Abstract
Delirium and dementia are two of the most common causes of cognitive impairment in older populations, yet their interrelationship remains poorly understood. Previous studies have documented that dementia is the leading risk factor for delirium; and delirium is an independent risk factor for subsequent dementia. However, a major area of controversy is whether delirium is simply a marker of vulnerability to dementia, whether the impact of delirium is solely related to its precipitating factors, or whether delirium itself can cause permanent neuronal damage and lead to dementia. Ultimately, it is likely that all of these hypotheses are true. Emerging evidence from epidemiological, clinicopathological, neuroimaging, biomarker, and experimental studies provide support for a strong interrelationship and for both shared and distinct pathological mechanisms. Targeting delirium for new preventive and therapeutic approaches may offer the sought-after opportunity for early intervention, preservation of cognitive reserve, and prevention of irreversible cognitive decline in ageing.
Introduction
With the unprecedented increases in the proportion of persons over age 75 in most industrialised countries, cognitive impairment is an increasingly frequent problem, calling for a thoughtful and effective approach to its recognition and management. Delirium and dementia are among the most common causes of cognitive impairment in clinical settings, yet they are often either unrecognised or mistaken for each other. Dementia, an insidious neurodegenerative condition, is characterised by chronic and progressive cognitive decline from a previous level of performance in one or more cognitive domains that interferes with independence in everyday activities.12 By contrast, delirium is a syndrome manifesting as an acute change in mental status that is characterised by inattention and disturbance in cognition that develops over a short period of time and tends to fluctuate. Delirium is a common, serious, and often fatal disorder that affects as many as 50% of elderly people in hospital. Typically, there is evidence of a medical and/or multifactorial aetiology.12 Delirium is preventable in about 30-40% of cases, and is consistently associated with increased mortality, cognitive impairment, and functional decline.2 Predisposing and precipitating factors for delirium derived from previously validated predictive models are shown in Table 2.2
Table 2.
Predisposing and precipitating factors for delirium*
| Predisposing Factors | Precipitating Factors |
|---|---|
| Dementia or pre-existing cognitive impairment | Medications:
|
| History of delirium | Use of physical restraints |
| Functional impairment | Use of bladder catheter |
Sensory impairment:
|
Physiologic and metabolic abnormalities:
|
| Comorbidity/severity of illness | Infection |
| Depression | Any iatrogenic event |
| History of transient ischaemia/stroke | Major surgery |
| Alcohol abuse | Trauma or urgent admission |
| Older age | Coma |
From validated predictive models for delirium2
Delirium and dementia can commonly coexist, with pre-existing dementia being a leading risk factor for delirium. While these conditions are recognised as substantially enmeshed, the nature of their interrelationship remains unclear. Moreover, shared pathophysiological mechanisms have been postulated for these syndromes, including cholinergic deficiency, inflammation, and reduced cerebral oxidative metabolism.1, 2 Fundamental understanding of the interface of delirium and dementia may provide an important opportunity to advance our conceptualisation and treatment approaches to both conditions.
In this review, we will first briefly discuss distinguishing delirium and dementia before examining the current epidemiological, clinical, neuroimaging, biomarker, and experimental evidence linking these disorders. In each of these areas, important gaps in knowledge and future directions for research will be highlighted. Finally, potential mechanisms underlying the links between delirium and dementia and their implications for treatment will be discussed.
Distinguishing delirium from dementia
To date, dementia and delirium have been conceptualised as distinct and mutually exclusive conditions. Indeed, DSM-5 states that dementia should not be diagnosed in the face of delirium, and that delirium should not be diagnosed when symptoms can be “better accounted for by a pre-existing, established, or evolving dementia.”12 Distinguishing the two diagnoses in the clinical setting can be difficult, even for experienced clinicians. Delirium symptoms can persist for months or even years,13-18 and the recognised conditions of “persistent delirium” and “reversible dementia” blur the boundaries between these previously demarcated syndromes of cognitive impairment.1 Distinguishing them is of critical importance, since their evaluation and clinical management are distinct. Signs and symptoms that can be useful to distinguish delirium from dementia are listed in Table 1.3, 19, 20 Most prominently, with delirium, the onset is typically abrupt over hours to days, whereas with dementia the onset is insidious and progressive over months to years. With delirium, attention and level of consciousness are reduced and fluctuating; with dementia these domains typically remain intact until the advanced stages of dementia. Ultimately, the differentiation may depend on the presence of an acute change in mental status or behaviour from baseline noted by an informed caregiver, or may be established only in retrospect by resolution of symptoms after precipitating factors have been removed or the acute illness has been treated. In the face of uncertainty, mental status changes should be treated as delirium, until proven otherwise.
Table 1.
Comparative features of delirium and dementia
| Feature | Delirium | Dementia |
|---|---|---|
| Onset | Abrupt, though initial loss of mental clarity may be subtle | Insidious and progressive |
| Duration | Hours to days (though can be prolonged) | Months to years |
| Attention | Reduced ability to focus, sustain, or shift attention is a hallmark feature, occurring early in presentation | Normal unless severe dementia |
| Consciousness (awareness of the environment) | Fluctuating (making assessment at multiple timepoints necessary), reduced level of consciousness and impaired orientation | Generally intact |
| Speech | Incoherent, disorganised; distractible in conversation | Ordered, may develop anomia or aphasia |
| Other features | Caused by underlying medical condition, substance intoxication, or medication side effect; Hyperactive, hypoactive, and mixed forms of psychomotor disturbance are possible; disruption in sleep duration and architecture; perceptual disturbances | Caused by underlying neurological process (e.g. beta-amyloid plaque accumulation in Alzheimer’s disease), with symptoms varying depending on underlying pathologies (e.g. fluctuations in cognition are a feature of Lewy body dementia) |
Note that there is substantial overlap between these syndromes; they may coexist in an individual patient.
Evidence linking delirium and dementia
A major area of controversy is whether delirium is simply a marker of vulnerability to dementia, whether delirium unmasks unrecognised dementia, whether the impact of delirium is solely related to its precipitating factors, or whether delirium itself can cause permanent neuronal damage and lead to dementia. Clinically, the development of delirium may have direct “toxic” effects related to periods of lethargy, psychomotor retardation or agitation, and unsafe behaviours. The lethargy and psychomotor retardation may result in immobility and related complications, including but not limited to aspiration pneumonia, respiratory compromise, decreased oral intake with dehydration or malnutrition, pressure ulcers, urinary tract infection, deep venous thrombosis and pulmonary emboli. Psychomotor agitation and unsafe behaviour may lead to falls and use of antipsychotics and other sedative medications or physical restraints, along with their attendant complications. Thus, the occurrence of delirium itself may set off a cascade of noxious stimuli that may adversely impact the brain.
To date, a number of mechanisms have been hypothesised on how delirium may contribute to permanent neuronal damage and dementia. This includes neurotoxicity (e.g., drugs, anaesthesia, endotoxins), inflammation, chronic stress, neuronal damage (e.g., prolonged ischaemia, hypoglycaemia, shock, sepsis), acceleration of dementia pathology (e.g., beta-amyloid (Aβ), tau), and diminished cognitive reserve (Figure 1).3-6 Certain insults, such as metabolic derangements or particular drugs (e.g., anticholinergics), may directly cause neuronal dysfunction via alterations in neurotransmitters (e.g., acetylcholine deficiency7 and/or dopamine excess8). Hypoxia or cerebral ischaemia may lead directly to cerebral dysfunction, via impaired cerebral blood flow and metabolism. Some anaesthetics may directly facilitate acceleration of Aβ accumulation, leading to apoptosis and cholinergic dysfunction, which in turn may further accelerate or initiate Aβ pathology.9 Infections or response to a stressor (e.g., surgery or acute illness) can cause neuronal dysfunction through activation of inflammatory mechanisms.10 Neuronal injury in these cases can occur indirectly through a variety of mechanisms, including altered neurotransmission, apoptosis, and/or activation of microglia and astrocytes, which lead to the production of free radicals, complement factors, glutamate, and nitric oxide.11 Emerging evidence from epidemiological, clinicopathological, neuroimaging, biomarker, and experimental studies provide support for a strong interrelationship and for both shared and distinct pathological mechanisms.
Figure 1.
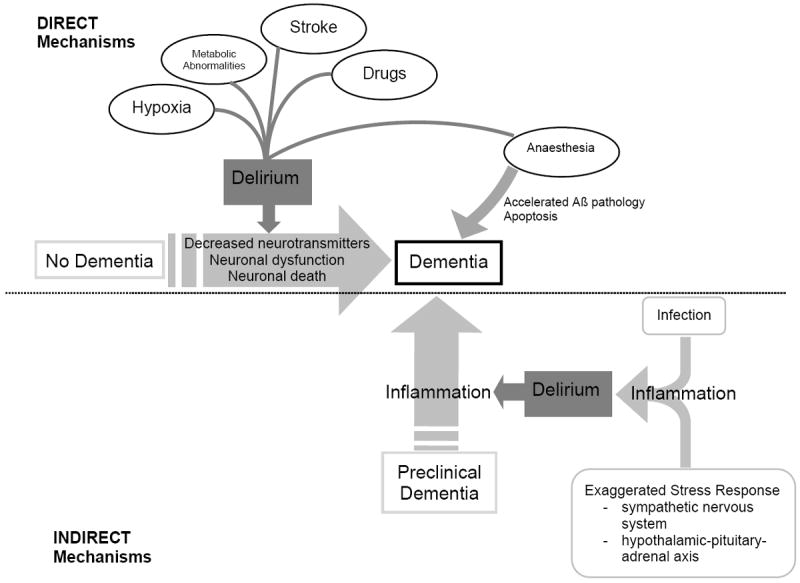
A hypothetical model for the pathophysiologic interrelationship between delirium and dementia. Delirium is a known risk factor for new onset dementia, and this may arise via direct mechanisms such as hypoxia, metabolic abnormalities, stroke, or medications. In turn, delirium is associated with neuronal dysfunction, alterations in neurotransmitters, and neuronal death and this could lead directly to dementia. There is also growing evidence that certain anesthetics associated with postoperative delirium may alter Aβ, which in turn may indicate a role for new onset dementia. Delirium is also likely to be a marker of vulnerability in patients with pre-existing dementia, and might accelerate existing dementia. This may occur indirectly, for example, via inflammation triggered by systemic infection or exaggerated response to a stressor.
Ref.
Maclullich AM, Anand A, Davis DH, Jackson T, Barugh AJ, Hall RJ, Ferguson KJ, Meagher DJ, Cunningham C. New horizons in the pathogenesis, assessment and management of delirium. Age Ageing. 2013 Nov;42(6):667-74.
Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol 2009; 5: 210-220. PMCID: PMC3065676
Epidemiological evidence
Large cohort studies suggest that cognitive impairment and dementia are substantial risk factors for delirium. In the majority of these studies, delirium has been assessed in populations that include patients with dementia. Table 3 summarises studies from a comprehensive review that have examined pre-existing cognitive impairment or dementia as risk factors for delirium in validated predictive models that include adjustment for important confounding variables.21-31 The studies include 5,166 participants with mean ages ranging from 68-85 years, recruited from diverse settings, including hospital medical or geriatric medicine wards, emergency department, and surgical services. Cognitive baseline status was determined by a variety of approaches, including brief cognitive screening tests (e.g., Short Portable Mental Status Questionnaire (SPMSQ),32 Mini-Mental State Examination (MMSE),33 proxy-based measures (e.g., Informant Questionnaire for Cognitive Decline in the Elderly (IQCODE),34 Blessed Dementia Rating Scale (BDRS)35; clinician diagnosis; or chart documentation of dementia. Delirium was also measured by a variety of approaches, including the Confusion of Assessment Method (CAM),36 Diagnostic and Statistical Manual (DSM) Versions III, IIIR, and IV,37-39 and the Delirium Observation Screening Scale (DOSS).40 The rate of delirium ranged from 9% to 44% across these studies. Baseline cognitive impairment or dementia is a substantial independent risk factor for delirium, consistently increasing delirium risk by 2- to 5-fold (Table 3).
Table 3.
Baseline cognitive impairment/dementia as independent risk factor for delirium from predictive models
| Study (year) | Population | Cognitive baseline | Delirium measure | Mean age (years) | % delirium | Effect size (adjusted) (95% CI) |
|---|---|---|---|---|---|---|
| Kennedy (2014)21 | Emergency department, age ≥ 65 years (n=700) | Documented dementia by chart | Prevalent delirium by CAM | 77 | 9% | OR 4.3 (2.2 to 8.5) |
| Koster (2013)22 | Elective cardiac surgery, age ≥ 70 years (n=300) | MMSE < 23 | DOSS | 74 | 17% | OR 4.5 (1.9 to 13) |
| Moerman (2012)23 | Acute hip fracture, age ≥ 65 years (n=378) | Clinical diagnosis of dementia | Prevalent delirium by DSM-IV | 84 | 27% | OR 2.8 (1.7 to 4.6) |
| Bo (2009)24 | Patients age ≥ 70 years admitted to medical or geriatric ward (n=252) | SPMSQ for presence and severity of cognitive impairment | Incident delirium by CAM | 82 | 11% | RR 2.1 (1.6 to 2.6) |
| Rudolph (2009)25 | Planned cardiac surgery, age ≥60 years (development n=122; validation=109). | Pre-operative MMSE ≤ 23 | Incident delirium by CAM | 75 | 44% | RR 1.3 (1.0 to 1.7) |
| Kalisvaart (2006)26 | Elective hip surgery, age ≥ 70 years (n=603) | Pre-operative MMSE <24 | Postoperative delirium by DSM-IV and CAM | 78 | 12% | RR 5.5 (3.6 to 8.6) |
| Wilson (2005)27 | Patients aged ≥ 75 years admitted to an acute medical ward (n=100) | IQCODE to establish presence of cognitive change over time | Incident delirium by DSM-III | 85 | 12% | OR 3.2 (1.2 to 9.0) |
| O’Keeffe (1996)28 | Acute medical admissions to geriatric medicine unit (n=225) | Clinical diagnosis of dementia or BDRS ≥4 | Incident delirium by DSM-III | 82 | 28% | OR 4.8 (2.0 to 11.6) |
| Marcantonio (1994)29 | Elective surgical admissions, age ≥ 50 years (n=1341) | TICS <30 | Post-operative delirium by CAM | 68 | 9% | OR 4.2 (2.4 to 7.3) |
| Pompei (1994)30 | Acute hospital medical and surgical admissions, age ≥ 65 years with no delirium (development n=432; validation n=323) | MMSE < 24 (education adjusted) | Incident delirium by DSM-III-R | 74 | 15% | OR 3.6 (2.1 to 6.2) |
| Inouye (1993)31 | Acute hospital medical admissions, age ≥ 70 years with no dementia or delirium (development n=107; validation n=174) | MMSE < 24 on admission | Incident delirium by CAM | 79 | 25% | RR 2.8 (1.2 to 6.7) |
BDRS Blessed Dementia Rating Scale; CAM Confusion Assessment Method; DOSS Delirium Observation Screening Scale; IQCODE Informant Questionnaire for Cognitive Decline in the Elderly; MMSE Mini-Mental State Examination; OR odds ratio; RR relative risk; SPMSQ Short Portable Mental Status Questionnaire; TICS Telephone Interview for Cognitive Status.
Delirium is an independent risk factor for long-term cognitive decline and dementia, according to a comprehensive review of studies representing a total of 4,745 individuals (Table 4).41-48 The studies vary in design, including population-based approaches, retrospective analyses of outpatients such as memory clinic patients, evaluation of ICU inpatients and those undergoing elective surgery. Nonetheless, these multiple studies consistently suggest that an episode of delirium carries substantial dementia risk, as well as an altered trajectory of cognitive recovery following surgical procedures. Cognitive outcomes were determined using a variety of measures, including neuropsychological assessments (e.g., Automated Geriatric Examination for Computer Assisted Taxonomy (AGECAT),49 Repeatable Battery for the Assessment of Neuropsychological Status (RBANS),50 Blessed Information-Memory-Concentration (IMC),35 MMSE,33 clinician diagnosis, or consensus panel diagnosis. Despite the multiple methods for operationalising delirium and dementia, the findings are consistent and robust across studies. For example, delirium was consistently associated with a significantly increased risk of both long-term cognitive decline (substantial declines by cognitive testing) and dementia (odds ratios from 6-41), with follow-up periods ranging from 1 to 5 years after baseline evaluation. A meta-analysis51 involving two studies with 241 total patients demonstrated that delirium was associated with an increased rate of incident dementia, even after controlling for relevant confounders (adjusted relative risk, RR, 5.7, 95% confidence interval, CI, 1.3-24.0). In another study of 225 cardiac surgery patients,44 delirium resulted in a punctuated decline in cognitive function, followed by recovery over 6-12 months in most patients; however, a substantial proportion, particularly those with prolonged delirium, never returned to baseline. In a study of 821 intensive care unit patients, a longer duration of delirium was independently associated with significantly worse global cognition and worse executive function scores based on a neuropsychological battery at 3 and 12 months follow-up.42 Moreover, clinical trial evidence has suggested that treatment of delirium was associated with better cognition during follow-up.52 While not directly linked to delirium, the literature on postoperative cognitive dysfunction also suggests persistent long-term impairment following surgery.53-55
Table 4.
Delirium as an independent risk factor for long term cognitive decline and dementia
| Study (year) | Population | Delirium measure | Cognitive outcome | Mean age at baseline (years) | % delirium | Effect size (adjusted) (95% CI) |
|---|---|---|---|---|---|---|
| CFAS (2014)41 | Population-based; multi-centre sampling from Health Authority lists (n=2197) | Algorithmic operationalisation of DSM-IV based on Geriatric Mental State examination | AGECAT-defined dementia at 2 years | 77 | 6% | OR 8.8 (2.8 to 28) |
| BRAIN-ICU (2013)42 | Multi-centre ICU admissions (n=821) | CAM-ICU | RBANS score at 1 year | 61 | 74% | -5.6 (-9.5 to -1.8) points per day of delirium |
| Gross (2012)43* | Memory clinic patients with clinically diagnosed Alzheimer’s dementia (n=263) | Retrospective diagnosis of delirium from case notes (validated algorithm) | Worsening on Blessed IMC score over up to 5 years | 78 | 56% | Additional 1.2 (0.5 to 1.8) points per year |
| Saczynski (2012)44 | Elective CABG or valve surgery patients age ≥60 years (n=225) | CAM | Trajectory of MMSE change over 1 year | 73 | 46% | Prolonged impairment in recovery |
| Vantaa 85+ (2012)45 | Population-based; all residents age ≥85 (n=553) | Participant and informant interview, along with medical record review | Dementia (DSM-III-R; individual clinician) at 2.5 years | 89 | 13% | OR 8.7 (2.1 to 35) |
| Fong (2009)46* | Memory clinic patients with clinically diagnosed Alzheimer’s dementia (n=408) | Retrospective diagnosis of delirium from case notes (validated algorithm) | Worsening on Blessed IMC score over 0.7 years | 74 | 18% | Additional 2.4 (1.0 to 3.8) points |
| Bickel (2008)47 | Elective hip surgery patients age ≥60 years (n=200) | CAM | Cognitive impairment and/or dementia | 74 | 21% | OR 41 (4.3 to 396) |
| Lundstrom (2003)48 | Acute hip fracture patients, dementia-free, age ≥ 65 years (n=78) | DSM-IV | Consensus diagnosis of dementia at 5 years | 79 | 38% | OR 5.7 (1.3 to 24) |
Related analyses with some overlap of data. AGECAT Automated Geriatric Examination for Computer Assisted Taxonomy; Blessed IMC Blessed Information-Memory-Concentration scale; BRAIN-ICU Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors; CABG Coronary artery bypass grafting; CAM Confusion Assessment Method; CAM-ICU Confusion Assessment Method-ICU; CFAS Cognitive Function and Ageing Study; DSM Diagnostic and Statistical Manual of the American Psychiatric Association; RBANS Repeatable Battery for the Assessment of Neuropsychological Status.
Careful follow-up studies have documented that persons with dementia who develop delirium have worse outcomes than those with dementia alone,56 including increased rates of re-hospitalisation, institutionalisation, mortality, and subsequent cognitive decline.57-61 In one study of 771 community-dwelling patients with Alzheimer’s disease (AD), after adjustment for confounders, delirium was associated with a greatly increased adjusted risk of death, relative risk of 5.4 (95% CI 2.3-12.5) or institutionalisation, relative risk of 9.3 (95% CI 5.5-15.7). At one year, 21% of those with cognitive decline, 15% of institutionalisations and 6% of deaths were attributable to delirium.59 In another study of 263 patients with AD, despite the trajectories being similar prior to an index hospitalisation, delirium resulted in a fundamental alteration in the trajectory of cognitive decline with a 2-fold acceleration in rate of decline over the year following hospitalisation, and accelerated decline persisting over the entire the 5-year follow-up period.43 This study was highly significant in demonstrating that in persons with AD, delirium resulted in a dramatic increase in the rate of cognitive decline over time, and that this change appeared to be irreversible.
Additional long-term follow-up studies looking at outcomes of delirium are still needed to fully understand the impact of this condition. For example, long-term follow-up of a well-characterised cohort who are initially free of dementia at baseline may help to clarify whether incident delirium can lead to new-onset dementia. The patient’s individual experience with delirium, including distress, and development of post-traumatic stress disorder has not been fully examined as outcome measures. Lastly, genetic and other important determinants of delirium risk and risk stratification to identify particularly high-risk individuals should be explored. Ultimately, these data will allow for greater support for early identification, prevention, and treatment of delirium.
Clinicopathological evidence
The interaction between delirium and dementia has been shown in a population-based study, Vantaa 85+, examining the impact of delirium (retrospectively determined) on cognitive and functional outcomes.45 In this cohort of 553 individuals age 85 years and older, delirium increased the risk of incident dementia (odds ratio 8.7, 95% CI 2.1-35). Moreover, consistent with the literature on cognitive trajectories, delirium was associated with worsening dementia severity, new functional deficits, and accelerated decline in cognitive scores. This study also examined the neuropathological correlates of dementia in the presence or absence of a history of delirium. The relationship between dementia and measures of neurofibrillary tau, amyloid burden, apolipoprotein (ApoE) ε4, vascular lesions and Lewy body pathology were strongest in the absence of a delirium history. However, when these pathological markers were assessed in relation to dementia where delirium was also part of the history, no associations were detectable. Although not powered to be conclusive, the results suggest that when delirium is part of the dementia trajectory, the pathological substrates may be different from conventional dementia pathology, such as Alzheimer’s, vascular or Lewy body pathology. These findings raise the intriguing possibility that the acceleration of cognitive decline following delirium might result from an alternative mechanism leading to neuronal damage.
Studies that include markers of AD pathology, such as CSF or tau and beta amyloid imaging, as well as additional post-mortem studies, will yield significant insight into the fundamental pathophysiology of delirium and may ultimately help with development of effective treatments.
Neuroimaging evidence
Despite its routine use in clinical practice and a growing number of studies utilising neuroimaging to investigate the pathophysiology and consequences of delirium, there are few studies that provide long-term follow-up or convincing evidence of permanent neurological changes attributable to delirium. Most studies to date have been limited by small sample sizes, inadequate control groups, and the lack of baseline scans prior to delirium.62, 63 Two studies on the same sample of 47 intensive care unit survivors used volumetric and diffusion tensor imaging at hospital discharge and 3 month follow-up.64, 65 In the volumetric analysis, longer duration of delirium was significantly associated with greater brain atrophy at hospital discharge and at 3 month follow-up. In addition, duration of delirium was significantly associated with white matter disruption at both hospital discharge and at 3 month follow-up.
The lack of baseline scans in previous studies precludes any strong conclusions about whether the development of delirium itself contributed to subsequent neuroimaging findings. Future studies, with larger cohorts, baseline characterisation, careful selection of controls, and advanced neural anatomic and functional neural imaging measures, may lead to greater understanding of the anatomic and functional links between delirium and dementia.
Biomarker evidence
A range of serum and cerebrospinal fluid (CSF) biomarkers has been considered in the search to understand delirium pathogenesis. Previous work in ICU patients found that elevated levels of baseline inflammatory markers were associated with subsequent delirium.64, 66 In a pilot study of patients who were critically ill due to infection, the proinflammatory cytokine interleukin (IL)-8 was associated with delirium,67 whereas in non-infected patients, the antiinflammatory cytokine IL-10 was associated with delirium. These findings suggest that the underlying mechanisms governing the development of delirium in patients with inflammation may differ from those without inflammation.68 Others have found cytokines such as insulin-like growth factor (IGF)-1, IL-1β and IL-1 receptor antagonist (RA) to be associated with delirium,69-71 and high levels of interferon (IFN-γ) with low levels of IGF-1 were associated with delirium severity.72 S100B, a marker of astrocyte damage, has been shown to be elevated in delirium, both in plasma and in CSF.68, 73, 74 It is not known if these changes in biomarkers are a direct consequence of delirium, a consequence of a separate dementia with progressive neurodegeneration, or both.
Several studies have looked for a direct association between AD biomarkers and delirium. In a cohort of 76 individuals admitted for emergency hip fractures, levels of Aβ1-42, tau, and phosphorylated-tau from CSF were not associated with delirium status, nor did they correlate significantly with IQCODE score, despite a strong association of postoperative delirium with premorbid cognitive decline (as measured by IQCODE).75 Given the limited sample size, however, the results must be interpreted with caution.
In a more recent study of 557 non-demented patients age ≥70 undergoing major non-cardiac surgery, after adjusting for age, sex, surgical procedure, and preoperative cognitive function, ApoE ε4 and ε2 carrier status were not associated with postoperative delirium. Further, there was no observed association between ApoE and delirium severity or number of delirium episodes. Thus, in a sample with careful exclusion of persons with underlying dementia, ApoE genotype does not appear to confer either risk or protection for postoperative delirium incidence, severity, or duration.76 The results of both studies are consistent with the Vantaa 85+ epidemiological study of cerebral pathology,45 suggesting that postoperative delirium might arise through pathophysiological pathways distinct from AD.
In contrast, however, other studies (that did not specifically exclude persons with dementia) have shown a possible association between AD biomarkers and postoperative delirium.9 In a study of 153 older adults undergoing elective total hip or knee replacement, CSF was obtained during initiation of spinal anaesthesia, and patients were monitored post-operatively for the development and severity of delirium. A significantly higher incidence of delirium was seen among participants with preoperative CSF Aβ40/Tau and Aβ42/Tau ratios in the lowest quartile versus all other quartiles (32% vs. 17%, P=.05 for both comparisons), suggesting a possible threshold effect in the relationship between preoperative AD biomarkers and postoperative delirium. After adjusting for age and sex, lower preoperative CSF Aβ40/Tau and Aβ42/Tau ratios were associated with significantly higher scores on a delirium severity scale (β = -0.12 ± 0.05, P=0.018 and β = -0.62 ± 0.27, P=0.022, respectively), suggesting that lower CSF Aβ/Tau ratios, similar to ratios seen in AD, are associated with greater delirium severity.9 Others have found elevated serum Aβ1-42/40 levels are associated with delirium occurrence and correlates with subjective complaints of cognitive-impairment 18-months after the delirium episode.68 Taken together, these findings suggest that there may be a role for Aβ and Tau in the neuropathogenesis of postoperative delirium, and that delirium may represent the first sign of a (subclinical) dementia process in some cases.
Although these studies are generally small and require cautious interpretation, the accumulating evidence lends support for the impact of delirium itself contributing to and/or being a mediator of permanent cognitive impairment. Future human studies with careful baseline assessment of cognitive function, control for confounding factors such as age and pre-existing dementia, and long-term follow-up with characterisation by neuropsychological testing and neuroimaging, are needed to better address this important area.
Animal models and neuronal tissue culture
Important recent work involving animal models relevant for delirium have demonstrated that in vulnerable animals, systemic inflammatory insults can cause punctuated cognitive decline typical of delirium, followed by persistent acceleration in disease progression typical of dementia.77 Many experiments have tried to take a clinically relevant experimental approach to delirium by capturing both predisposing and precipitating factors. In these models, underlying pathology/brain vulnerability has been induced by either neurodegeneration associated with prion infection,78 or through selective and partial lesioning of the cholinergic projections of the basal forebrain.79 Subsequent to this, the animals are exposed to an inflammatory challenge to simulate bacterial or viral infection (e.g. lipopolysaccharide (LPS) or polyinosinic: polycytidylic acid (poly I:C), respectively).80, 81 In these models, acute peripheral inflammation induced by LPS or poly I:C leads to acute deficits in cognition and motor function, analogous to delirium, and similar deficits are observed with inflammation superimposed upon either of these underlying neurodegenerative models. Thus, such animal models provide an opportunity to probe specific pathophysiological pathways in delirium and dementia.82 Other studies using a single dose of LPS to induce an inflammatory insult comparable to sepsis in humans, a frequent contributor to delirium, have found that inflammation via inducible nitric oxide synthase contributes to neuronal death, microglial activation, decreased regional blood flow, and loss of cholinergic activation,83-85 with persistent cognitive deficits in attention, executive function, and working memory.
Microglial priming has been demonstrated in chronic neurodegeneration78 and ageing,86 whereby microglia elaborate a more aggressive inflammatory response to peripheral inflammation compared with either younger or non-diseased animals. The acute insult triggers acute, transient81 and fluctuating87 cognitive deficits during T-maze testing, and further neurodegeneration78 and acceleration of disease trajectory is observed.77 Other studies using this model have shown microglia express cyclo-oxygenase (COX) 1 and synthesise prostaglandins. Selective inhibition of COX-1 or non-selective inhibition with ibuprofen are protective against systemic LPS or IL-1β-induced cognitive deficits respectively.88 Inflammation was sufficient, but microglial priming was not essential, for similar deficits reproduced in cholinergic-deficient mice, which could be blocked by donepezil.80 This suggests an important interplay between acetylcholine deficiency and systematic inflammation but the observation that worsening neurodegeneration makes animals progressively more susceptible to the cognitively disrupting effects of LPS87 implicates several different neuronal networks.
Previous studies in human neuronal cell culture have demonstrated that exposure to some inhalational anaesthetics (e.g., isoflurane, sevoflurane) may induce neurotoxicity, including apoptosis, caspase activation, A-β oligomerisation and accumulation, neuroinflammation, and mitochondrial dysfunction,6, 89 whereas this effect is not seen with other agents (e.g. desflurane, nitrous oxide and propofol).90
Animal models and neuronal tissue culture studies have already begun to explore pathophysiological pathways that may identify future targets for intervention. Other areas will need to be explored, including neurotransmitter dysregulation, oxidative stress, and aberrant stress response. Advancing these mechanistic studies will be critical, and ultimately will represent the primary means for understanding the pathophysiology of delirium. Initial studies focused on inflammation have suggested the impact of delirium itself may contribute to and/or be a mediator of permanent cognitive impairment. Taken together, these experimental studies provide strong support for the pathophysiological linkage between delirium mechanisms and long-term cognitive impairment or dementia, and further studies are necessary to confirm and extend these findings.
Conclusion
Ultimately, it is likely that delirium serves multiple roles: it is a marker of vulnerability, unmasks unrecognised dementia, mediates the effects of noxious insults, and itself leads to permanent neuronal damage and dementia. There is little doubt that occurrence of an episode of delirium can signal underlying vulnerability of the brain with decreased cognitive reserve and increased risk for future dementia.91 Delirium reflects a decompensated cognitive state under stress conditions, and its presence implies diminished cognitive reserve. In some cases, delirium may bring previously unrecognised cognitive impairment to medical attention. Moreover, there is no question that severe precipitating factors of delirium, such as prolonged hypoglycaemia or hypoxaemia, can lead to neuronal death and permanent cognitive impairment. It is also possible that delirium may mediate the impact of many factors, such as general surgery, anaesthesia, critical illness, acute respiratory distress syndrome, prolonged intubation, or sepsis, on long-term cognitive outcomes.
Unraveling the inter-relationship of delirium and dementia poses myriad challenges highlighting the barriers to addressing this important area. Given the lengthy prodromal stage of dementia along with its unpredictable progression, knowledge of the baseline state and trajectory of any cognitive changes are essential. The target population often is frail, with multiple medical co-morbidities, and delirium may go undetected, thus active surveillance is essential. Refinement of distinct diagnostic criteria and demarcation of the overlap syndrome will be critical to differentiate the two conditions. Identification of the contribution of the presence of delirium is a paramount first step; however, a dose-response relationship with delirium severity and duration will help to strengthen causal inference. Appropriate control for confounding factors, without over-controlling, will be necessary to evaluate the contribution of delirium itself, as well as the mediation effects of other precipitating insults by delirium. Moreover, the presence of delirium poses numerous logistical challenges, including informed consent, ethical dilemmas, and challenges to conducting procedures and neuroimaging in the face of older adults with agitation, behavioural disturbances, severe illness, multi-morbidity, and frailty.
Acknowledging delirium as a determinant of chronic cognitive impairment obliges us to broaden our understanding of dementia. Recognising that slowly evolving neurodegenerative processes may be accelerated by delirium necessitates the consideration of the long-term impact of acute illness and other precipitants on the vulnerable brain. Thus, delirium may serve as an important model system for research, offering a unique approach to advance our understanding of cognitive disorders and dementias more generally. The frequency and acuity of delirium and its associated serious adverse outcomes make it a highly promising area for investigation. The development of delirium may help to identify persons who are vulnerable to cognitive decline through genetic predisposition, diminished cognitive reserve, or the presence of unrecognised dementia. Investigation of delirium also provides a window to observe the link between brain pathophysiology and behavioural manifestations, which may hold broader implications for other neurological and psychiatric disorders. Moreover, advancing the understanding of the pathogenesis of delirium will be critical to identify preventable factors which can lead directly to neuronal injury, and thus, permanent cognitive sequelae. Implementing therapies for prevention of delirium holds particular relevance for their potential to delay or alter both the typical cognitive ageing process as well as the progression of cognitive decline in persons with dementia. Finally, targeting delirium for new therapeutic approaches may offer the much sought-after opportunity for early intervention, preservation of cognitive reserve capacity and prevention of irreversible cognitive decline in ageing.
PANEL
Search strategy and selection criteria
We conducted an initial systematic search of Medline, Ovid SP, Embase, and Science Citation Index from 1950-2012. The Ovid search terms included “exp Delirium/ep [Epidemiology]” “delirium.mp” “acute confusion”.mp “metabolic encephalopathy”.mp, with equivalent terms used in the other databases. There were no language restrictions. Articles were selected by hand-review of the results of the search on the basis of relevance to delirium and dementia. Subsequently, an updated systematic search was conducted in PubMed from 2000 – 2015 using the following search strategy: (“dementia”[MeSH Terms] OR “dementia”[All Fields]) AND (“delirium”[MeSH Terms] OR “delirium”[All Fields]). For all articles, including systematic and comprehensive reviews, tables and reference listings generated were reviewed for additional pertinent articles.
Acknowledgments
The funding sources had no role in the study design, analysis or interpretation of the data, writing of the report, or decision to submit the paper for publication.
The authors gratefully acknowledge Dr. Eva Schmitt and Ms. Dulce Pina for assistance with coordinating the work. This work is dedicated to the memory of Joshua Bryan Inouye Helfand.
Grant Funding: This work was supported in part by Grants No. P01AG031720 (SKI), R01AG044518 (SKI), and K07AG041835 (SKI) from the National Institute on Aging and by the Milton and Shirley F. Levy Family Chair. The funding sources had no role in the study design, analysis or interpretation of the data, writing of the report, or decision to submit the paper for publication.
Footnotes
Author Contributions
All authors contributed to the search strategy, selection of articles, synthesis of information identified in the search, drafting and editing the manuscript or relevant sections thereof. All authors have seen and approved the final version. Dr. Inouye had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of Interests
The authors have no conflicts of interest to disclose.
References
- 1.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–65. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 2.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–22. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5:210–20. doi: 10.1038/nrneurol.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maclullich AM, Anand A, Davis DH, et al. New horizons in the pathogenesis, assessment and management of delirium. Age Ageing. 2013;42:667–74. doi: 10.1093/ageing/aft148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308:73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Z, Dong Y, Maeda U, et al. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1300–6. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 7.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63:764–72. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez-Bermudez J, Ruiz-Chow A, Perez-Neri I, et al. Cerebrospinal fluid homovanillic acid is correlated to psychotic features in neurological patients with delirium. Gen Hosp Psychiatry. 2008;30:337–43. doi: 10.1016/j.genhosppsych.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z, Swain CA, Ward SA, et al. Preoperative cerebrospinal fluid beta-Amyloid/Tau ratio and postoperative delirium. Ann Clin Transl Neurol. 2014;1:319–28. doi: 10.1002/acn3.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65:229–38. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simone MJ, Tan ZS. The role of inflammation in the pathogenesis of delirium and dementia in older adults: a review. CNS Neurosci Ther. 2011;17:506–13. doi: 10.1111/j.1755-5949.2010.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition (DSM-5) Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 13.Cole M, McCusker J, Dendukuri N, Han L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–60. doi: 10.1046/j.1365-2389.2003.51255.x. [DOI] [PubMed] [Google Scholar]
- 14.Levkoff SE, Evans DA, Liptzin B, et al. Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992;152:334–40. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 15.Levkoff SE, Liptzin B, Evans DA, et al. Progression and Resolution of Delirium in Elderly Patients Hospitalized for Acute Care. Am J Geriatr Psychiatry. 1994;2:230–8. doi: 10.1097/00019442-199400230-00007. [DOI] [PubMed] [Google Scholar]
- 16.Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–24. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 17.McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med. 2003;18:696–704. doi: 10.1046/j.1525-1497.2003.20602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockwood K. The occurrence and duration of symptoms in elderly patients with delirium. J Gerontol. 1993;48:M162–6. doi: 10.1093/geronj/48.4.m162. [DOI] [PubMed] [Google Scholar]
- 19.Downing LJ, Caprio TV, Lyness JM. Geriatric psychiatry review: differential diagnosis and treatment of the 3 D’s - delirium, dementia, and depression. Curr Psychiatry Rep. 2013;15:365. doi: 10.1007/s11920-013-0365-4. [DOI] [PubMed] [Google Scholar]
- 20.Gower LE, Gatewood MO, Kang CS. Emergency department management of delirium in the elderly. West J Emerg Med. 2012;13:194–201. doi: 10.5811/westjem.2011.10.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy M, Enander RA, Tadiri SP, Wolfe RE, Shapiro NI, Marcantonio ER. Delirium risk prediction, healthcare use and mortality of elderly adults in the emergency department. J Am Geriatr Soc. 2014;62:462–9. doi: 10.1111/jgs.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koster S, Hensens AG, Schuurmans MJ, van der Palen J. Prediction of delirium after cardiac surgery and the use of a risk checklist. Eur J Cardiovasc Nurs. 2013;12:284–92. doi: 10.1177/1474515112450244. [DOI] [PubMed] [Google Scholar]
- 23.Moerman S, Tuinebreijer WE, de Boo M, Pilot P, Nelissen RG, Vochteloo AJ. Validation of the Risk Model for Delirium in hip fracture patients. Gen Hosp Psychiatry. 2012;34:153–9. doi: 10.1016/j.genhosppsych.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Bo M, Martini B, Ruatta C, et al. Geriatric ward hospitalization reduced incidence delirium among older medical inpatients. Am J Geriatr Psychiatry. 2009;17:760–8. doi: 10.1097/jgp.0b013e3181a315d5. [DOI] [PubMed] [Google Scholar]
- 25.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–36. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalisvaart KJ, Vreeswijk R, de Jonghe JF, van der Ploeg T, van Gool WA, Eikelenboom P. Risk factors and prediction of postoperative delirium in elderly hip-surgery patients: implementation and validation of a medical risk factor model. J Am Geriatr Soc. 2006;54:817–22. doi: 10.1111/j.1532-5415.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 27.Wilson K, Broadhurst C, Diver M, Jackson M, Mottram P. Plasma insulin growth factor-1 and incident delirium in older people. Int J Geriatr Psychiatry. 2005;20:154–9. doi: 10.1002/gps.1265. [DOI] [PubMed] [Google Scholar]
- 28.O’Keeffe ST, Lavan JN. Predicting delirium in elderly patients: development and validation of a risk-stratification model. Age Ageing. 1996;25:317–21. doi: 10.1093/ageing/25.4.317. [DOI] [PubMed] [Google Scholar]
- 29.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–9. [PubMed] [Google Scholar]
- 30.Pompei P, Foreman M, Rudberg MA, Inouye SK, Braund V, Cassel CK. Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42:809–15. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 31.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119:474–81. doi: 10.7326/0003-4819-119-6-199309150-00005. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–53. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 35.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 36.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third Edition (DSM-III) Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 38.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third Edition, revised (DSM-IIIR) Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 39.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition (DSM-IV) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 40.Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The Delirium Observation Screening Scale: a screening instrument for delirium. Research & Theory for Nursing Practice. 2003;17:31–50. doi: 10.1891/rtnp.17.1.31.53169. [DOI] [PubMed] [Google Scholar]
- 41.Davis DH, Barnes LE, Stephan BC, et al. The descriptive epidemiology of delirium symptoms in a large population-based cohort study: results from the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) BMC Geriatr. 2014;14:87. doi: 10.1186/1471-2318-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandharipande PP, Girard TD, Ely EW. Long-term cognitive impairment after critical illness. N Engl J Med. 2014;370:185–6. doi: 10.1056/NEJMc1313886. [DOI] [PubMed] [Google Scholar]
- 43.Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172:1324–31. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135:2809–16. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–5. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bickel H, Gradinger R, Kochs E, Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord. 2008;26:26–31. doi: 10.1159/000140804. [DOI] [PubMed] [Google Scholar]
- 48.Lundstrom M, Edlund A, Bucht G, Karlsson S, Gustafson Y. Dementia after delirium in patients with femoral neck fractures. J Am Geriatr Soc. 2003;51:1002–6. doi: 10.1046/j.1365-2389.2003.51315.x. [DOI] [PubMed] [Google Scholar]
- 49.Copeland JR, Dewey ME, Griffiths-Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med. 1986;16:89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- 50.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of Clinical & Experimental Neuropsychology. 1998;20:310–9. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 51.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–51. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 52.Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Multicomponent geriatric intervention for elderly inpatients with delirium: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61:176–81. doi: 10.1093/gerona/61.2.176. [DOI] [PubMed] [Google Scholar]
- 53.Selnes OA, Gottesman RF, Grega MA, Baumgartner WA, Zeger SL, McKhann GM. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012;366:250–7. doi: 10.1056/NEJMra1100109. [DOI] [PubMed] [Google Scholar]
- 54.Avidan MS, Evers AS. Review of clinical evidence for persistent cognitive decline or incident dementia attributable to surgery or general anesthesia. J Alzheimers Dis. 2011;24:201–16. doi: 10.3233/JAD-2011-101680. [DOI] [PubMed] [Google Scholar]
- 55.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106:572–90. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 56.Watt D, Koziol K, Budding D. Delirium and confusional states. In: Noggle C, Dean R, editors. Disorders in Neuropsychiatry. New York: Springer Publishing Company; 2012. [Google Scholar]
- 57.Baker FM, Wiley C, Kokmen E, Chandra V, Schoenberg BS. Delirium episodes during the course of clinically diagnosed Alzheimer’s disease. J Natl Med Assoc. 1999;91:625–30. [PMC free article] [PubMed] [Google Scholar]
- 58.Fick D, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26:30–40. doi: 10.3928/0098-9134-20000101-09. [DOI] [PubMed] [Google Scholar]
- 59.Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156:848–56. W296. doi: 10.7326/0003-4819-156-12-201206190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165:575–83. [PMC free article] [PubMed] [Google Scholar]
- 61.Rockwood K, Cosway S, Carver D, Jarrett P, Stadnyk K, Fisk J. The risk of dementia and death after delirium. Age Ageing. 1999;28:551–6. doi: 10.1093/ageing/28.6.551. [DOI] [PubMed] [Google Scholar]
- 62.Alsop DC, Fearing MA, Johnson K, Sperling R, Fong TG, Inouye SK. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci. 2006;61:1287–93. doi: 10.1093/gerona/61.12.1287. [DOI] [PubMed] [Google Scholar]
- 63.Soiza RL, Sharma V, Ferguson K, Shenkin SD, Seymour DG, Maclullich AM. Neuroimaging studies of delirium: a systematic review. J Psychosom Res. 2008;65:239–48. doi: 10.1016/j.jpsychores.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 64.Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40:2022–32. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morandi A, Rogers BP, Gunther ML, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: The VISIONS prospective cohort magnetic resonance imaging study*. Crit Care Med. 2012;40:2182–9. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGrane S, Girard TD, Thompson JL, et al. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care. 2011;15:R78. doi: 10.1186/cc10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MacLullich AM, Edelshain BT, Hall RJ, et al. Cerebrospinal fluid interleukin-8 levels are higher in people with hip fracture with perioperative delirium than in controls. J Am Geriatr Soc. 2011;59:1151–3. doi: 10.1111/j.1532-5415.2011.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van den Boogaard M, Kox M, Quinn KL, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011;15:R297. doi: 10.1186/cc10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cape E, Hall RJ, van Munster BC, et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1beta in delirium after hip fracture. J Psychosom Res. 2014;77:219–25. doi: 10.1016/j.jpsychores.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pearson A, de Vries A, Middleton SD, et al. Cerebrospinal fluid cortisol levels are higher in patients with delirium versus controls. BMC Res Notes. 2010;3:33. doi: 10.1186/1756-0500-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westhoff D, Witlox J, Koenderman L, et al. Preoperative cerebrospinal fluid cytokine levels and the risk of postoperative delirium in elderly hip fracture patients. J Neuroinflammation. 2013;10:122. doi: 10.1186/1742-2094-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adamis D, Lunn M, Martin FC, et al. Cytokines and IGF-I in delirious and non-delirious acutely ill older medical inpatients. Age Ageing. 2009;38:326–32. doi: 10.1093/ageing/afp014. [DOI] [PubMed] [Google Scholar]
- 73.Hall RJ, Ferguson KJ, Andrews M, et al. Delirium and cerebrospinal fluid S100B in hip fracture patients: a preliminary study. Am J Geriatr Psychiatry. 2013;21:1239–43. doi: 10.1016/j.jagp.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 74.van Munster BC, Korevaar JC, Korse CM, Bonfrer JM, Zwinderman AH, de Rooij SE. Serum S100B in elderly patients with and without delirium. Int J Geriatr Psychiatry. 2010;25:234–9. doi: 10.1002/gps.2326. [DOI] [PubMed] [Google Scholar]
- 75.Witlox J, Kalisvaart KJ, de Jonghe JF, et al. Cerebrospinal fluid beta-amyloid and tau are not associated with risk of delirium: a prospective cohort study in older adults with hip fracture. J Am Geriatr Soc. 2011;59:1260–7. doi: 10.1111/j.1532-5415.2011.03482.x. [DOI] [PubMed] [Google Scholar]
- 76.Vasunilashorn SNL, Kosar CM, Fong TG, Jones RN, Inouye SK, Marcantonio ER. Does Apolipoprotein E Genotype Increase Risk of Postoperative Delirium? Am J Geriatr Psychiatry. 2015 doi: 10.1016/j.jagp.2014.12.192. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cunningham C, Campion S, Lunnon K, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–12. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–84. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Field RH, Gossen A, Cunningham C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J Neurosci. 2012;32:6288–94. doi: 10.1523/JNEUROSCI.4673-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Field R, Campion S, Warren C, Murray C, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain, Behavior, & Immunity. 2010;24:996–1007. doi: 10.1016/j.bbi.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murray C, Sanderson DJ, Barkus C, et al. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging. 2012;33:603–16 e3. doi: 10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cunningham C, Maclullich AM. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain, Behavior, & Immunity. 2013;28:1–13. doi: 10.1016/j.bbi.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cunningham C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochem Soc Trans. 2011;39:945–53. doi: 10.1042/BST0390945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–57. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Weberpals M, Hermes M, Hermann S, et al. NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J Neurosci. 2009;29:14177–84. doi: 10.1523/JNEUROSCI.3238-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Godbout JP, Chen J, Abraham J, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 87.Davis DH, Skelly DT, Murray C, et al. Worsening Cognitive Impairment and Neurodegenerative Pathology Progressively Increase Risk for Delirium. Am J Geriatr Psychiatry. 2014 doi: 10.1016/j.jagp.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Griffin EW, Skelly DT, Murray CL, Cunningham C. Cyclooxygenase-1-dependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction. J Neurosci. 2013;33:15248–58. doi: 10.1523/JNEUROSCI.6361-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Xu Z, Wang H, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–98. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie Z, Xu Z. General anesthetics and beta-amyloid protein. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:140–6. doi: 10.1016/j.pnpbp.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17:593–601. doi: 10.1017/S1355617710001748. [DOI] [PMC free article] [PubMed] [Google Scholar]


