Abstract
In a rat model of status epilepticus (SE) induced by lithium and pilocarpine and refractory to midazolam, deep hypothermia (20°C for 30 min.) reduced EEG power over 50-fold, stopped SE within 12 minutes, and reduced EEG spikes by 87%. Hypothermia deserves further investigation as a treatment of last resort for refractory SE.
Keywords: Status epilepticus, hypothermia, EEG power
1. Introduction
Refractory and super-refractory status epilepticus (RSE, SRSE) are an increasingly common therapeutic challenge in our intensive care units (ICU), at enormous financial and human costs. This increase may be in part due to greater availability of EEG monitoring and to increased recognition of ”subtle” SE, to improvements in ICU care, and to the aging of the population, since SE and RSE are common in the elderly. Drugs fail to stop SE in 31–53% of cases [1–3]. In the VA Cooperative Study 47% of SE patients had RSE [3]. New methods for treating RSE and SRSE are clearly needed.
During SE, pharmacoresistance develops progressively. All currently available drugs display this phenomenon in experimental SE, with the possible exception of NMDA antagonists in some models [4]. In humans, early treatment of SE is much more effective than late treatment, suggesting that pharmacoresistance may be present as well. In the VA Cooperative Study [3], four treatments were randomly rotated. The first treatment (regardless of which one of the 4 was selected) was successful in 53% of patients. The third treatment given was successful in 2% of patients. Time-dependent pharmacoresistance is the most likely explanation for these results. We need alternative treatments for RSE/SRSE, and a way to overcome pharmacoresistance. Here we suggest that hypothermia, which acts by completely different mechanisms than anticonvulsant drugs, may be able to stop the pharmacoresistant seizures of RSE/SRSE.
Hypothermia activates many anticonvulsant and neuroprotective mechanisms. It reduces cerebral metabolic rate by 6–7% per degree Celsius, so that at 20° C, the human cerebral oxygen consumption was measured at one-fifth of normothermic values [5]. It alters the function of ion pumps [6, 7] intrinsic membrane properties and voltage-gated ion channels [8–10]. It slows release of excitatory neurotransmitters [11, 12] and modifies gene expression [13].
These actions reduce excitatory drive and would be expected to inhibit seizure activity. They also activate several neuroprotective mechanisms: reduction of the cerebral demand for oxygen and glucose [14]; preservation of ATP and energy stores and of tissue pH; reduction of release of excitotoxic amino acids [15] and of calcium influx into neurons [16, 17]; inhibition of early gene expression and stress response; induction of the expression of heatshock and other stress proteins [18, 19]; and inhibition of early molecular cascades involved in neuronal apoptosis.
Mild to moderate hypothermia has been shown to reduce seizure activity in experimental animals and in humans [20, 21], although seizures frequently recur upon re-warming. Maeda et al [22] induced SE with intra-amygdalar kainic acid injection and found that mild hypothermia (30° C) reduced seizure frequency and severity. Schmitt et al [23] used mild hypothermia (≥29° C) to treat SE induced by perforant path stimulation. Hypothermia alone reduced motor but not EEG seizures. The combination of mild hypothermia with low-dose diazepam reduced all measures of seizure activity, but seizures returned upon re-warming. Liu et al [24] induced SE with kainate in rats kept hypothermic for 4 hours, and in normothermic controls. Seizure reduction was much greater during hypothermia at 23° C. than at 28° C, suggesting that the depth of hypothermia increases its efficacy [25].
Anecdotal reports of successful treatment of clinical SE with hypothermia have been published [21, 26]. Re-cooling stopped seizure activity which developed upon re-warming in an infant treated for hypoxic-ischemic encephalopathy [27]. Cold saline perfusion suppressed interictal spike foci during electrocorticography [20] and was used to stop seizures triggered by intraoperative cortical stimulation.
The neuroprotective role of hypothermia has been well-documented in focal hypoxia-ischemia, in traumatic brain injury (TBI), and in global cerebral ischemia. It has also been seen with seizure-associated neuronal injury [28], seizure-associated leaks in the blood-brain barrier (BBB) [29] and other conditions [30].
In neonatal hypoxic-ischemic encephalopathy, hypothermia improves developmental and radiological outcome [31–33]. Bernard et al [34] and The Hypothermia after Cardiac Arrest Study Group [35] showed benefits of hypothermia after cardiac arrest due to ventricular fibrillation. In focal ischemia, many animal models showed improved outcome after hypothermia [36] but adverse effects of deep hypothermia have been reported [37]. Hypothermia reverses many ischemia-induced changes in gene expression, and alters the expression of genes involved in protein synthesis, in cell cycle and in apoptotic pathways [38].
Hypothermia protects from TBI-induced neuronal injury [39–41] and changes in gene expression [42]. It reduces blood-brain barrier (BBB) disruption and cell-mediated inflammation [43, 44]. Hypothermia decreases TBI-induced inflammatory cytokines, cell-mediated immune responses and activation of immune transcription factors [45, 46]. Mild hypothermia after TBI mitigated the TBI-associated reduction in pentylenetetrazol seizure threshold 12 weeks later [47].
Recent developments in ICU technology have reduced the complications of hypothermia. Mild hypothermia has become routine treatment for neonatal hypoxic-ischemic encephalopathy [48, 49], for traumatic brain injury [50], for post-cardiac arrest encephalopathy [34], and may have potential for stroke [51]. Deep hypothermia is routinely used to protect the brain or spinal cord when circulatory arrest is needed in cardiac surgery [52], vascular surgery [53] and neurosurgery [54]. Most of the complications reported after deep hypothermia are the result of induced circulatory arrest, not the result of hypothermia itself [55, 56], although the potential for adverse effects, especially for very deep hypothermia, is significant [57].
Our results suggest that deep hypothermia is quite effective in stopping seizures in this experimental model of RSE, and may deserve further study.
2. Materials and Methods
SE was induced with lithium (3 mEq/kg ip 16 hrs prior) and pilocarpine (60 mg/kg ip) + methylscopolamine (1 mg/kg ip) in adult male Wistar rats (200–250 gm) previously implanted with skull screw electrodes. Control animals were given an equal volume of saline i.p. Only lithium/pilocarpine-treated rats displaying behavioral/EEG manifestations of seizures were used. EEG was recorded from skull-implanted electrodes. “Brain surface” temperature was recorded from a probe pre-implanted near the surface of parietal cortex. When full-blown SE was established (12 min. after the 2d stage ≥3 seizure) midazolam (3 mg/kg ip) was injected. The second stage ≥3 seizure is a discrete and easily recognizable event. The time between the second stage 3 or higher seizure and the onset of continuous polyspikes is both short and reproducible (1.28 ± 1.1 min., n=16). Choosing the second stage 3 seizure as our time anchor and recording 12 min. of EEG before treating guarantees that all our rats are in full-blown SE when treatment begins. In this study, we initiated cooling right after completing the midazolam injection. In later experiments (not reported here) we introduced an additional 15 min. delay between midazolam and the initiation of hypothermia, in order to eliminate from the study animals which respond to midazolam, but in the current study we cannot rule out a midazolam contribution to the behavioral or EEG response to treatment,
All animals received scopolamine (2 mg/kg) at the same time as midazolam. One of the problems of chemical models of SE is the difficulty of obtaining clean results because of the presence of a convulsant in the animal. A specific treatment might stop the seizures, but the convulsant could immediately restart them if it is not neutralized. Morrissett et al [58] were the first to show that an amount of atropine or scopolamine which blocked SE as pretreatment was unable to stop SE after seizures became established. The seizures had become independent of their original trigger (probably because of receptor trafficking). We took advantage of this feature to block the effects of pilocarpine without altering the course of SE. In preliminary experiments, we studied the amount of antimuscarinic agent which stops 100% of seizures when given as pretreatment but blocks 0% of seizures when given after seizure onset. Scopolamine 2 mg/kg reached that goal.
Cooling was then started with whole body ice packs. Rectal temperature closely approximated “brain surface” temperature. When rectal temperature reached 20° C., it was held for 30 min. at that temperature ± 1° C., then ice packs were replaced by a warming blanket with circulating 37° C. water, until rectal temperature reached 36° C. Mean cooling time from 37° C to 20° C was 39.5 min. Mean re-warming time from 20° C to 36° C was 61.5 min. In “cool cap” animals, only the head and neck of the animals were packed with ice.
Acute seizures were monitored by video/EEG for 24 hours. The video/EEGs was analyzed using our standard methods for quantifying many components of seizure severity [59, 60]. Relative EEG power was the ratio of EEG power at the time of measurement to EEG power before seizure induction in the same rat. It increased approximately 100-fold during early SE, then declined as a function of time, treatment and temperature.
ANOVA was used for statistical testing if the data were normally distributed with similar variance, otherwise either data transforms to improve normality or nonparametric tests such as the Kruskal-Wallis test were used. P values were considered significant at the 0.05 level. Post-hoc tests consisted of Scheffe’s or Tukey’s in the case of ANOVA and Dunn’s in the case of Kruskal-Wallis. The Graph-Pad/Prism statistical package was used for these tests.
3. Results
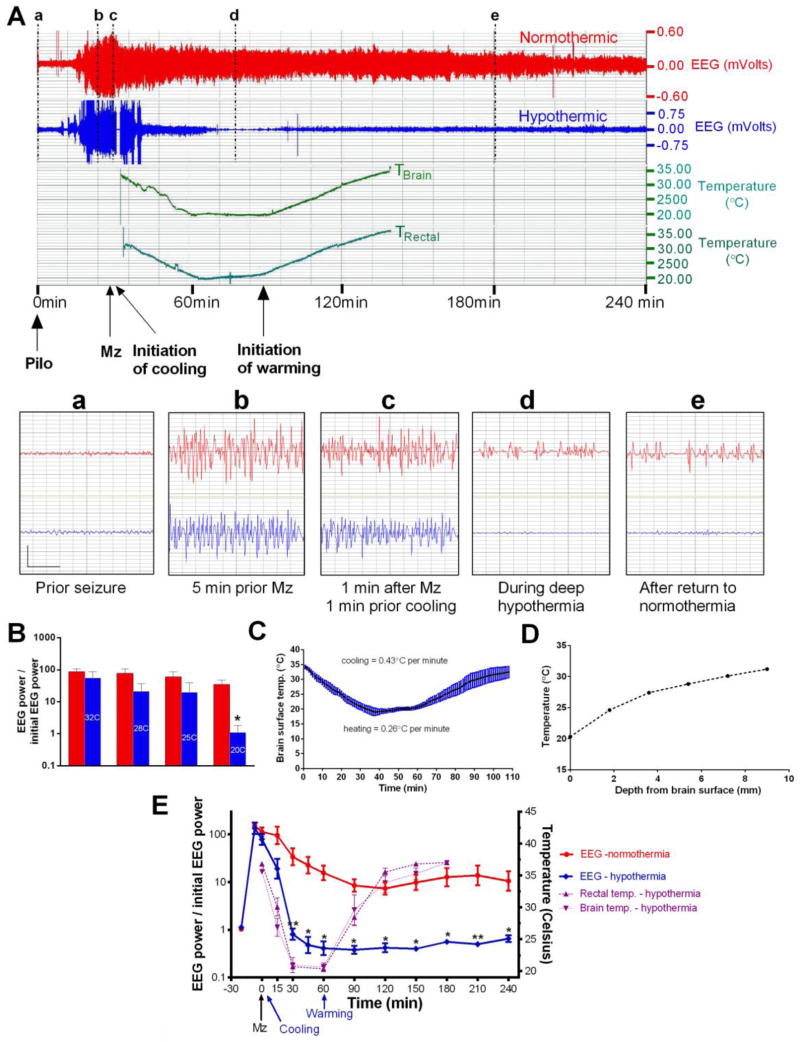
Pilocarpine injection was followed by an increase in EEG power and in behavioral activity, then by the appearance of EEG spikes, followed by individual EEG seizures with behavioral arrest, twitching of face and vibrissae, or clonic forelimb activity. The EEG seizures (Figure 1A) merged rapidly into nearly continuous polyspikes, while behavioral seizures increased in frequency, severity and duration but remained intermittent. The injection of midazolam (41 ± 19 min. after pilocarpine) was followed by a mild decrease in EEG power in both groups, but SE was not interrupted until animals became hypothermic. Hypothermia stopped seizures after approximately 25 min of SE (1483 ± 291 sec.), or 12 min. (761± 243 sec.; n=5) after initiation of hypothermia (Figure 1A, E), while SE continued for 17.9 ± 4.6 hours after initiation of cooling in paired normothermic animals (n=5; p<0.0001). The average brain and rectal temperature at the time of seizure termination was 31° C. ± 0.6 (Fig. 1C, E). In hypothermic animals, EEG power decreased with cooling (Fig. 1B), and by the time they reached 20° C, relative power in hypothermic animals was 3% of that of the normothermic group measured at the same time (p<0.01). It was below pre-pilocarpine baseline and showed no seizure or residual paroxysmal activity. It remained below pre-seizure baseline through rewarming and in most animals, stayed close to baseline values for the remaining of the 24 hours, although some late spikes and sharp wave activity did return. Manual review of EEGs confirmed the absence of seizures after re-warming in most rats. In one animal, 12 late seizures were observed 8.2 to 11.9 hours after the initiation of re-warming. Many of these seizures were brief (mean duration 45 ± 6 seconds), and SE did not recur. In normothermic rats, EEG power decreased from pre-treatment SE (probably in part due to midazolam), but remained significantly above baseline for many hours, reflecting continuation of SE (Fig. 1E). In most normothermic rats, semi-periodic spike bursts which slowly decreased in amplitude over time were separated by stretches of low-voltage background activity which increased progressively in duration (Fig. 1A), and this pattern often continued for the whole 24 hours of recording.
Figure 1. Deep cooling durably reduces relative EEG power.
A) The upper panel shows the compressed 4-hr EEG of a normothermic (red) and a hypothermic rat (blue, 20ºC for 30 min.) with brain and rectal temperature. Both rats received midazolam 3 mg/kg ip (arrow) and cooling was initiated 2 min later. The lower panel shows the magnified 4-sec EEG traces marked by vertical lines (a–d) (vertical bar = 0.75 mV; horizontal bar = 1sec).
B) Relative EEG power (EEG power/baseline EEG power) in normothermic (red bars) and hypothermic rats (blue bars, temperature in degrees Celsius) at the same time points. Relative EEG power was reduced by hypothermia in a dose-dependent fashion. The 20º C point and the slope were significantly different from normothermics (* p<0.01).
C) “Brain surface” temperature during cooling and re-warming.
D) Brain temperature as a function of electrode depth with the “cool cap” method. Steep temperature gradients suggest that deep structures such as the hippocampus may remain warmer than the cortical surface.
E) The left y-axis of this graph shows the ratio of EEG power at each time points to initial EEG power at baseline, before pilocarpine injection. Note the logarithmic scale. Every time point beyond 30 min. showed a significant difference between normothermic and hypothermic rats (** p < 0.01; * p < 0.05). The right y-axis shows the rectal and brain temperature scales. Note that the temperature of a probe positioned at the surface of parietal cortex was close to the rectal temperature.
Most measures of the severity of SE were reduced in the hypothermic group compared to their normothermic counterparts. The number of EEG spikes per 24 hours, including those which occurred before the initiation of cooling, was 7440 ± 1999 in the normothermic group (N) and 1004 ± 296 in the hypothermic group (H) (p< 0.01). The time it took for EEG power to decline to twice pre-pilocarpine values was 4.97 ± 1.89 hours after pilocarpine injection in the normothermic group against 0.8 ± 0.13 hour in the hypothermic group (p<0.05). Hjorth function integral [61] for the first 6 hours after pilocarpine injection was 9879 ± 1939 in normothermics versus 3990 ± 618 in hypothermics (p = 0.01).
4. Discussion
These results suggest that deep hypothermia is a powerful tool for seizure termination in this animal model, and deserves further evaluation as a potential treatment of last resort for RSE. In the current study, hypothermia reduced EEG power over 100-fold, and reduced the duration of SE from a mean of 17.9 hours (median 24 hrs) to a mean of 0.2 hours (median 0.16 hour). It terminated RSE in all animals. Seizures stopped well before reaching our target temperature (range: 29–33° C.) and returned in only one rat, many hours after the completion of re-warming. Our experiments were designed to mimic the clinical situation where hypothermia is likely to be used only after drug treatment. We induced SE with lithium and pilocarpine and treated with midazolam when SE was well-established. Midazolam reduced EEG power and seizure severity but did not stop SE in normothermic animals. In this study, hypothermia was applied just after midazolam injection. Pilot experiments showed that the temperature of a probe positioned on the surface of parietal cortex was close to rectal temperature, so the latter was used in most experiments. Temperature was brought down and maintained near 20° C. for the relatively short period of 30 min. Warming was then initiated by wrapping the rats in an electrical heating pad. Mean cooling time (39.5 min.) was shorter than mean re-warming time (61.5 min.). All animals tolerated hypothermia well.
Deep hypothermia (13–20° C) is used in vascular and cardiac surgery and neurosurgery, with good results for periods of circulatory arrest of 25–50 min. [62], and the equipment and expertise needed are available in most large surgical centers. Its potential for treating RSE has never been evaluated, although results in experimental SE were encouraging [23, 25]. Our results suggest that cooling to 20°C can be very effective in stopping seizures. While surgical applications use hypothermia as a method to allow circulatory arrest during repair of aortic or cerebral aneurysms or congenital heart disease [63, 64], its use as a treatment for RSE would be free of the complications of circulatory arrest. It would add a non-pharmacological option to our treatment regimens for RSE as well as for cholinergic seizures induced by nerve agents or organophosphate insecticides, and deserves further study.
Computer-generated seizure counts were reviewed manually, to eliminate frequent duplicate counts, but underestimate seizure activity since the computer counts long periods of high-amplitude polyspikes (frequently seen in the early phase of SE) as single seizures, and does not count as seizures the long periods of semi-periodic bursts on a low-amplitude background which is seen in most normothermic animals in the late phase of SE. Preliminary experiments using “Cool Cap” hypothermia were disappointing. The depression of seizure activity by head cooling was transient, and in these adult rats “cool cap” hypothermia did not block seizures from returning upon re-warming. In “cool cap” animals we found steep temperature gradients between parietal cortex and deep brain, as shown in Fig. 1D. At a depth of 5 mm from the cortical surface, brain temperature was over 8°C warmer than cortex. This result is not surprising since adult brain tissue has a high fat content and would be expected to be an excellent thermal insulator, and steep temperature gradients after local cooling have been observed in the mature primate brain [65]. This could result in inadequate cooling of key brain regions, such as ventral hippocampus, where seizure activity could persist during cortical cooling and from which it could spread (and restart SE) upon re-warming.
The rationale for injecting scopolamine in all animals at the time of treatment was that two components are involved in chemically-induced SE: the initial pilocarpine stimulation of muscarinic receptors, and a later component of self-sustaining seizures which are independent of the original trigger. Since the dose of scopolamine used here blocks all seizure activity when used as pretreatment, it should be sufficient to prevent residual pilocarpine from re-starting the seizures if a treatment stops self-sustaining SE. It enables us to study self-sustaining SE without the confusing presence of a chemical convulsant on board [60, 66].
5. Conclusion
This present study show that deep hypothermia (20ºC for 30 min.) is an efficient treatment to stop RSE. Hypothermia is very neuroprotective in animal models of ischemic or traumatic brain injury [67], and our preliminary data (not shown) indicate a strong reduction of neuronal injury in RSE as well. Further studies of its risk/benefit ratio of hypothermia in RSE and SRSE seem worth undertaking.
Highlights.
We treated midazolam-refractory status epilepticus with deep hypothermia
Deep hypothermia (20°C for 30 min.) was well-tolerated.
Deep hypothermia stopped status epilepticus within 12 minutes.
Status epilepticus did not return upon re-warming.
Deep hypothermia reduced EEG power over 50-fold and reduced EEG spikes by 87%.
Acknowledgments
Supported by VHA Research Service (CW) and NINDS (grant UO1 NS074926; CW).
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holtkamp M, Othman J, Buchheim K, Meierkord H. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry. 2005;76:534–9. doi: 10.1136/jnnp.2004.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–10. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- 3.Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, Handforth A, Faught E, Calabrese VP, Uthman BM, Ramsay RE, Mamdani MB. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–8. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 4.Mazarati AM, Wasterlain CG. N-methyl-D-asparate receptor antagonists abolish the maintenance phase of self-sustaining status epilepticus in rat. Neurosci Lett. 1999;265:187–90. doi: 10.1016/s0304-3940(99)00238-4. [DOI] [PubMed] [Google Scholar]
- 5.McCullough JN, Zhang N, Reich DL, Juvonen TS, Klein JJ, Spielvogel D, Ergin MA, Griepp RB. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg. 1999;67:1895–9. doi: 10.1016/s0003-4975(99)00441-5. discussion 1919–21. [DOI] [PubMed] [Google Scholar]
- 6.Aihara H, Okada Y, Tamaki N. The effects of cooling and rewarming on the neuronal activity of pyramidal neurons in guinea pig hippocampal slices. Brain Res. 2001;893:36–45. doi: 10.1016/s0006-8993(00)03285-6. [DOI] [PubMed] [Google Scholar]
- 7.Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol. 2000;522(Pt 1):59–76. doi: 10.1111/j.1469-7793.2000.0059m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiff SJ, Somjen GG. The effects of temperature on synaptic transmission in hippocampal tissue slices. Brain Res. 1985;345:279–84. doi: 10.1016/0006-8993(85)91004-2. [DOI] [PubMed] [Google Scholar]
- 9.Thompson SM, Masukawa LM, Prince DA. Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci. 1985;5:817–24. doi: 10.1523/JNEUROSCI.05-03-00817.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen KF, Schwartzkroin PA. Effects of temperature alterations on population and cellular activities in hippocampal slices from mature and immature rabbit. Brain Res. 1988;475:305–16. doi: 10.1016/0006-8993(88)90619-1. [DOI] [PubMed] [Google Scholar]
- 11.Volgushev M, Kudryashov I, Chistiakova M, Mukovski M, Niesmann J, Eysel UT. Probability of transmitter release at neocortical synapses at different temperatures. J Neurophysiol. 2004;92:212–20. doi: 10.1152/jn.01166.2003. [DOI] [PubMed] [Google Scholar]
- 12.Yang XF, Ouyang Y, Kennedy BR, Rothman SM. Cooling blocks rat hippocampal neurotransmission by a presynaptic mechanism: observations using 2-photon microscopy. J Physiol. 2005;567:215–24. doi: 10.1113/jphysiol.2005.088948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller BJ. Gene expression in response to low temperatures in mammalian cells: a review of current ideas. Cryo Letters. 2003;24:95–102. [PubMed] [Google Scholar]
- 14.Soukup J, Zauner A, Doppenberg EM, Menzel M, Gilman C, Young HF, Bullock R. The importance of brain temperature in patients after severe head injury: relationship to intracranial pressure, cerebral perfusion pressure, cerebral blood flow, and outcome. J Neurotrauma. 2002;19:559–71. doi: 10.1089/089771502753754046. [DOI] [PubMed] [Google Scholar]
- 15.Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989;20:904–10. doi: 10.1161/01.str.20.7.904. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson P, Laursen H, Hillered L, Hansen AJ. Calcium movements in traumatic brain injury: the role of glutamate receptor-operated ion channels. J Cereb Blood Flow Metab. 1996;16:262–70. doi: 10.1097/00004647-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Colbourne F, Grooms SY, Zukin RS, Buchan AM, Bennett MV. Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc Natl Acad Sci U S A. 2003;100:2906–10. doi: 10.1073/pnas.2628027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Yenari MA. Therapeutic hypothermia: neuroprotective mechanisms. Front Biosci. 2007;12:816–25. doi: 10.2741/2104. [DOI] [PubMed] [Google Scholar]
- 19.Yenari MA, Han HS. Influence of hypothermia on post-ischemic inflammation: role of nuclear factor kappa B (NFkappaB) Neurochem Int. 2006;49:164–9. doi: 10.1016/j.neuint.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Sartorius CJ, Berger MS. Rapid termination of intraoperative stimulation-evoked seizures with application of cold Ringer’s lactate to the cortex. Technical note. J Neurosurg. 1998;88:349–51. doi: 10.3171/jns.1998.88.2.0349. [DOI] [PubMed] [Google Scholar]
- 21.Karkar KM, Garcia PA, Bateman LM, Smyth MD, Barbaro NM, Berger M. Focal cooling suppresses spontaneous epileptiform activity without changing the cortical motor threshold. Epilepsia. 2002;43:932–5. doi: 10.1046/j.1528-1157.2002.03902.x. [DOI] [PubMed] [Google Scholar]
- 22.Maeda T, Hashizume K, Tanaka T. Effect of hypothermia on kainic acid-induced limbic seizures: an electroencephalographic and 14C-deoxyglucose autoradiographic study. Brain Res. 1999;818:228–35. doi: 10.1016/s0006-8993(98)01269-4. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis. 2006;23:689–96. doi: 10.1016/j.nbd.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Gatt A, Mikati M, Holmes GL. Effect of temperature on kainic acid-induced seizures. Brain Res. 1993;631:51–8. doi: 10.1016/0006-8993(93)91185-u. [DOI] [PubMed] [Google Scholar]
- 25.Kowski AB, Kanaan H, Schmitt FC, Holtkamp M. Deep hypothermia terminates status epilepticus--an experimental study. Brain Res. 2012;1446:119–26. doi: 10.1016/j.brainres.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Orlowski JP, Erenberg G, Lueders H, Cruse RP. Hypothermia and barbiturate coma for refractory status epilepticus. Crit Care Med. 1984;12:367–72. doi: 10.1097/00003246-198404000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Kendall GS, Mathieson S, Meek J, Rennie JM. Recooling for rebound seizures after rewarming in neonatal encephalopathy. Pediatrics. 2012;130:e451–5. doi: 10.1542/peds.2011-3496. [DOI] [PubMed] [Google Scholar]
- 28.Lundgren J, Smith ML, Blennow G, Siesjo BK. Hyperthermia aggravates and hypothermia ameliorates epileptic brain damage. Exp Brain Res. 1994;99:43–55. doi: 10.1007/BF00241411. [DOI] [PubMed] [Google Scholar]
- 29.Oztas B, Kaya M. The effect of profound hypothermia on blood-brain barrier permeability during pentylenetetrazol-induced seizures. Epilepsy Res. 1994;19:221–7. doi: 10.1016/0920-1211(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 30.Elmas I, Kucuk M, Kalayci RB, Cevik A, Kaya M. Effects of profound hypothermia on the blood-brain barrier permeability in acute and chronically ethanol treated rats. Forensic Sci Int. 2001;119:212–6. doi: 10.1016/s0379-0738(00)00429-1. [DOI] [PubMed] [Google Scholar]
- 31.Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–7. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 33.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH National Institute of Child H, Human Development Neonatal Research N. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 34.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 35.Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 36.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–69. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 37.Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke. 1992;23:1454–62. doi: 10.1161/01.str.23.10.1454. [DOI] [PubMed] [Google Scholar]
- 38.Nagel S, Papadakis M, Pfleger K, Grond-Ginsbach C, Buchan AM, Wagner S. Microarray analysis of the global gene expression profile following hypothermia and transient focal cerebral ischemia. Neuroscience. 2012;208:109–22. doi: 10.1016/j.neuroscience.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 39.Fay T. Observations on generalized refrigeration in cases of severe cerebral trauma. Assoc Res Nerv Ment Dis Proc. 1945;24:611–619. [Google Scholar]
- 40.Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–6. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truettner JS, Alonso OF, Bramlett HM, Dietrich WD. Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. J Cereb Blood Flow Metab. 2011;31:1897–907. doi: 10.1038/jcbfm.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lotocki G, de Rivero Vaccari JP, Perez ER, Sanchez-Molano J, Furones-Alonso O, Bramlett HM, Dietrich WD. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J Neurotrauma. 2009;26:1123–34. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oda Y, Gao G, Wei EP, Povlishock JT. Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood-brain barrier dysfunction after traumatic brain injury in rat. J Cereb Blood Flow Metab. 2011;31:1143–54. doi: 10.1038/jcbfm.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Truettner JS, Suzuki T, Dietrich WD. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res Mol Brain Res. 2005;138:124–34. doi: 10.1016/j.molbrainres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Tomura S, de Rivero Vaccari JP, Keane RW, Bramlett HM, Dietrich WD. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J Cereb Blood Flow Metab. 2012;32:1939–47. doi: 10.1038/jcbfm.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkins CM, Truettner JS, Lotocki G, Sanchez-Molano J, Kang Y, Alonso OF, Sick TJ, Dietrich WD, Bramlett HM. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur J Neurosci. 2010;32:1912–20. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azzopardi D, Strohm B, Edwards AD, Halliday H, Juszczak E, Levene M, Thoresen M, Whitelaw A, Brocklehurst P, Steering G participants TCR. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94:F260–4. doi: 10.1136/adc.2008.146977. [DOI] [PubMed] [Google Scholar]
- 49.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD Eunice Kennedy Shriver NNRN. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamps M, Bisschops LA, van der Hoeven JG, Hoedemaekers CW. Hypothermia does not increase the risk of infection: a case control study. Crit Care. 2011;15:R48. doi: 10.1186/cc10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312–7. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- 52.Gega A, Rizzo JA, Johnson MH, Tranquilli M, Farkas EA, Elefteriades JA. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg. 2007;84:759–66. doi: 10.1016/j.athoracsur.2007.04.107. discussion 766–7. [DOI] [PubMed] [Google Scholar]
- 53.Rubens FD, Nathan H. Lessons learned on the path to a healthier brain: dispelling the myths and challenging the hypotheses. Perfusion. 2007;22:153–60. doi: 10.1177/0267659107078142. [DOI] [PubMed] [Google Scholar]
- 54.Todd MM, Hindman BJ, Clarke WR, Torner JC Intraoperative Hypothermia for Aneurysm Surgery Trial I. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352:135–45. doi: 10.1056/NEJMoa040975. [DOI] [PubMed] [Google Scholar]
- 55.Fleck TM, Czerny M, Hutschala D, Koinig H, Wolner E, Grabenwoger M. The incidence of transient neurologic dysfunction after ascending aortic replacement with circulatory arrest. Ann Thorac Surg. 2003;76:1198–202. doi: 10.1016/s0003-4975(03)00832-4. [DOI] [PubMed] [Google Scholar]
- 56.Parissis H, Hamid U, Soo A, Al-Alao B. Brief review on systematic hypothermia for the protection of central nervous system during aortic arch surgery: a double-sword tool? J Cardiothorac Surg. 2011;6:153. doi: 10.1186/1749-8090-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bickler PE, Warren DE, Clark JP, Gabatto P, Gregersen M, Brosnan H. Anesthetic protection of neurons injured by hypothermia and rewarming: roles of intracellular Ca2+ and excitotoxicity. Anesthesiology. 2012;117:280–92. doi: 10.1097/ALN.0b013e318260a7b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrisett RA, Jope RS, Snead OC., 3rd Effects of drugs on the initiation and maintenance of status epilepticus induced by administration of pilocarpine to lithium-pretreated rats. Exp Neurol. 1987;97:193–200. doi: 10.1016/0014-4886(87)90293-7. [DOI] [PubMed] [Google Scholar]
- 59.Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814:179–85. doi: 10.1016/s0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- 60.Suchomelova L, Baldwin RA, Kubova H, Thompson KW, Sankar R, Wasterlain CG. Treatment of experimental status epilepticus in immature rats: dissociation between anticonvulsant and antiepileptogenic effects. Pediatr Res. 2006;59:237–43. doi: 10.1203/01.pdr.0000196333.16608.30. [DOI] [PubMed] [Google Scholar]
- 61.Hjorth B. Principles for transformation of scalp EEG from potential field into source distribution. J Clin Neurophysiol. 1991;8:391–6. doi: 10.1097/00004691-199110000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Prêtre RTM. Deep hypothermic circulatory arrest. In: Cohn L, Edmunds L, editors. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2003. pp. 401–412. [Google Scholar]
- 63.Bellinger DC, Newburger JW, Wypij D, Kuban KC, duPlesssis AJ, Rappaport LA. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19:86–97. doi: 10.1017/S1047951108003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G, Lin M, Bellinger DC. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 65.King C, Robinson T, Dixon CE, Rao GR, Larnard D, Nemoto CE. Brain temperature profiles during epidural cooling with the ChillerPad in a monkey model of traumatic brain injury. J Neurotrauma. 2010;27:1895–903. doi: 10.1089/neu.2009.1178. [DOI] [PubMed] [Google Scholar]
- 66.Brandt C, Tollner K, Klee R, Broer S, Loscher W. Effective termination of status epilepticus by rational polypharmacy in the lithium-pilocarpine model in rats: Window of opportunity to prevent epilepsy and prediction of epilepsy by biomarkers. Neurobiol Dis. 2015;75:78–90. doi: 10.1016/j.nbd.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 67.Darwazeh R, Yan Y. Mild hypothermia as a treatment for central nervous system injuries: Positive or negative effects. Neural Regen Res. 2013;8:2677–86. doi: 10.3969/j.issn.1673-5374.2013.28.010. [DOI] [PMC free article] [PubMed] [Google Scholar]