Abstract
BACKGROUND
Although screening of human immunodeficiency virus (HIV)-positive individuals for anal intraepithelial neoplasia (AIN; a precursor of anal cancer) has been practiced in San Francisco among HIV health care providers since the early 1990s, to the authors’ knowledge no study to date has focused on evaluating recent AIN trends.
METHODS
Cases of high-grade AIN 3 and invasive anal cancer from 2000 to 2009 were obtained from the San Francisco/Oakland Surveillance, Epidemiology, and End Results (SEER) population-based cancer registry. Age-standardized rates of AIN 3 and anal cancer were calculated overall and by demographic characteristics (sex, race, and age group). Log-linear regression calculated annual percent change in rates during 2000 to 2009, and rate ratios (RRs) and 95% confidence intervals (95% CIs), evaluated differences in rates during 2000 through 2004 and 2005 through 2009.
RESULTS
During 2000 through 2009, the majority of AIN 3 cases occurred among men (1152 of 1320 men; 87.3%). Rates of AIN 3 during the corresponding period increased by 11.48% per year (P <.05) among men and were stable among women. Comparing rates among men during 2000 to 2004 with those during 2005 to 2009, the largest increases were noted among those aged 50 years to 64 years (RR, 2.47; 95% CI, 1.93–3.17) and among black individuals (RR, 3.49; 95% CI, 2.14–5.85). During the same period, anal cancer rates were stable among men and women.
CONCLUSIONS
Rates of AIN 3 increased in San Francisco during 2000 through 2009, in conjunction with an anal cytology screening program for high-risk groups, whereas rates of invasive anal cancer were unchanged. Continued surveillance is necessary to evaluate the impact of screening and human papillomavirus vaccination on the prevention of human papillomavirus-related AIN and anal cancer.
Keywords: anal cancer, anal intraepithelial neoplasia, incidence, screening
INTRODUCTION
Anal cancer is biologically similar to cervical cancer, in that both are due to persistent infection of epithelial cells with oncogenic human papillomavirus (HPV) types (ie, 16/18) and are preceded by precursor lesions that may be detected with cytologic screening.1 The potential for high-grade anal intraepithelial neoplasia (the presumptive precursor lesion, AIN 3, hereafter referred to as AIN) to progress to invasive anal cancer has been documented,2–4 and immune suppression due to human immunodeficiency virus (HIV) infection is an important cofactor. Many studies have evaluated AIN and anal cancer among HIV-positive and HIV-negative men who have sex with men (MSM), who are at high risk for both outcomes.5–8 Furthermore, based on evidence provided in the current HIV treatment guidelines, AIN screening and treatment are considered to provide clinical benefits comparable to those of other opportunistic infection prevention measures among HIV-positive individuals.9 In San Francisco County (an area with a high burden of HIV infection),10 cytologic screening (ie, anal Papanicolaou [Pap] testing) for AIN provided by many HIV health care practitioners to individuals with risk factors for anal cancer has been available since the early 1990s. Individuals with a previous HPV-related anogenital lesion or abnormal anal cytology are referred by primary care providers to the University of California at San Francisco Anal Neoplasia Clinic. Biopsy-confirmed high-grade AIN is treated to reduce the risk of progression to invasive anal cancer.11
In June 2012, the LAST (Lower Anogenital Squamous Terminology) Project consensus statement was issued by the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology12 that standardized histopathologic terminology for AIN diagnosis by creating a 2-tiered nomenclature system (high-grade and low-grade) based on current knowledge of the biology of HPV-related lesions of the lower anogenital region and by providing consistent terminology among HPV-related lesions at different anogenital sites. Due to the high burden of anal cancer in San Francisco,13,14 the availability of AIN screening and treatment, and the recent focus on standardization of reporting, we used population-based cancer registry data to evaluate trends in high-grade AIN to inform diagnosis, reporting, and cancer control activities in the region, as well as to inform the potential future impact of the LAST recommendations.
MATERIALS AND METHODS
Data regarding cases of high-grade AIN (ie, AIN 3) and anal cancer diagnosed between 2000 and 2009 were obtained from the San Francisco/Oakland area population-based cancer registry, which participates in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. We focused on cases occurring between 2000 and 2009 because this was the most recent 10-year period for which data were available and because AIN reporting was likely to have been stable during this period because the familiarity of health care workers with anal cancer screening increased and reporting became more routine. Consistent with previously established methodology, histologically confirmed cases of AIN and anal cancer were selected according to anatomic site and histology, using the third edition of the International Classification of Diseases for Oncology (ICD-O-3) site codes C21.0 (anus), C21.1 (anal canal), C20.9 (rectum), and 21.8 (overlapping lesion of the rectum, anus, and anal canal), and were restricted to squamous cell carcinoma (SCC; histology codes 8050–8084 and 8120–8131) because this subtype is associated with persistent HPV infection.15,16 Rectal cancers are typically adenocarcinomas, and rectal SCCs appearing in registry data are frequently misclassified cases of AIN and anal cancer, and therefore rectal SCCs were included in all analyses.17 Cases of AIN 3 were identified using the behavior code in situ to differentiate between invasive anal cancers, and both were classified by sex, age, and race. Cases were divided by the corresponding population denominators and expressed as rates per 100,000 population, and age-standardized to the 2000 US standard population using SEER*Stat software.18
We evaluated temporal trends between 2000 and 2009 by fitting a weighted least squares regression model to the log-transformed annual age-standardized rates, weighted by the inverse of their variance.19 The annual percent change (APC) was considered to be statistically significant if the 2-sided P value for the parameter was <.05. To assess changes in rates by demographic characteristics, we calculated 5-year average rates for stability, and compared rates during 2000 to 2004 with those for 2005 through 2009 via the Poisson rate ratio (RR), with corresponding 95% confidence intervals (95% CIs).20
RESULTS
A total of 1319 diagnoses of high-grade AIN were identified in the San Francisco/Oakland cancer registry during 2000 through 2009. Table 1 shows the distribution of patients by demographic characteristics and calendar period. During 2000 through 2004 and 2005 through 2009, most diagnoses were made among those aged 35 to 49 years and 50 to 64 years and among those who were white. Greater than 85% of AIN 3 diagnoses occurred among males during both time periods.
TABLE 1.
Demographic Characteristics of AIN 3 Cases in the San Francisco/Oakland Cancer Registry by Calendar Period (N=1319)
| Characteristic | 2000–2004 n=466 |
2005–2009 n=853 |
|---|---|---|
| Age, y, no. (%) | ||
| 20–34 | 46 (9.87) | 102 (11.96) |
| 35–49 | 291 (62.45) | 424 (49.71) |
| 50–64 | 112 (24.03) | 291 (34.11) |
| ≥65 | 17 (3.65) | 36 (4.22) |
| Sex, no. (%) | ||
| Male | 398 (85.41) | 753 (88.28) |
| Female | 68 (14.59) | 100 (11.72) |
| Race, no. (%) | ||
| White | 373 (80.04) | 634 (74.33) |
| Black | 37 (7.94) | 95 (11.14) |
| Other/unknown | 56 (12.02) | 124 (14.54) |
Abbreviation: AIN 3, anal intraepithelial neoplasia 3.
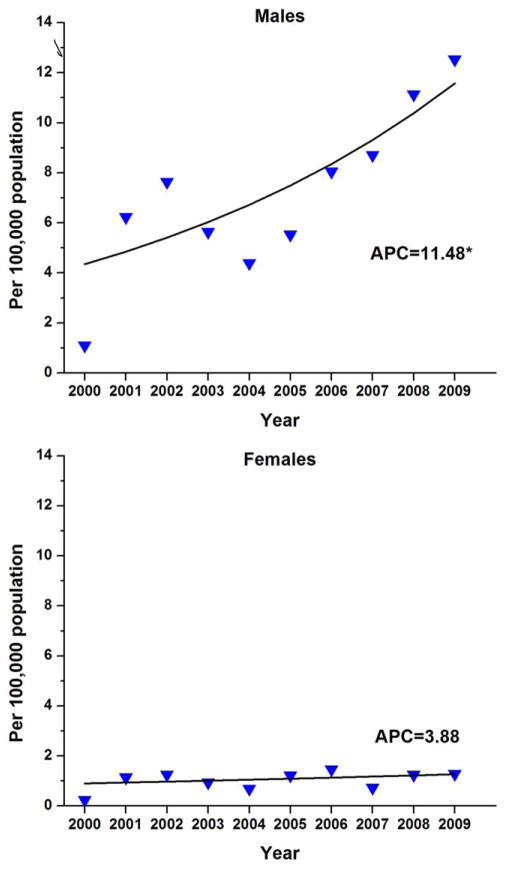
Among men, overall high-grade AIN incidence rates increased by 11.48% per year between 2000 and 2009 (P <.05) (Fig. 1). By calendar period, incidence rates nearly doubled from 5.03 to 9.24 per 100,000 between the 2 time periods of 2000 through 2004 and 2005 through 2009 (RR, 1.84; 95% CI, 1.62–2.08) (Table 2). Significant increases were also noted for men within each age stratum <65 years. Rates of high-grade AIN were found to significantly increase among men of all races, with the largest increase noted between 2000 and 2004 and 2005 and 2009 among black men (RR, 3.49; 95% CI, 2.14–5.85).
Figure 1.
Temporal trends in age-adjusted incidence rates of anal intraepithelial neoplasia 3 are shown for 466 men and 853 women in the San Francisco/Oakland Cancer Registry between 2000 and 2009. APC indicates annual percent change. Triangles represent observed rates and the solid line represents the modeled rates based on log-linear regression. The asterisk indicates P <.05.
TABLE 2.
Age-Adjusted Incidence Rates of AIN 3 in the San Francisco/Oakland Cancer Registry by Sex, Age Group, Race, and Calendar Period
| Characteristic | Rate per 100,000 Population
|
RR (95% CI) | P | |
|---|---|---|---|---|
| 2000–2004 | 2005–2009 | |||
| Overall | 2.95 | 5.24 | 1.78 (1.59–2.00) | <.001 |
| Males | 5.03 | 9.24 | 1.84 (1.62–2.08) | <.001 |
| Age, y | ||||
| 20–34 | 1.57 | 3.77 | 2.41 (1.63–3.59) | <.001 |
| 35–49 | 9.96 | 15.24 | 1.53 (1.30–1.80) | <.001 |
| 50–64 | 5.40 | 13.33 | 2.47 (1.93–3.17) | <.001 |
| ≥65 | 0.95 | 1.97 | 2.08 (0.94–4.91) | .073 |
| Race | ||||
| White | 5.95 | 10.12 | 1.70 (1.48–1.96) | <.001 |
| Black | 3.20 | 11.18 | 3.49 (2.14–5.85) | <.001 |
| Other/unknown | 2.78 | 5.90 | 2.13 (1.49–3.06) | <.001 |
| Females | 0.85 | 1.19 | 1.40 (1.02–1.94) | .038 |
| Age, y | ||||
| 20–34 | 0.24 | 0.57 | 2.37 (0.83–7.63) | .123 |
| 35–44 | 1.34 | 1.41 | 1.05 (0.63–1.73) | .937 |
| 50–64 | 1.19 | 1.91 | 1.60 (0.92–2.87) | .102 |
| ≥65 | 0.50 | 0.92 | 1.84 (0.68–5.48) | .273 |
| Race | ||||
| White | 0.86 | 1.24 | 1.45 (0.98–2.16) | .067 |
| Black | 1.77 | 2.26 | 1.28 (0.60–2.80) | .611 |
| Other/unknown | 0.42 | 0.66 | 1.57 (0.61–4.34) | .428 |
Abbreviation: 95% CI, 95% confidence interval; AIN 3, anal intraepithelial neoplasia 3; RR, rate ratio.
Among women, the overall incidence of high-grade AIN increased during 2000 through 2009 by 3.88% per year, but this increase was not statistically significant (P =.31) (Fig. 1). By calendar period, rates increased from 0.85 to 1.19 per 100,000 between the 2 time periods of 2000 through 2004 and 2005 through 2009, respectively (RR, 1.40; 95% CI, 1.02–1.94) (Table 2). Rates also increased nonsignificantly for women of every age group and race.
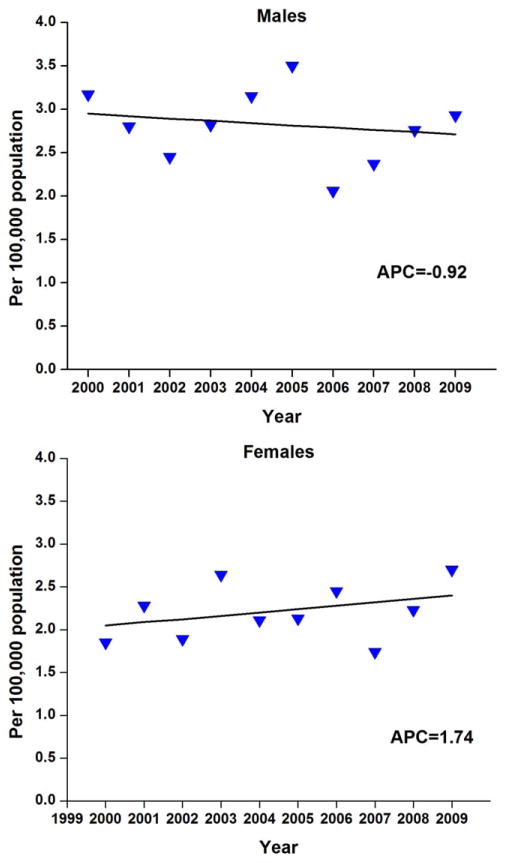
Overall, invasive anal cancer rates were found to be statistically stable for both men and women during 2000 through 2009 (Fig. 2), and there were no changes noted by demographic subgroups (Tables 3 and 4). By the end of this period, the year 2009, the incidence rate of AIN was 10-fold higher among men (12.5) versus women (1.3), whereas rates of invasive anal cancer were nearly the same (2.9 and 2.7, respectively, among men and women).
Figure 2.
Temporal trends in age-adjusted incidence rates of anal cancer are shown for 417 men and 378 women in the San Francisco/Oakland Cancer Registry between 2000 and 2009. APC indicates annual percent change. Triangles represent observed rates and the solid line represents the modeled rates based on log-linear regression. The asterisk indicates P <.05.
TABLE 3.
Demographic Characteristics of Invasive Anal Cancer in the San Francisco/Oakland Cancer Registry by Calendar Period (N=795)
| 2000–2004 | 2005–2009 | |
|---|---|---|
| Characteristica | n=383 | n=412 |
| Sex, no. (%) | ||
| Male | 206 (53.8) | 211 (51.2) |
| Female | 177 (46.2) | 201 (48.8) |
| Race, no. (%) | ||
| White | 320 (83.6) | 341 (82.8) |
| Black | 46 (12.0) | 48 (11.7) |
| Other/unknown | 17 (4.4) | 23 (5.5) |
Age-specific information is not displayed due to small cell counts.
TABLE 4.
Age-Adjusted Incidence Rates of Invasive Anal Cancer in the San Francisco/Oakland Cancer Registry by Sex, Age Group, Race, and Calendar Period
| Rate per 100,000 Population | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | 2000–2004 | 2005–2009 | RR (95% CI) | P |
| Overall | 2.51 | 2.50 | 1.00 (0.86–1.15) | .97 |
| Males | 2.88 | 2.72 | 0.95 (0.77–1.15) | .60 |
| Age, y | ||||
| 20–34 | a | a | ||
| 35–49 | 2.52 | 2.36 | 0.94 (0.65–1.35) | .78 |
| 50–64 | 4.98 | 4.91 | 0.99 (0.73–1.37) | .73 |
| ≥65 | 5.34 | 5.00 | 0.94 (0.63–1.40) | .81 |
| Race | ||||
| White | 3.38 | 3.27 | 0.97 (0.78–1.20) | .81 |
| Black | 4.27 | 3.52 | 0.82 (0.45–1.50) | .60 |
| Other/unknown | a | a | ||
| Females | 2.16 | 2.26 | 1.04 (0.85–1.29) | .71 |
| Age, y | ||||
| 20–34 | a | a | ||
| 35–44 | 1.44 | 1.00 | 0.70 (0.40–1.19) | .20 |
| 50–64 | 4.17 | 4.27 | 1.02 (0.74–1.42) | .95 |
| ≥65 | 4.58 | 5.83 | 1.28 (0.91–1.78) | .16 |
| Race | ||||
| White | 2.71 | 2.79 | 1.03 (0.82–1.30) | .85 |
| Black | 2.28 | 2.82 | 1.24 (0.63–2.44) | .61 |
| Other/unknown | a | a | ||
Abbreviation: 95% CI, 95% confidence interval; RR, rate ratio.
Data are not shown for rates based on fewer than 16 cases.
DISCUSSION
We observed significant increases in the population-based incidence rates of AIN 3 among men but not women in the San Francisco/Oakland cancer registry. These trends should be considered in the context of both the availability of screening and treatment for high-grade AIN and the high burden of invasive anal cancer among both men and women living in the San Francisco area.11,13,14,17 AIN 3 is reportable to cancer registries statewide in California and in other SEER program registry areas.21,22 However, strong collaborative efforts between cancer surveillance programs, clinicians conducting anal cytology screening, high-resolution anoscopy (HRA) and HRA-guided biopsy and treatment of high-grade AIN, and pathologists involved with interpreting anal cytology and histology are needed to improve the accurate monitoring of AIN trends at the community level. Communication among these groups would promote the clinical care and management of patients with AIN, which might prevent many cases from progressing to invasive cancer. Routine screening of high-risk individuals would then also warrant evaluations at the broader population level. The increasing AIN 3 trends documented herein coincide with stable rates of anal cancer in the region. The impact of screening programs on invasive cancers and mortality outcomes is not known.
The current AIN incidence trends are likely to be the result of several factors, including the high incidence of HIV infection in the preceding 20 years and the focused nature of these infections among MSM (whom are also at high risk of HPV infections and anal neoplasia).5–8,23 Although HIV infection incidence and mortality have decreased over time, HIV prevalence has increased as a function of effective HIV therapies, thereby resulting in a large population at an increased risk of anal neoplasia in the region. To our knowledge, there are no data regarding trends in HPV infections occurring San Francisco, although anal HPV infections are detected in a very high percentage of HIV-infected adults.24 The elevated AIN 3 rates among men compared with women reflect the excess of diagnoses among HIV-positive men and higher relative rates of screening compared with women, regardless of HIV status, in San Francisco. Screening of high-risk patients likely increased over time, although data are not available for a more systematic evaluation of contemporary screening rates.
The findings of the current study provide a baseline for monitoring the effect of increased screening, and potentially the future impact of HPV vaccination. Although HPV vaccination coverage levels remain low, they are increasing.25 Routine vaccination is recommended for girls and boys aged 11 years or 12 years.26–28 Vaccination is also recommended for unvaccinated females aged 13 years through 26 years and males aged 13 years through 21 years. The vaccine is also recommended for gay and bisexual men (or any man who has sex with men) and men with compromised immune systems (including HIV) through age 26 years.28 Vaccination should ideally occur before sexual debut. During 2011, approximately 1.3% of males aged 13 years to 17 years had received the full recommended 3-dose series of the HPV vaccine.29 As coverage levels increase and vaccinated cohorts enter age groups in which AIN and anal cancer risks are highest, the effect of vaccination would be most likely to manifest first as declines in population-based rates of high-grade AIN.1,22
The characteristics of screening and treatment coupled with trends in AIN and anal cancer rates in the current study underscore the need for studies to assess anal cytology practices in other areas and evaluations of the impact of such screening on these outcomes. In 1999, Goldie et al found it to be both clinically effective and cost-effective to screen HIV-positive MSM with annual anal Pap smears,30 and a recent systematic review31 summarized AIN screening practices among HIV-positive individuals. To the best of our knowledge, no studies to date have evaluated the efficacy of screening to reduce mortality from anal cancer.14 A cohort study in San Francisco demonstrated that 38% of HIV-positive and 17% of HIV-negative MSM developed AIN during 4 years of follow-up.7 A more recent Canadian study found 37% of HIV-positive MSM developed AIN over 3 years.5 However, to the best of our knowledge, data regarding AIN risk for longer time periods are not yet available.8 Studies by de Pokomandy et al and Palefsky et al5,7 as well as others6,32 found HIV infection, age, nadir CD4-positive T-lymphocyte count, number of HPV infections and specific HPV types (ie, 16/18), and other characteristics were independent predictors of AIN risk among MSM, although risk factors among women, and more generally, among individuals without HIV infection or men who are not MSM, remain unclear. Additional research is also needed to determine rates of progression from high-grade AIN to invasive cancer and to determine the host characteristics and mechanistic pathways involved in anal carcinogenesis.
The largest increase in AIN 3 incidence rates during the 10-year study period was noted among black men and the reason for this strong increase is unclear. In part, the trend may reflect improved access to care (especially for black HIV-positive MSM who are now more engaged in the health care system due to focused HIV programs). However, it is interesting to note that AIN 3 rates during 2005 through 2009 were similar among black (11.18 per 100,000 population) and white (10.12 per 100,000 population) men, underscoring the importance of interventions for all men at risk of developing AIN. Another recent study also found increases in the incidence of AIN 3 in San Francisco, although it did not provide a detailed evaluation of trends by sex, race, and age groups and focused on different outcomes.33
The current evaluation of trends in AIN rates also should be considered in the context of the new LAST Project pathology recommendations.12 The recommendations provide guidance for pathologists diagnosing and clinicians screening for AIN, and are meant to promote the use of common terminology across disciplines using a 2-tiered nomenclature system that classifies AIN as either high grade or low grade. Importantly, to be reported to cancer surveillance programs, such diagnoses still need to be coded as AIN 3.21 The LAST Project statement also provides guidance for p16 (a cell regulatory protein) testing, which might be beneficial in improving the diagnostic accuracy of high-grade AIN. In terms of AIN treatment, the protocol used by many health care providers in San Francisco (mainly those treating HIV-positive patients) calls for abnormal cytology to be confirmed with biopsy via HRA and for those individuals with AIN 2 or AIN 3 to be treated (AIN 2 is a lower-grade lesion but some may progress to AIN 3 and eventually invasive cancer, and therefore they are also treated).34 Treatment can be challenging because of tolerability and side effects, and to the best of our knowledge only limited efficacy data exist. Modalities range from topical and ablative therapies to surgical excision for extensive disease.34 The optimal surveillance interval after treatment for recurrent lesions is unknown.
To our knowledge, this assessment of temporal trends in high-grade AIN using high-quality, population-based surveillance data are the first to provide detailed information focused solely on AIN 3 rates by sex, race, and age group in an area with an existing screening program. The current analysis also has limitations. We had no data available regarding HIV infection, although many of the AIN cases included in the current study were likely to have been HIV-positive based on previous studies of AIN and anal cancer in the region.13,17 In addition, although the cancer registry is population-based, the completeness of AIN reporting likely increased over time as screening became more widespread in San Francisco and clinician awareness of AIN grew, therefore complicating the interpretation of temporal trends.11 This issue may have been partly addressed by restricting our analysis to the most recent 10-year period for which there were available data. In addition, AIN is a rare outcome and this was evident in the analysis of incidence rates among women, for whom the APC analysis (of annual rates) revealed a nonsignificant increase likely due to a lack of statistical power (P =.31), but the RR analysis (of 5-year average annual rates) demonstrated a significant increase just below the decision threshold. In addition, we focused on the San Francisco area because of our prior knowledge of an AIN screening program and this selection limits the generalizability of our findings. Overall AIN 3 rates significantly increased in 4 other SEER 9 registries during the same time period (Connecticut, Detroit, Iowa, and Seattle; data not shown). The findings of the current study demonstrate the usefulness of cancer registry data for monitoring anal cancer precursor lesions at the population level.
The results of the current study demonstrated increases in the occurrence of AIN among men between 2000 and 2009 in the San Francisco/Oakland cancer registry. These increases occurred within the context of an AIN screening program that was focused mainly on HIV-positive individuals, and coincided with stable incidence rates of invasive anal cancer. This baseline evaluation of AIN rates provides data for the future monitoring of the effect of more widely disseminated screening and the delayed effect of increasing HPV vaccination coverage levels in the prevention of AIN and anal cancer.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICT OF INTEREST DISCLOSURES
Dr. Clarke has received a grant from the National Cancer Institute. Dr. Palefsky is a member of the boards of Merck and Company and Pharmajet, and has received travel support from Merck. He has also received grants from Merck and Hologic and has acted as a member of the Speakers’ Bureau for Hologic. Dr. Palefsky also owns stock in Aura Biosciences.
References
- 1.Schiffman M, Kjaer SK. Chapter 2: natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003;(31):14–19. doi: 10.1093/oxfordjournals.jncimonographs.a003476. [DOI] [PubMed] [Google Scholar]
- 2.Kreuter A, Potthoff A, Brockmeyer NH, et al. Anal carcinoma in human immunodeficiency virus-positive men: results of a prospective study from Germany. Br J Dermatol. 2010;162:1269–1277. doi: 10.1111/j.1365-2133.2010.09712.x. [DOI] [PubMed] [Google Scholar]
- 3.Scholefield JH, Castle MT, Watson NF. Malignant transformation of high-grade anal intraepithelial neoplasia. Br J Surg. 2005;92:1133–1136. doi: 10.1002/bjs.4994. [DOI] [PubMed] [Google Scholar]
- 4.Watson AJ, Smith BB, Whitehead MR, Sykes PH, Frizelle FA. Malignant progression of anal intra-epithelial neoplasia. ANZ J Surg. 2006;76:715–717. doi: 10.1111/j.1445-2197.2006.03837.x. [DOI] [PubMed] [Google Scholar]
- 5.de Pokomandy A, Rouleau D, Ghattas G, et al. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis. 2011;52:1174–1181. doi: 10.1093/cid/cir064. [DOI] [PubMed] [Google Scholar]
- 6.Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 7.Palefsky JM, Holly EA, Ralston ML, Jay N, Berry JM, Darragh TM. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. AIDS. 1998;12:495–503. doi: 10.1097/00002030-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Severini A. Anal intraepithelial neoplasia in men living with HIV in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52:1182–1183. doi: 10.1093/cid/cir070. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. quiz CE1–CE4. [PubMed] [Google Scholar]
- 10.San Francisco Department of Public Health HIV/AIDS Epidemiology Annual Report. [Accessed June 25, 2013];HIV Epidemiology Section. sfdph.org/dph/files/reports/RptsHIVAIDS/AnnualReport2011.pdf.
- 11.Pineda CE, Berry JM, Jay N, Palefsky JM, Welton ML. High-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: a ten-year experience. Dis Colon Rectum. 2008;51:829–835. doi: 10.1007/s10350-008-9233-4. discussion 835–837. [DOI] [PubMed] [Google Scholar]
- 12.Darragh TM, Colgan TJ, Cox JT, et al. Members of the LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266–1297. doi: 10.5858/arpa.LGT200570. [DOI] [PubMed] [Google Scholar]
- 13.Hessol NA, Pipkin S, Schwarcz S, Cress RD, Bacchetti P, Scheer S. The impact of highly active antiretroviral therapy on non-AIDS-defining cancers among adults with AIDS. Am J Epidemiol. 2007;165:1143–1153. doi: 10.1093/aje/kwm017. [DOI] [PubMed] [Google Scholar]
- 14.Katz KA, Clarke CA, Bernstein KT, Katz MH, Klausner JD. Is there a proven link between anal cancer screening and reduced morbidity or mortality? Ann Intern Med. 2009;150:283–284. doi: 10.7326/0003-4819-150-4-200902170-00020. author reply 284–285. [DOI] [PubMed] [Google Scholar]
- 15.Watson M, Saraiya M, Ahmed F, et al. Using population-based cancer registry data to assess the burden of human papillomavirus-associated cancers in the United States: overview of methods. Cancer. 2008;113(suppl 10):2841–2854. doi: 10.1002/cncr.23758. [DOI] [PubMed] [Google Scholar]
- 16.Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer. 2008;113(suppl 10):2892–2900. doi: 10.1002/cncr.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cress RD, Holly EA. Incidence of anal cancer in California: increased incidence among men in San Francisco, 1973–1999. Prev Med. 2003;36:555–560. doi: 10.1016/s0091-7435(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance Research Program, National Cancer Institute. [Accessed June 25, 2013];SEER*-Stat software. Version 7.0.5. seer.cancer.gov/seerstat.
- 19.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Rothman J, Greenland S, Lash T. Modern Epidemiology. Philadelphia, PA: Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 21.Surveillance Systems Branch, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute. [Accessed June 25, 2013];SEER Program Coding and Staging Manual. seer.cancer.gov/manuals/2011/SPCSM_2011_maindoc_09272011.pdf.
- 22.Saraiya M, Goodman MT, Datta SD, Chen VW, Wingo PA. Cancer registries and monitoring the impact of prophylactic human papillomavirus vaccines: the potential role. Cancer. 2008;113(suppl 10):3047–3057. doi: 10.1002/cncr.23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia Q, Nonoyama A, Molitor F, Webb D, Osmond D. Recent decline in the incidence of human immunodeficiency virus infection among California men who have sex with men. Am J Epidemiol. 2011;174:203–210. doi: 10.1093/aje/kwr054. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez AL, Efird JT, Holly EA, Berry JM, Jay N, Palefsky JM. Risk factors for anal human papillomavirus infection type 16 among HIV-positive men who have sex with men in San Francisco. J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0b013e3182968f87. published online ahead of print April 22, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC). . National and state vaccination coverage among adolescents aged 13 through 17 years-United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117–1123. [PubMed] [Google Scholar]
- 26.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP) Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). . FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–629. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC). . Recommendations on the use of quadrivalent human papillomavirus vaccine in males-Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–1708. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC). . National and state vaccination coverage among adolescents aged 13–17 years-United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–677. [PubMed] [Google Scholar]
- 30.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281:1822–1829. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 31.Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006;43:223–233. doi: 10.1086/505219. [DOI] [PubMed] [Google Scholar]
- 32.Salit IE, Tinmouth J, Chong S, et al. Screening for HIV-associated anal cancer: correlation of HPV genotypes, p16, and E6 transcripts with anal pathology. Cancer Epidemiol Biomarkers Prev. 2009;18:1986–1992. doi: 10.1158/1055-9965.EPI-08-1141. [DOI] [PubMed] [Google Scholar]
- 33.Amirian ES, Fickey PA, Jr, Scheurer ME, Chiao EY. Anal cancer incidence and survival: comparing the greater San-Francisco bay area to other SEER cancer registries. PLoS One. 2013;8:e58919. doi: 10.1371/journal.pone.0058919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park IU, Palefsky JM. Evaluation and management of anal intraepithelial neoplasia in HIV-negative and HIV-positive men who have sex with men. Curr Infect Dis Rep. 2010;12:126–133. doi: 10.1007/s11908-010-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]