditure, is important to understand because it typically accounts for the largest portion of total energy needs (6). RMR is typically defined as the energy required by the body in a resting condition (3). The definition can be further refined as the amount of energy expended when the individual is awake, in a postabsorptive, thermoneutral state while having not exercised for typically 12 h (10). RMR has been measured either in the sitting or supine positions, with a minimum of 15 min of rest, sometimes up to an overnight rest. Resting and basal metabolic rate (BMR) are similar and only differ in that BMR is usually measured in the morning, after an overnight fast, no exercise for the previous 24 h, free from emotional stress, familiar with the apparatus, and the subject completely rested (27). In general, RMR may be a better indicator of daily energy needs than BMR (15). Although there have been comparative studies on the influence of sex, age, and obesity status on BMR, which have resulted in prediction equations, there is little comprehensive comparative data on the influence of sex, age, and obesity status on RMR. Studies have shown differences in RMR between men and women (4,12,13,19,27), between obese and nonobese adults (6,11), and possibly racial/ethnic differences (25). Furthermore, older adults (>70 yr) have lower RMRs than younger adults (30,33) by as much as 20%–25% (30). However, to our knowledge, there has not been an examination of combined effects of sex, age, and obesity status on RMR to approximate group characteristics normally encountered in public health efforts.
The metabolic equivalent (MET) is a common term used by exercise physiologists, epidemiologists, and the medical community to express RMR. In addition, energy demands of various physical activities have been represented by multiples of a MET, made relative to RMR (1–3). The concept of a MET has been in use for quite some time (17), but the exact derivation is not known (13). The conventional definition of one MET is 3.5 mL oxygen per kilogram body mass per minute (3.5 mL·kg−1·min−1) and is assumed to be approximately equal to 1 kcal·kg−1·h−1 or 4.184 kJ·kg−1·h−1 (1–3). In all three articles by Ainsworth et al. (1–3), the energy expenditure of a MET is noted to be imprecise and seen only as a means of classifying activities based on the expected intensity of typical activity participation when expressed as a multiple of 1 MET (1–3). Work by Byrne et al. (13) suggests that the use of the conventionally defined MET value often reflects an overestimate that does not apply well to all individuals nor to population subgroups. Unfortunately, applying a standard MET value to all individuals has attained widespread acceptance but has been questioned in the past decade by the scientific community (13,29,30).
A perusal of the RMR literature reveals that considerable information on studies of specific population subgroups (e.g., men, women, children, obese, and patient populations) is based primarily on relatively small sample sizes, or studies that were not intended to be population based (21). Although reviews have been completed (6,25,43), there has not been any comprehensive effort to assemble RMR estimates from existing studies to identify the combined effect of common demographic (sex and age) and obesity status (body mass index) characteristics that apply to groups of individuals encountered in the delivery of public health interventions. Such an effort is important because the convention of applying a single estimate of RMR to an entire population subgroup is likely to misrepresent expected energy costs of physical activity promotion intended to achieve, for example, energy balance among groups of men or women who are overweight or obese. In addition, the issue is relevant to public health efforts that target groups of individuals for the delivery of physical activity programs, to say, older adult, overweight women versus younger, and obese men, in hopes of thwarting the growing diabetes epidemic (14). Thus, the purposes of this article were to examine the literature on RMR and to determine the extent to which age, sex, and obesity status relate to RMR as compared with the commonly accepted value for a MET.
METHODS
We perused scientific articles published between 1980 and 2011 to identify studies that measured RMR using PubMed, BIOSIS Previews, NTIS, EMBASE, MEDLINE, and Pascal databases. Several search terms were used, including RMR, resting energy expenditure, resting oxygen uptake (or V̇O2), healthy adults, and older healthy adults. To be included, the studies had to have directly assessed RMR using either oxygen uptake or a metabolic chamber in healthy adults. For purposes of this review, RMR was required to be measured in an awake adult, at least 3 h postprandial, in a thermoneutral state with no exercise for the previous 8 h (10). These criteria, although general, seemed to adequately represent normal daily life for an adult. Studies that examined cohorts having specific maladies were not included, unless they offered separate data for a healthy adult control group, which we could use separately in our analyses. RMR in units of kilocalories per kilograms per hour had to be available or be able to be computed from the data presented. For example, studies that reported their data in kilocalories per day, kilocalories per kilograms, or kilocalories per kilogram of fat-free mass (FFM) per day that also reported data for weight and body fat allowed us to compute RMR in kilocalories per kilograms per hour. In addition, the studies had to be published in peer-reviewed journals in English. While reading many of the papers, particularly their “methods and materials” sections, it became obvious that RMR data from the same subjects were frequently reported in two or more papers. Therefore, we used the first reported results and excluded the redundant paper reporting the same information. We made no attempt to find and use nonpublished data that may be available to avoid positive publication bias, nor did we attempt to contact authors to answer questions we might have about the data that was presented. When intervention studies reported baseline and outcome measures of RMR, we chose only to abstract the former estimates to facilitate comparisons with all other nonintervention studies under review. Differing specific methods for estimating RMR were used by studies (e.g., prior rest of 15–60 min, postprandial state of 3–12 h, refraining from exercise 8–24 h, and supine versus sitting position). We recognize this as an inherent potential problem in making study-to-study comparisons, for which we could not control and we did not endeavor such comparisons with the groups of estimates we examined.
If a paper cited previous publications that provided unique information on RMR, we went back to that study to assess the relevance of its data for our review. In this manner, we identified publications from as far back as 1921, which we added to our systematic survey (dated before 1980 = 7 studies or 12 [<3%] of the publication estimates). The low number of studies was a result of 1) methods that measured basal and not RMR and 2) an incomplete search of all studies as mentioned previously. The search identified more than 600 publications, but once the previously mentioned criteria were applied, only 197 studies remained. The 197 studies resulted in 410 publication estimates of RMR that could represent a specific cohort or population subgroup. Of the 410 estimates, 13 did not provide standard errors and were eliminated yielding 397 population estimates. The studies included 11,951 subjects, and the reported sex distribution was as follows: 52% as women, 39% as men, and 9% with no indication of sex status. The ages of the participants ranged from 18 to older than 80 yr. We found limited studies particularly for oldest adult groups (≥75 yr) among whom RMR information for 80- to 90-yr-old adults was virtually absent. We did not consider the existing studies for this latter age group because they focused only on BMR and not RMR; hence, they failed to meet the inclusion criteria for our review. For a complete listing of all articles used for the analyses, contact the corresponding author.
The studies included a wide range of ages and sample sizes; therefore, we used several differing approaches to examine the data. To partition RMR publication estimates to examine the influence of sex and age, we first stratified all publication estimates by sex and then by 10-yr incremental age groupings using the mean age reported by a study. The relationship of relative weight with RMR was examined by stratifying publication estimates according to standard World Health Organization categories of BMI (kg·m−2): <25, normal; 25–29.9, overweight; and ≥30, obese (47), and by sex. To examine the combinations of sex, age, and obesity status, we first stratified publication estimates by sex and BMI categories and then divided them into three classifications based on the mean age (yr) of the publication estimates: young (20–39 yr), middle age (40–54 yr), and older adult (55–74 yr). These age groupings seemed logical based on activity/life stages known to occur among adults (e.g., active early career and family, declining activity and more established career and family, and periretirement age, and postretirement or senescence). We acknowledge that this is an artificially crude set of distinctions compared with simply dividing the sample by decade of age, or many other potential approaches. However, we attempted to achieve a balance of meaningful distinctions to reflect population subgroups by age against partitioning the publication estimates so fine that error variances would render comparisons moot. As such, our process and final inclusion of studies should not be considered an “exhaustive,” fully standardized effort, and we readily acknowledge any unintended selection bias resulting from our decisions. Finally, because methodology and equipment have changed over the decades (15), we explored possible trends in the data by decade of study publication.
We used the Comprehensive Meta-Analysis statistical software (7) to estimate weighted means and 95% confidence intervals (95% CI) for each of the subgroups using the inverse variance weighting technique (32). Q-statistics were computed to evaluate the heterogeneity between sets of study estimates for contrasts of interest (e.g., RMR differences between all men and women). In the presence of significant heterogeneity, we used a random effects model, which occurred for all of the contrasts we explored. We used 95% CI to compare subgroups and conservatively inferred that when CI did not overlap, the mean estimates were significantly different (16). Doing so has the advantage of partially controlling for the overinterpretation that occurs when making multiple comparisons.
RESULTS
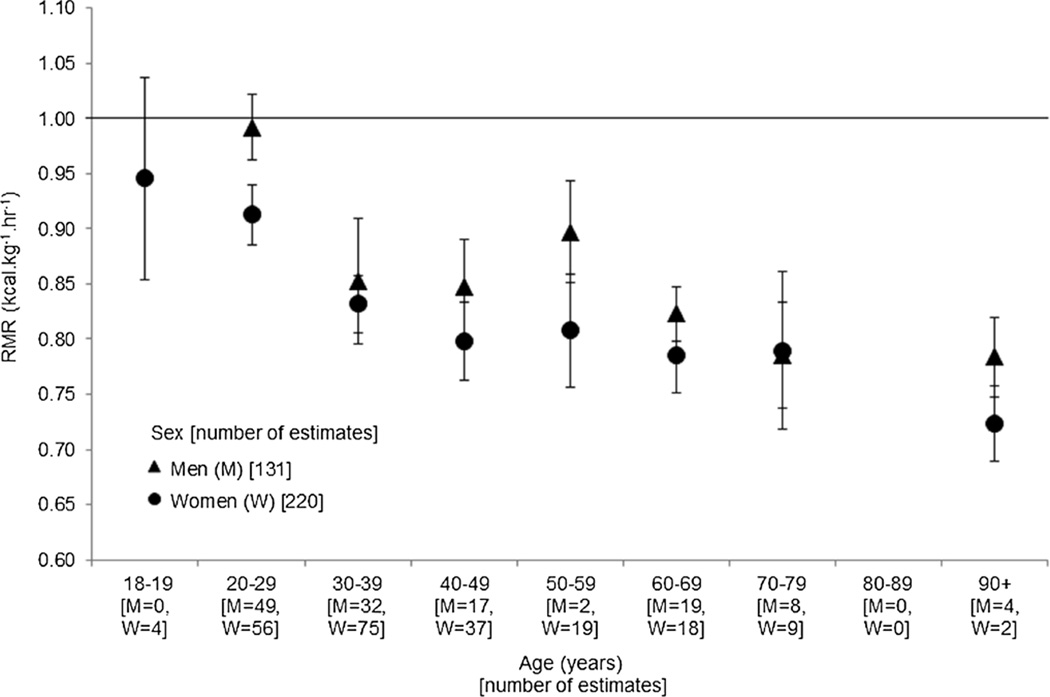
The overall mean value for RMR from the 397 publication estimates for adults was 0.863 kcal·kg−1·h−1 (95% CI = 0.852–0.874). Restricting our analyses to the available 351 sex-specific publication estimates, the RMR of the women (n = 220) was found to be lower than that for the men (n = 131): 0.839 kcal·kg−1·h−1 (95% CI = 0.825–0.853) versus 0.892 kcal·kg−1·h−1 (95% CI = 0.872–0.912), respectively. As anticipated, RMR decreased with age in both sexes; the 95% CI of the younger group (20–29 yr) did not overlap the two older groups (Fig. 1).
FIGURE 1.
Adjusted mean with 95% CI for the RMR (kcal·kg−1·h−1) for adults from 351 publication estimates presented by nine age groupings and sex. The number of publication estimates in each category is included on the x-axis for men (M) and women (W). Solid line = 1.0 kcal·kg−1·h−1.
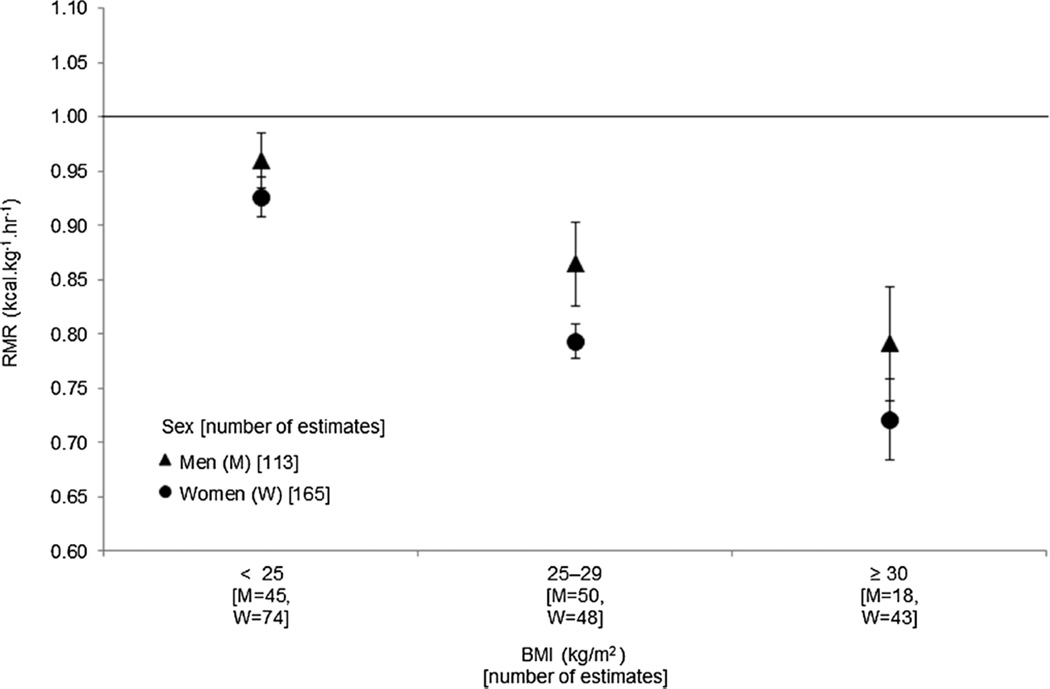
Figure 2 depicts RMR with respect to BMI group and sex for 278 publication estimates (165 women and 113 men). For both sexes, RMR (kcal·kg−1·h−1) was highest in the normal weight group (women = 0.926, 95% CI = 0.908–0.945; men = 0.960, 95% CI = 0.934–0.985), whereas the obese groups had the lowest RMR (women = 0.721, 95% CI = 0.684–0.758; men = 0.791, 95% CI = 0.738–0.843).
FIGURE 2.
Adjusted mean with 95% CI for the RMR (kcal·kg−1·h−1) for adults from 278 publication estimates, based on BMI (kg·m−2) categories of normal (< 25), overweight (25–29.9), and obese (≥ 30) adults presented by sex. The number of publication estimates in each category is included on the x-axis for men (M) and women (W). Solid line = 1.0 kcal·kg−1·h−1.
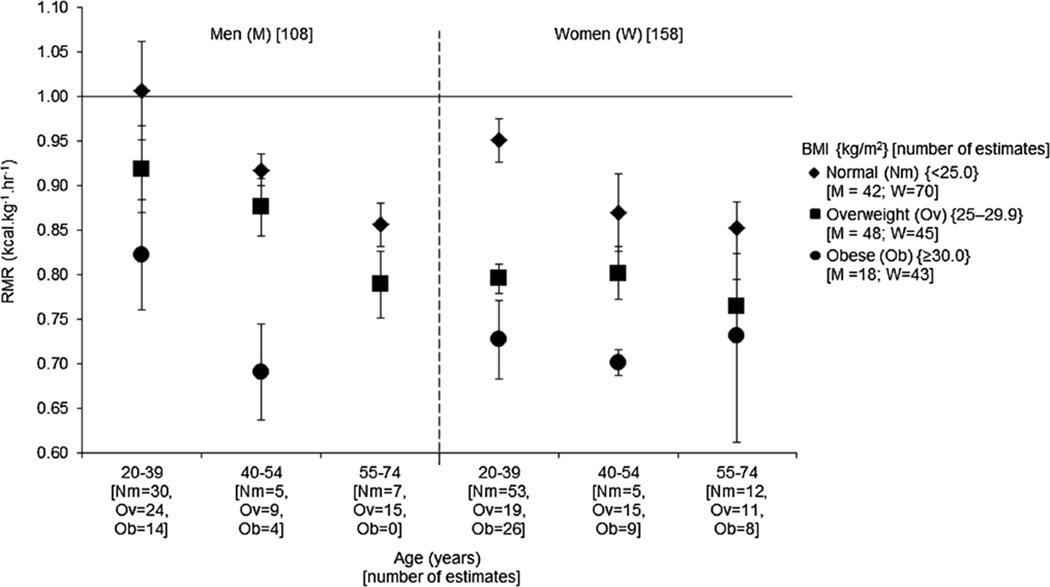
Figure 3 combines age and BMI effects on RMR. For men, the RMR of those who were young and normal weight was the highest, for example, 1.007 kcal·kg−1·h−1 (95% CI = 0.952–1.062), and as age group and BMI group increased, the RMR declined monotonically. For women, the trend was inconsistent. Like men, the RMR was highest for young, normal-weight women: 0.918 kcal·kg−1·h−1 (95% CI = 0.869–0.958). RMR tended to decline with increasing BMI category within all three age groups. However, for the women who were in the two highest BMI categories, there appeared to be little influence of age.
FIGURE 3.
Adjusted mean with 95% CI for the RMR (kcal·kg−1·h−1) for adults from 266 publication estimates, presented by sex, three age groupings, and BMI. The number of publication estimates for BMI groups is included on the x-axis for normal weight (Nm), overweight (Ov), and obese (Ob). Solid line = 1.0 kcal·kg−1·h−1.
DISCUSSION
These results from hundreds of study estimates suggest that there is considerable variability in the RMR of adults such that one standard value should not reasonably be used for adults of varying ages, sex, or obesity status. This opinion has been previously suggested by others (6,9,13) using more restricted data samples. As expected, the RMR of women was lower than that for men (4,12,13,19,27), and the RMR of older adults was less than that for younger adults (9,13,22). Some of the differences between the sexes and age groupings could be related to muscle mass being lower (e.g., less metabolically active tissue) in women and in older adults. RMR is mostly dependent on the amount of metabolically active tissue in an individual; mainly muscle mass (18,35). Our presentation of findings makes this pertinent for public health purposes by offering RMR estimates for groups of men and women by age and relative weight groups.
The results further suggest that RMR per total body mass among obese adults is lower than for normal-weight adults for both women and men (6,22,42). This makes sense because total mass is made up of both fat and FFM, with fat mass not appreciably contributing to metabolism (22,28). Hence, when, for example, one compares an obese person to a normal-weight person having an identical amount of FFM, the obese person has a larger amount of fat mass that produces a greater total body mass. In this comparative example, RMR expressed per total FFM would be equal, whereas overall RMR, expressed per total body mass, must be smaller for the obese person having the greater total body mass. Intriguingly, there seems to be some interaction of obesity with age in women as one study of severely obese women found that their absolute RMR (kcal·min−1) may remain stable with increasing cross-sectional age compared with declines found among leaner counterparts (34). Monda et al. (34) speculated that “the excessive body weight of severely obese women could be similar to a heavy-resistance strength training10 on the legs, so that the muscle mass of the legs does not decrease during aging,” which might potentially preserve FFM over time. Monda et al. (34) also suggested a potentially greater sympathetic tone, which contributes to the maintenance of muscle mass. Another possibility is brown adipose tissue, as Pfannenberg et al. (37) have shown that there is no BMI or age-related decline in the metabolic activity of brown adipose fat in women. These last two mechanisms are controversial and in need of further verification (41).
Overall, the effect of obesity on RMR has implications for determining the energy demands and needs for obese individuals, when using an RMR constant based on a normal-weight adult may result in biased estimates. On the basis of our results, the energy expenditure per kilogram of total body mass would be overestimated by approximately 20%–30% (Fig. 3). Extended over a period of a day, this could sum to a considerable amount of energy. In such cases, one should consider using absolute RMR (kcal·min−1 or kJ·min−1) rather than energy expenditure per kilogram body mass.
Body composition, both FFM and fat mass, contributes to RMR (4,19,33,35,36,40). Although several studies reported either body fat or FFM, we were unable to complete any meaningful systematic analysis because of dissimilar distributions of sex and age in those studies; both characteristics are known to influence RMR (4,10, 21,23). Specifically, we found that for the same FFM of 50–59 kg, 21 publication estimates yielded an average of 0.736 (95% CI = 0.679–0.793) mL·kgFFM−1·min−1 for women whose mean ± SD age was 34.2 ± 4.96 yr and for 26 publication estimates of men with an average of 0.848 mL·kgFFM−1·min−1 (95% CI = 0.824–0.872); however, their mean age was 59.3 ± 20.5 yr. Thus, large-scale studies on the interactions of sex, age, and FFM on RMR are needed.
We explored the relationship of vintage of the data to our results because selected samples and research methods have changed considerably over the time period for which we had publication estimates. The mean RMR from studies post-1980 appears to be lower than that for pre-1980 studies, roughly 0.86 versus 1.0 kcal·kg−1·h−1, respectively. There are several possible explanations for these differences. First, earlier studies were completed mostly on young, relatively lean men, with very few studies focused on women. Men at that earlier point in time tended also to be leaner (with a lower BMI) and have a correspondingly higher RMR on a total body mass basis. Second, the U.S. population since the 1980s is more overweight than previous generations of adults (45), which would result in a lower RMR per kilogram body mass, as we have seen. Third, many methodological changes may have occurred in equipment and better standardization of procedure (15) that could account for some of the difference. Thus, our results cannot be interpreted to imply a definitive reduction in RMR per kilogram body mass over the decades. Intriguingly, the RMR of those earliest six studies approximates 1 kcal·kg−1·h−1, which might account for the development and use of the MET convention being set at that level, a concept that has persevered for almost three quarters of a century.
Although the MET may be a good method to classify intensities of activities, our results suggest that the current definition of a MET may significantly overestimate the RMR of adults, particularly among women and older adults; thus, our findings agree with others using smaller bodies of evidence (13,29,30). When attempting to estimate energy expenditure of adults using the MET approach, if one knew nothing of the effects of age (or BMI) on RMR, then a better estimate may be 0.89 kcal·kg−1·h−1 for men and 0.84 kcal·kg−1·h−1 for women. Doing so would result in an estimate of oxygen uptake that would more closely approximate 3 mL·kg−1·min−1 for men and 2.8 mL·kg−1·min−1 for women. However, efforts beyond the one described here, particularly using individual RMR data to properly address both intra- and interindividual variability in the metric, are needed to develop appropriate values for the extremes in age and body fat for each sex. We believe the additional effort may be worthwhile because recent public health interventions, such as the National Diabetes Prevention Program (14) seek to prevent or delay the development of diabetes among high-risk adults via lifestyle intervention that includes physical activity to be targeted to groups of adults who may vary on sex, age, and overweight/obesity status. Also, given the recent interest in the role of sitting in the development of chronic diseases such as diabetes and cardiovascular disease (46), it is important to recognize just how low rates of energy expenditure might be for groups in the rested sitting state to appreciate how much activity breaks may help to raise energy expenditure to a higher level.
Our review has several limitations. The studies included cannot be considered to be a totally synoptic review of all RMR measurement studies that have been undertaken and published. Many studies measured RMR as a descriptive variable, not as their main focus. Although we made an attempt to eliminate studies using highly questionable methodology, there is really no way to guarantee that data from marginal studies have not affected our findings. We eliminated studies that specifically reported BMR. Many studies did not report the duration of postprandial state (e.g., 3 h vs 6 h vs 9 h, etc.) or prior rest. However, when this information was available, we eliminated studies with less than 3-h postprandial because the greatest thermic effect occurs during this period (39), or with less than 15 min of rest before measurement. Regardless, to our knowledge, our review is the only one to take a public health perspective that identifies potential population group differences in RMR for combinations of demographic and anthropometric characteristics. As with any review of this type, various methods and equipment were used to measure RMR in each study, which changed over time. For example, some older studies may not meet “best practice”methodology as currently defined (15), whereas studies that measured RMR as an ancillary variable may not have followed a rigorous measurement protocol for RMR assessment.
Other issues influencing RMR
Race/ethnicity may influence RMR. In a study of children matched for BMI or body fat, African Americans had approximately a 10%–20% lower RMR than Caucasians (25,31). African Americans also have been found to have less fat than Caucasians or Hispanics (31) and organ weights that vary significantly among different ethnic groups (23,24). Each of these ethnic factors may influence RMR. However, the literature on RMR is quite clear that the location of fat mass in the body is also important in understanding RMR (5,8,24,28,35,42). Furthermore, aerobic fitness (20), or physical activity level (43,44), may influence RMR, but the absolute change is controversial (26,38). We did not have available to us any observations relating organ-specific metabolic rates, location of body fat, consistent ethnic identifiers, nor physical activity or physical fitness parameters for all participants to examine these potential influences. This is not to be a critical limitation because the purpose of our review was to examine the effect of three characteristics commonly encountered in the delivery of public health interventions (e.g., age, sex, and obesity status) on RMR and how it compares to the commonly accepted value for a MET. This may, nonetheless, be fruitful areas for future research.
In conclusion, our review identified hundreds of publication estimates of measured RMR to reflect groups of individuals that vary according to sex, age, and obesity status to be congruent with a public health perspective. We found that adhering to the nearly universally accepted convention of defining 1 MET as 1.0 kcal·kg−1·h−1 (or 3.5 mL O2·kg−1·min−1) may lead to the overestimation of the RMR of approximately 10% for men and almost 15% for women and may reach as high as 20%–30% in some instances for groups varying by these three common demographic and anthropometric characteristics. Given these errors in estimating RMR, one must carefully consider the longstanding adherence to using the conventional MET value for RMR. Even 2% error is a large imbalance taken over an extended time period. Because of existing public health efforts to address things such as diabetes epidemic, having better estimations of RMR, particularly for groups of women and older adults, may help better to plan and achieve intervention outcomes of intervention programs that target such groups of individuals. It is possible that failure to do so may result in important miscalculations of the expected gains from diabetes and other chronic disease prevention efforts that rely on lifestyle interventions based, in part, on physical activity promotion.
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC or the EPA.
No funding was received for this project.
Footnotes
The authors report no conflicts of interest.
The results of this study do not constitute endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 2.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 4.Arciero PJ, Goran MI, Poehlman ET. Resting metabolic rate is lower in women than in men. J Appl Physiol. 1993;75(6):2514–2520. doi: 10.1152/jappl.1993.75.6.2514. [DOI] [PubMed] [Google Scholar]
- 5.Armellini F, Robbi R, Zamboni M, Todesco T, Castelli S, Bosello O. Resting metabolic rate, body-fat distribution, and visceral fat in obese women. Am J Clin Nutr. 1992;56(6):981–987. doi: 10.1093/ajcn/56.6.981. [DOI] [PubMed] [Google Scholar]
- 6.Astrup A, Gotzsche PC, van de Werken K, et al. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr. 1999;69(6):1117–1122. doi: 10.1093/ajcn/69.6.1117. [DOI] [PubMed] [Google Scholar]
- 7.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta Analysis Software, Version 2. Englewood (NJ): Biostat; 2005. [Google Scholar]
- 8.Bosy-Westphal A, Plachta-Danielzik S, Dorhofer RP, Muller MJ. Short stature and obesity: positive association in adults but inverse association in children and adolescents. Br J Nutr. 2009;102(3):453–461. doi: 10.1017/S0007114508190304. [DOI] [PubMed] [Google Scholar]
- 9.Bosy-Westphal A, Wolf A, Buhrens F, et al. Familial influences and obesity-associated metabolic risk factors contribute to the variation in resting energy expenditure: the Kiel Obesity Prevention Study. Am J Clin Nutr. 2008;87(6):1695–1701. doi: 10.1093/ajcn/87.6.1695. [DOI] [PubMed] [Google Scholar]
- 10.Bray GA, Bouchard C, James WPT. Handbook of Obesity. New York (NY): Marcel Dekker, Inc; 2004. pp. 446–448. [Google Scholar]
- 11.Buchowski MS, Sun M. Energy expenditure, television viewing and obesity. Int J Obes Relat Metab Disord. 1996;20(3):236–244. [PubMed] [Google Scholar]
- 12.Butte NF, Treuth MS, Mehta NR, Wong WW, Hopkinson JM, Smith EO. Energy requirements of women of reproductive age. Am J Clin Nutr. 2003;77(3):630–638. doi: 10.1093/ajcn/77.3.630. [DOI] [PubMed] [Google Scholar]
- 13.Byrne NM, Hills AP, Hunter GR, Weinsier RL, Schutz Y. Metabolic equivalent: one size does not fit all. J Appl Physiol. 2005;99(3):1112–1119. doi: 10.1152/japplphysiol.00023.2004. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. National Diabetes Prevention Program. 2012 Available at http://www.cdc.gov/diabetes/prevention/index.htm.
- 15.Compher C, Frankenfield D, Keim N, Roth-Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106(6):881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Davies HT, Crombie K. What are confidence intervals and p-values? London: Hayward Medical Communications; 2009. pp. 1–8. Available at http://www.medicine.ox.ac.uk/bandolier/painres/download/whatis/what_are_conf_inter.pdf. [Google Scholar]
- 17.Dill DB. The economy of muscular exercise. Physiol Rev. 1936;16:203. [Google Scholar]
- 18.DiPietro L. Physical activity, body weight, and adiposity: an epidemiologic perspective. Exerc Sport Sci Rev. 1995;23:275–303. [PubMed] [Google Scholar]
- 19.Dionne I, Després JP, Bouchard C, Tremblay A. Gender difference in the effect of body composition on energy metabolism. Int J Obes Relat Metab Disord. 1999;23(3):312–319. doi: 10.1038/sj.ijo.0800820. [DOI] [PubMed] [Google Scholar]
- 20.Dolezal BA, Potteiger JA. Concurrent resistance and endurance training influence basal metabolic rate in nondieting individuals. J Appl Physiol. 1998;85(2):695–700. doi: 10.1152/jappl.1998.85.2.695. [DOI] [PubMed] [Google Scholar]
- 21.Dos Anjos LA, Machado JM, Wahrlich V, De Vasconcellos MT, Caspersen CJ. Absolute and relative energy costs of walking in a Brazilian adult probability sample. Med Sci Sports Exerc. 2011;43(11):2211–2218. doi: 10.1249/MSS.0b013e31821f5798. [DOI] [PubMed] [Google Scholar]
- 22.Fukagawa NK, Bandini LG, Young JB. Effect of age on body composition and resting metabolic rate. Am J Physiol. 1990;259(2 pt 1):E233–E238. doi: 10.1152/ajpendo.1990.259.2.E233. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher D, Albu J, He Q, et al. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr. 2006;83(5):1062–1067. doi: 10.1093/ajcn/83.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol Endocrinol Metab. 1998;275(2 Pt 1):E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 25.Gannon B, DiPietro L, Poehlman ET. Do African Americans have lower energy expenditure than Caucasians? Int J Obes Relat Metab Disord. 2000;24(1):4–13. doi: 10.1038/sj.ijo.0801115. [DOI] [PubMed] [Google Scholar]
- 26.Geliebter A, Maher MM, Gerace L, Gutin B, Heymsfield SB, Hashim SA. Effects of strength or aerobic training on body composition, resting metabolic rate, and peak oxygen consumption in obese dieting subjects. Am J Clin Nutr. 1997;66(3):557–563. doi: 10.1093/ajcn/66.3.557. [DOI] [PubMed] [Google Scholar]
- 27.Henry CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 2005;8(7A):1133–1152. doi: 10.1079/phn2005801. [DOI] [PubMed] [Google Scholar]
- 28.Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282(1):E132–E138. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- 29.Kozey S, Lyden K, Staudenmayer J, Freedson P. Errors in MET estimates of physical activities using 3.5 ml × kg(−1) × min(−1) as the baseline oxygen consumption. J Phys Act Health. 2010;7(4):508–516. doi: 10.1123/jpah.7.4.508. [DOI] [PubMed] [Google Scholar]
- 30.Kwan M, Woo J, Kwok T. The standard oxygen consumption value equivalent to one metabolic equivalent (3.5 ml/min/kg) is not appropriate for elderly people. Int J Food Sci Nutr. 2004;55(3):179–182. doi: 10.1080/09637480410001725201. [DOI] [PubMed] [Google Scholar]
- 31.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2(6):e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MarNn-MartNnez F, Sanchez-Meca J. Weighting by inverse variance or by sample size in random effects meta-analysis. Educ Psychol Meas. 2010;70(1):56–73. [Google Scholar]
- 33.Melzer K, Laurie Karsegard V, Genton L, Kossovsky MP, Kayser B, Pichard C. Comparison of equations for estimating resting metabolic rate in healthy subjects over 70 years of age. Clin Nutr. 2007;26(4):498–505. doi: 10.1016/j.clnu.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Monda M, Messina G, Mangoni C, De Luca B. Resting energy expenditure and fat-free mass do not decline during aging in severely obese women. Clin Nutr. 2008;27(4):657–659. doi: 10.1016/j.clnu.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Muller MJ, Bosy-Westphal A, Kutzner D, Heller M. Metabolically active components of fat-free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev. 2002;3(2):113–122. doi: 10.1046/j.1467-789x.2002.00057.x. [DOI] [PubMed] [Google Scholar]
- 36.Nookaew I, Svensson PA, Jacobson P, et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J Clin Endocrinol Metab. 2013;98(2):E370–E378. doi: 10.1210/jc.2012-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfannenberg C, Werner MK, Ripkens S, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–1793. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poehlman ET, Melby CL, Badylak SF. Relation of age and physical exercise status on metabolic rate in younger and older healthy men. J Gerontol. 1991;46(2):B54–B58. doi: 10.1093/geronj/46.2.b54. [DOI] [PubMed] [Google Scholar]
- 39.Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr. 1996;63(2):164–169. doi: 10.1093/ajcn/63.2.164. [DOI] [PubMed] [Google Scholar]
- 40.Saris WH. Fit fat and fat free: the metabolic aspects of weight control. Int J Obes Relat Metab Disord. 1998;22(2 Suppl):S15–S21. [PubMed] [Google Scholar]
- 41.Tam CS, Lecoultre V, Ravussin ER. Brown adipose tissue: mechanisms and potential therapeutic targets. Circulation. 2012;125:2782–2791. doi: 10.1161/CIRCULATIONAHA.111.042929. [DOI] [PubMed] [Google Scholar]
- 42.Tataranni PA, Larson DE, Ravussin E. Body fat distribution and energy metabolism in obese men and women. J Am Coll Nutr. 1994;13(6):569–574. doi: 10.1080/07315724.1994.10718449. [DOI] [PubMed] [Google Scholar]
- 43.Toth MJ, Poehlman ET. Effects of exercise on daily energy expenditure. Nutr Rev. 1996;54(4 Pt 2):S140–S148. doi: 10.1111/j.1753-4887.1996.tb03910.x. [DOI] [PubMed] [Google Scholar]
- 44.Tremblay A, Fontaine E, Poehlman ET, Mitchell D, Perron L, Bouchard C. The effect of exercise-training on resting metabolic rate in lean and moderately obese individuals. Int J Obes. 1986;10(6):511–517. [PubMed] [Google Scholar]
- 45.Voelker R. Escalating obesity rates pose health, budget threats. JAMA. 2012;308(15):1514. doi: 10.1001/jama.2012.13712. [DOI] [PubMed] [Google Scholar]
- 46.Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–2905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. BMI classifications. Available at http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.