Abstract
Psychological stress is thought to arise from appraisal processes that ascribe threat-related meaning to experiences that tax or exceed our coping ability. Neuroimaging research indicates that these appraisal processes originate in brain systems that also control physiological stress reactions in the body. Separate lines of research in health psychology and behavioral medicine indicate that these physiological stress reactions confer risk for physical disease. Accordingly, integrative research that cuts across historically separated disciplines may help to define the brain-body pathways linking psychological stress to physical health. We describe recent studies aimed at this goal, focusing on studies of the brain bases of stressor-evoked cardiovascular system reactions and heart disease risk. We also outline an interpretive framework for these studies, as well as needs for next-generation models and metrics to better understand how the brain encodes and embodies stress in relation to health.
Keywords: appraisal, health, neuroimaging, stress
How does psychological stress influence physical health? This perennial question is timely for many reasons. One is that psychological stress confers risk for chronic diseases that pose the greatest burden to public health, including heart disease (Cohen, Janicki-Deverts, & Miller, 2007). Yet, although psychological stress may undermine health, we still lack a mechanistic understanding of how this might occur. A barrier to this understanding is our incomplete knowledge of how the brain generates states of psychological stress and bodily stress reactions—particularly physiological stress reactions—that may lead to physical disease or increase disease vulnerability. Critically, however, the integration of neuroimaging into human stress science is providing new opportunities to expand this knowledge. This integration is enabling us to better understand the interplay of psychological and physiological mechanisms of stress-health relationships, which can be conceptualized as involving links across at least four elements: (1) brain appraisal systems; (2) visceromotor outputs from the brain to organs and tissues; (3) peripheral physiology and pathophysiology; and (4) viscerosensory input from the body back to brain appraisal systems (Fig. 1). Here, we provide an interpretive context for recent neuroimaging studies bearing on these links. We focus on studies of disease-related cardiovascular system reactions to acute psychological stressors as example approaches to studying brain-body-health relationships.
Figure 1.
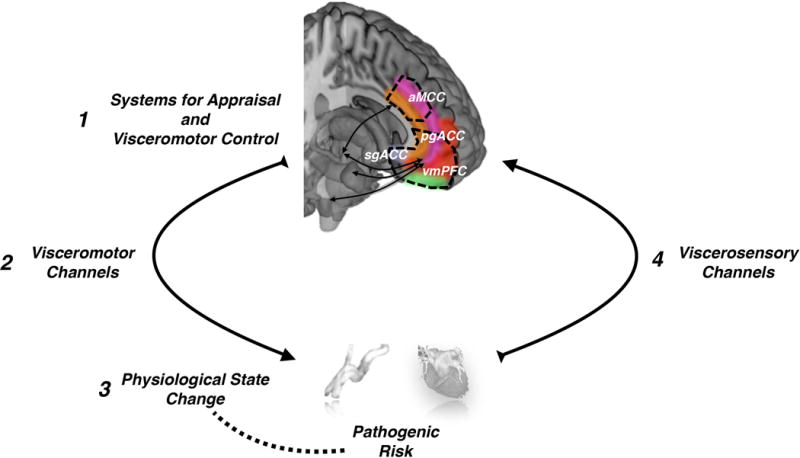
Four core elements comprise at least one kind of brain-body pathway linking psychological stress and physiological stress reactions to physical health. The first encompasses networked brain systems that appraise internal and external sources of information as threats that tax or exceed coping resources and translate these appraisals into visceromotor commands. These systems likely include the anterior mid-cingulate cortex (aMCC), pregenual anterior cingulate cortex (pgACC), subgenual anterior cingulate cortex (sgACC), and ventromedial prefrontal cortex (vmPFC). The second encompasses visceromotor (e.g., autonomic nervous system) channels that translate appraisal-based neural commands into a change in an end organ state in the body (e.g., a rise in blood pressure and heart rate). A corollary of this third component is that the patterning of the end-organ state change (e.g., a sizeable and sustained rise in blood pressure or heart rate) should plausibly relate to disease progression or risk. For example, chronically large or prolonged rises in blood pressure may exert shear or tensile stress on blood vessel walls over time, possibly accelerating atherosclerosis and influencing risk for later heart disease. The fourth component encompasses viscerosensory (e.g., autonomic nervous system) channels that relay feedback signals from the body, enabling the representation of end organ state changes in the brain. The interaction between appraisal systems and visceromotor and viscerosensory channels may thus represent part of the possible basis for stress-health relationships.
Appraisal Systems and Psychological Stress
Brain appraisal systems comprise the first element of a pathway linking psychological stress to health-related physiology and pathophysiology. Appraisal systems encode and evaluate events and experiences (e.g., thoughts, memories, and life situations) for their meaning and significance to the individual. Specific appraisals are thought to generate states of psychological stress, particularly those associated with threats to physical, social, and personal well-being that tax or exceed our coping ability (Lazarus, 1966; Monroe, 2008). In extension, because individuals differ in the meaning they ascribe to appraised events and their perceived coping resources, such individual differences may be linked to corresponding differences in stress reactions and health-related outcomes (Cohen et al., 2007; Lazarus, 1966; Monroe, 2008). Otherwise healthy people, for example, who appraise their life experiences as more taxing than others exhibit higher levels of blood pressure and a faster progression of atherosclerosis, a pathophysiological determinant of heart disease (Kamarck, Shiffman, Sutton-Tyrrell, Muldoon, & Tepper, 2012). Several mechanisms could mediate such health risks (Miller, Chen, & Cole, 2009). Of these, much historical attention has focused on the biological mechanisms by which threat appraisals might affect health via two primary stress response systems: the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis (Lane et al., 2009). But, how precisely do brain-based appraisals engage these systems and lead to physiological or pathophysiological effects on the body? Recent neuroimaging studies that measure brain and physiological activity together provide a way to address this question. Many of these kinds of studies focus on linking brain activity to changes in cardiovascular physiology mediated by the ANS (Fig. 2). Accordingly, researchers are becoming better able to characterize the human brain systems for appraisal and health-relevant stress reactions.
Figure 2.
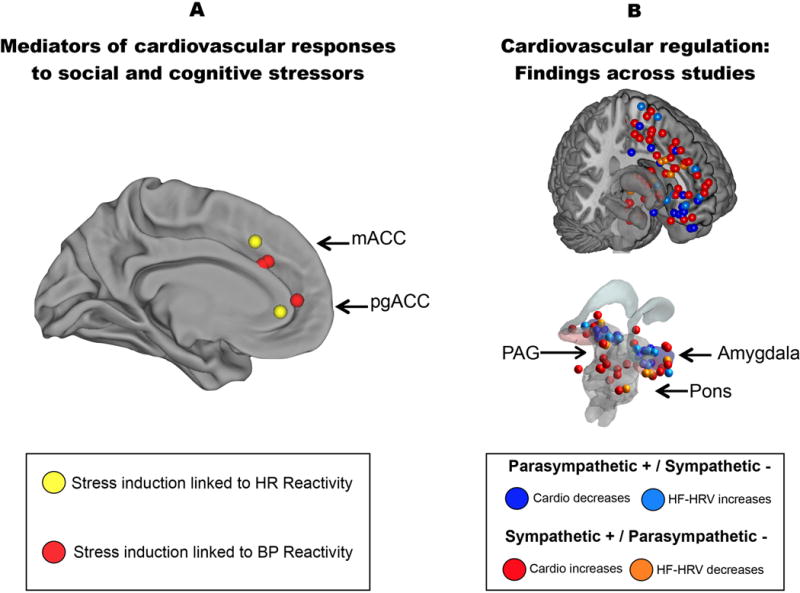
Brain regions where neural activity has been associated with cardiovascular and cardiac autonomic physiology in neuroimaging studies. A) Areas of the mPFC coded in yellow indicate where activity was associated with heart rate reactivity to social stressor involving evaluative threat (Wager et al., 2009, Neuroimage, 47, 821–835; Wager et al., 2009, Neuroimage, 47, 836–851); areas of the mPFC coded in red indicate where activity was associated with blood pressure reactivity to a cognitive stressor (Gianaros et al., 2005, Psychophysiology, 42, 627–635). B) An illustrative summary of brain regions where changes in cardiovascular and cardiac autonomic activity have been associated with neural activity in existing neuroimaging studies (a complete reference list of the studies contributing to this summary is available on request). Areas in warmer colors (red and yellow) correspond to brain coordinates where increases in neural activity have been associated with ‘pro-sympathetic’ or ‘anti-parasympathetic’ autonomic responding, as reflected by increases in heart rate and blood pressure and decreases in high-frequency heart rate variability (HRV). Areas in cooler colors (blue and light blue) correspond to brain coordinates where increases in neural activity have been associated with ‘anti-sympathetic’ or ‘pro-parasympathetic’ responding, as reflected by decreases in heart rate and blood pressure and increases in high-frequency heart rate variability (HRV). mACC, mid-anterior cingulate cortex; pgACC, pregenual anterior cingulate cortex.
Linking Appraisal to Physiology: Neuroimaging Studies of Cardiovascular Stress Reactions
Acute psychological stressors evoke cardiovascular reactions that unfold rapidly. These reactions result from brain-based changes in ANS visceromotor outflow to the heart and vasculature—typically characterized by increases in sympathetic and decreases in parasympathetic activity. Such changes usually lead to simultaneous increases in heart rate and blood pressure, which have long been viewed to provide metabolic support for adaptive behaviors that emerge from interacting cortical and subcortical brain systems (Charvat, Dell, & Folkow, 1964). Cannon (1932) viewed these cardiovascular changes as components of ‘emergency reactions’ that help us deal with perceived threats. In the fields of cardiovascular behavioral medicine and psychophysiology, these changes have also been viewed as arising from ‘central commands’ in brain systems that couple peripheral physiology with effortful somatic behavior (Obrist, 1981).
Importantly, stress-related changes in cardiovascular physiology that can be measured in neuroimaging studies have been linked in epidemiological studies to heart disease risk, establishing the relevance of their brain correlates for health. Hence, individuals who exhibit metabolically ‘exaggerated’ or prolonged stressor-evoked cardiovascular reactions (e.g., sizeable and sustained rises in blood pressure and heart rate) are at risk for hypertension, heart attacks, and premature death by heart disease (Carroll et al., 2012; Chida & Steptoe, 2010).
These cardiovascular reactions have their origins in the brain, with appraisals of threat based on prior experience and expectations from the current context (Fig. 1). This is demonstrated in neuroimaging studies that manipulate appraisal-related brain activity via standardized instructions (e.g., ‘This is a test of your performance that predicts your real-world success.’) or cognitive demand combined with failure feedback and time pressure. However, interpreting the brain changes that accompany such manipulations is problematic, as they can be resistant to tight experimental control. This is because psychological stress engages complex changes in cognitive, emotional, and physiological response systems. Thus, to enable more specific inferences about which brain systems are central to stressor-evoked cardiovascular reactivity, researchers focus on brain signals that correlate with the intensity of self-reported experiences and the patterning (e.g., magnitude) of cardiovascular reactions within and across individuals.
For example, in a series of studies focusing on heart rate reactivity, participants prepared a speech under the threat of negative social evaluation (Wager, Van Ast, et al., 2009; Wager, Waugh, et al., 2009). This stressor increased activity in several brain areas. However, the medial prefrontal cortex (mPFC)—encompassing the inner and anterior portion of the cerebral hemispheres—was the only area to show activity increases that persisted for the duration of heart rate increases. Moreover, mediation analyses showed that stressor-evoked mPFC activity (1) predicted heart-rate increases on a person-by-person basis and (2) mediated heart-rate reactions induced by the stressor. Notably, stressor-evoked mPFC activity linked to heart-rate reactivity in these studies was decomposed into two components. The first consisted of activity increases in more dorsal mPFC areas (anterior mid-cingulate cortex [aMCC], pregenual anterior cingulate cortex [pgACC]). The second consisted of activity decreases in more ventral mPFC areas (ventromedial prefrontal cortex [vmPFC], encompassing the subgenual ACC [sgACC]). The effects of both mPFC components on heart rate were, in turn, mediated by subcortical activity in the periaqueductal gray (PAG) and thalamus, areas important for stress- and threat-related ANS visceromotor control (Bandler, Keay, Floyd, & Price, 2000; Saper, 2002). Thus, these studies established a psychological stress-to-cardiovascular reactivity pathway spanning cortical (mPFC) and subcortical (PAG, thalamic) systems in humans.
In other work focusing on blood pressure reactivity, people completed demanding cognitive tasks that involved processing conflict, inhibiting overlearned responses, and receiving negative feedback under conditions of time pressure, low task control, and high task unpredictability (Gianaros & Sheu, 2009). As in the heart-rate studies above, stressor-evoked blood pressure increases were associated with increases in medial prefrontal activity (aMCC, pgACC), along with increases in the PAG and other subcortical ANS control areas. In earlier work also using demanding cognitive tasks (Gianaros, Van Der Veen, & Jennings, 2004), activity decreases in vmPFC were linked not only to increases in heart rate as in the speech stressor studies above, but also decreases in high-frequency heart-rate variability (HF-HRV), a marker of parasympathetic outflow. Notably, stressor-evoked decreases in HF-HRV are linked to concurrent rises in heart rate and blood pressure (Brindle, Ginty, Phillips, & Carroll, 2014), as well as heart disease risk (Gianaros et al., 2005). Importantly, animal models suggest that the mPFC responses observed in these studies likely play a causal role in mediating stressor-evoked cardiovascular reactivity (Resstel & Correa, 2006) via direct and indirect anatomical connections with subcortical ANS control centers (Gabbott, Warner, Jays, Salway, & Busby, 2005; Öngür & Price, 2000; Saper, 2002).
Together with foundational studies in this area (Critchley, 2005; Critchley et al., 2003), the above findings are converging on a picture of the brain systems for stressor-evoked cardiovascular reactivity (Fig. 3). Part of this picture includes generally—but not exclusively—contrasting autonomic effects linked to dorsal (e.g., aMCC/pgACC) vs. ventral (e.g., vmPFC/sgACC) mPFC areas. Dorsal mPFC areas are more often related to pro-threat/pro-sympathetic responses, whereas ventral mPFC areas are more often related to anti-threat/pro-parasympathetic responses, respectively (Fig. 2; cf., Critchley, Nagai, Gray, & Mathias, 2011). These contrasting dorsal vs. ventral mPFC patterns appear similar to those seen in neuroimaging studies of stressor-evoked immune and hormonal responses (Muscatell & Eisenberger, 2012).
Figure 3.
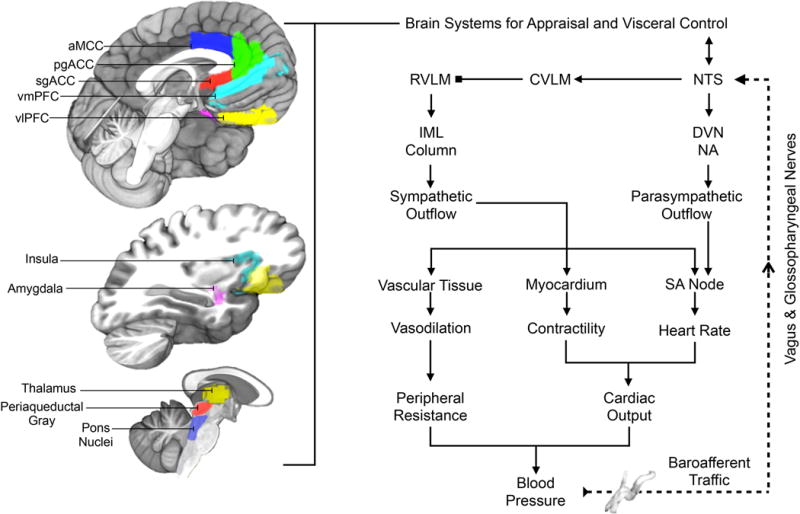
Illustration of selected brain areas engaged by psychological stressors and related to the expression of stressor-evoked cardiovascular (blood pressure and heart rate) responses, presumably via interactions with the baroreflex. On the left side of the figure are brain areas where stressor-evoked neural activity has been related to simultaneous changes in blood pressure, heart rate, and baroreflex measures (see text). Key areas include those presumably involved in mediating stressor appraisals and coordinating these appraisals with behavioral and physiological responding, including the dorsal anterior mid-cingulate cortex (aMCC), pregenual and subgenual areas of the anterior cingulate cortex (pgACC, sgACC), the ventromedial and ventrolateral prefrontal cortex (vmPFC, vlOFC), insula, and amygdala. These areas are networked with one another and with subcortical areas that play more proximal roles in the visceral regulation of cardiovascular function, including areas in the thalamus, periaqueductal gray, and pons. We note that this is not an exhaustive illustration of all brain areas involved in cardiovascular regulation. On the right side of the figure is a simplified illustration of the major motor and sensory limbs of the baroreflex, a homeostatic visceral control loop that regulates blood pressure. For clarity, not all components and connections are shown. Pressure sensitive cells (baroreceptors) that encode changes in blood pressure are concentrated in the major arteries (e.g., aorta, carotid artery). Their sensory signals (baroafferent traffic) are relayed via the vagal and glossopharyngeal nerves to a cell group in the medulla of the brainstem that influences both sympathetic and parasympathetic nervous system outflow: the NTS (nucleus of the solitary tract). The NTS not only projects to higher subcortical and cortical regions, but also to the dorsal vagal nucleus (DVN) and nucleus ambiguous (NA), which regulate parasympathetic outflow to the sinoatrial (SA) node of the heart to affect heart rate. The NTS also influences the rostral ventrolateral medulla (RVLM) indirectly via the caudal ventrolateral medulla (CVLM). The RVLM is a major source of excitatory drive to the sympathetic nervous system. Accordingly, indirect inputs from the NTS to the RVLM have the net effects of influencing sympathetic nervous system outflow to heart muscle (myocardium) to influence its contractility and the blood vessels (vascular tissue) to influence their level of dilation and resistance to blood flow in the periphery. These influences occur via RVLM projections to preganglionic cells of the sympathetic nervous system in the intermediolateral cell column (IML) of the spinal cord. Blood pressure itself is the product of the level of resistance to blood flow in the periphery and the volume of blood being pumped by the heart (cardiac output), which is determined by heart rate and myocardial contractility. Blocked endpoints denote inhibitory influences; arrowed endpoints denote excitatory influences. Broken lines correspond to ascending (afferent) visceral input, particularly from peripheral baroreceptors. The baroreflex is one example of a visceral control loop by which appraisal systems of the brain may link states of psychological stress and physiological responding to influence health.
Although definitive homologies with non-human animals are unclear, the dorsal/ventral mPFC distinction found in humans may parallel distinctions between dorsal prelimbic and ventral infralimbic areas in rat mPFC, which have divergent anatomical connectivity (Vertes, 2004) and possibly opposing functions related to threat processing and responding (Quirk & Beer, 2006; Roy, Shohamy, & Wager, 2012; cf., Ulrich-Lai & Herman, 2009). In speculation, the functional nature of contrasting dorsal vs. ventral mPFC patterns may correspond to a greater encoding of threat-related meaning and perhaps to stronger ‘central commands’ to subcortical areas for greater (e.g., exaggerated or prolonged) physiological stress responding (cf., Lovallo & Gerin, 2003). Thus, a provisional view is that a functional bias in mPFC areas to encode or appraise experiences as ‘threatening’ may represent a marker of physical (e.g., heart) disease risk.
This view, however, will undoubtedly evolve as more precise metrics are developed to quantify complex patterns of activity across cortical and subcortical visceral control systems (Table 1). Moreover, caution is warranted in inferring strict functional segregations of autonomic influences in the brain: there is much regional overlap and intermixing of the neural correlates of sympathetic and parasympathetic autonomic functionality (Fig. 2). This intermixing agrees with animal findings, and one consequence is that the same gross anatomical brain areas may contribute differently to cognitive, affective, and physiological stress reactions under different conditions. Furthermore, stressors can evoke complex patterns of independent, coactive, and reciprocal changes in sympathetic and parasympathetic activity (Berntson, Sarter, & Cacioppo, 1998), making it challenging to isolate unique brain correlates of each pattern of visceromotor output.
Table 1.
Open questions about brain-body pathways linking stress and health.
|
As the field advances, it will also be critical to carefully consider the subcortical systems that link stress-related cortical appraisals to downstream stress reactions. The mPFC, for instance, projects to the amygdala, PAG, and hypothalamus (among other regions), which are interconnected subcortical systems for generating threat responses and physiological stress reactions. Much of the human work on stress has focused on the amygdala, but its role is variable. In some work, amygdala activity relates to greater stressor-evoked cardiovascular reactivity (Gianaros & Sheu, 2009). In other work, amygdala activity is not increased by stress or related to cardiovascular reactivity or self-reported threat; rather, correlated mPFC-PAG activity predicts stressor-evoked cardiovascular reactivity, and mPFC activity predicts experienced threat (Wager, Van Ast, et al., 2009; Wager, Waugh, et al., 2009).
Such discordant findings create a discrepancy between studies emphasizing amygdala vs. PAG in stressor-evoked cardiovascular reactivity. This paradox may be addressed by considering the related functional roles of amygdala and PAG suggested by animal studies (McNally, Johansen, & Blair, 2011). Specifically, the PAG appears to support encoding primary aversive events (e.g., negative reinforcers) and providing teaching signals to the amygdala, likely in the form of prediction errors (McNally et al., 2011). The PAG also organizes patterned autonomic and behavioral responses to aversive events (Bandler et al., 2000). In contrast, the amygdala may be crucial for learning associations between aversive events (signaled in part by PAG) and other sensory cues (LeDoux, 2012). In this regard, the amygdala may trigger threat or stress responses based on learned cues, but it may not be critical for the experience of threat or fear itself. Thus, stress studies emphasizing sensory cues or task-related cognitive stimuli under threat (Gianaros et al., 2008) may increase or decrease amygdala activity, but studies in which the stressor is internal (e.g., in thought or memory) may not do so (Wager, Waugh, et al., 2009). Accordingly, existing findings demonstrate that the amygdala is not necessary for processing all threats or for generating stress-related physiological responses via cortical input. Instead, other systems, such as the PAG, may be equally important for such functions under some contexts. Moreover, we note that the subcortical circuits involved in threat appraisals and linked visceral control functions are not limited to the PAG and amygdala; however, our understanding of other such circuits in humans has been limited by conventional neuroimaging acquisition and analysis methods that are not optimized for studying small subcortical brain structures (see Table).
Closing the Loop: Viscerosensory Inputs To Appraisal Systems
Stressor-evoked cardiovascular reactions may arise from cortical appraisal systems that alter peripheral physiology via interactions with subcortical systems. However, we have not yet developed a clear understanding of how appraisal-based information processing relates to visceral control loops of the body, which more directly govern peripheral physiology to influence disease vulnerability. Visceral control loops are homeostatic mechanisms that regulate our internal organs and peripheral physiological systems, such as the cardiovascular system. These mechanisms are comprised of bidirectional communication pathways, in which events at one end of the loop can change events at the other end via feed-forward (i.e., visceromotor) and feedback (i.e., viscerosensory) signals. Findings on cardiovascular reactivity above, for example, can be considered from this perspective; namely, that brain appraisal systems generate and regulate health-relevant physiological stress responses by altering visceral control loops of the body.
To elaborate, heart rate and blood pressure are controlled by a visceral control loop, called the baroreflex, which maintains the momentary circulatory and metabolic requirements of the body. Specifically, the baroreflex governs blood pressure homeostasis on a heartbeat-to-heartbeat basis via (1) a viscerosensory limb that detects changes in blood pressure and (2) a visceromotor limb that adjusts sympathetic and parasympathetic outflow to the heart and vasculature to control heart rate, the force with which the heart beats, and the caliber (degree of constriction) of blood vessels (Fig. 3). Intriguingly, however, psychological stressors interrupt this visceral control loop, which is usually defined by the reciprocal coupling of heart rate to blood pressure (i.e., when blood pressure increases, heart rate decreases in a homeostatic fashion). This interruption results from a suppression of the baroreflex (Gianaros & Sheu, 2009). But, how is this possible? What is the basis by which a psychological event can suppress cardiovascular homeostasis to permit blood pressure and heart rate to rise together instead of relating to each other in their typical ‘push-pull’, control-loop fashion?
Answers to these questions were first suggested by animal work showing that mPFC areas that presumably mediate threat appraisals in homologue areas of the human brain send anatomical projections to subcortical areas that inhibit the baroreflex, effectively taking this control loop offline (Resstel & Correa, 2006; Verberne & Owens, 1998). This animal work was only recently extended to humans in a neuroimaging study showing that the degree of stressor-evoked baroreflex suppression related directly to the degree of stressor-evoked changes in cortical and subcortical areas previously linked to heart rate and blood pressure reactivity: the mPFC, amygdala, and PAG (Gianaros, Onyewuenyi, Sheu, Christie, & Critchley, 2012). Another area identified was the insula (see Fig. 3), a heterogeneous cortical region important for cardiovascular control and integrating multiple sources of viscerosensory information with the appraisal of personally-relevant stimuli (Craig, 2002; Critchley, 2005). Mechanistically, the brain systems engaged by psychological stressors could thus generate health-relevant physiological stress responses (e.g., coupled rises in blood pressure and heart rate) by affecting visceral control loops through appraisal-based, ‘top-down’ pathways.
In addition to ‘top-down’ pathways, it is clear that the magnitude of cardiovascular stress reactions in the body (e.g., the extent of a rise in blood pressure) can be encoded in the brain by ‘bottom-up’ viscerosensory pathways (Fig. 3). Thus, bodily stress reactions do not unfold in a vacuum: they are an embodied source of sensory information that can come to affect the same brain areas that initiated the reactions in the first place—thus closing a brain-body feedback loop (Berntson, Sarter, & Cacioppo, 2003; Critchley & Harrison, 2013). This sensory information may serve many functions. For example, it may represent a negative feedback or ‘teaching’ signal that delimits the magnitude or further duration of a cardiovascular stress reaction. Exaggerated or prolonged cardiovascular stress reactions that predict poor health may thus be viewed as arising from a maladaptive interplay between a specific visceral control loop and brain appraisal systems. Further, such sensory information could plausibly bias the encoding or appraisal of future stressors or otherwise personally-relevant stimuli. This bias could result from the recursive or experience-dependent updating of the meaning we ascribe to appraised events based on ongoing or prior visceral (e.g., cardiovascular) reactions. In view of these issues, our understanding of the brain systems that mediate stress appraisals and generate physiological stress responses linked to health will remain incomplete until we better account for ‘bottom-up’ influences of viscerosensory information within visceral control loops.
Summary
Evidence from health psychology and behavioral medicine has linked the two end-points of the psychological stress-health continuum. Emerging neuroimaging evidence has begun mapping the brain systems that generate states of psychological stress and link these states with visceromotor (feed-forward) and viscerosensory (feedback) mechanisms important for health. Although questions remain (Table 1), this brain-body evidence may increase our ability to predict individual differences in disease risk and provide new mechanistic information on the possible origins of stress-related disease vulnerability.
Recommended Reading.
Gianaros, P. J., & Sheu, L. K. (2009). (See References). A meta-analysis of neuroimaging studies of stressor-evoked blood pressure reactivity and a discussion of mechanisms linking cardiovascular reactivity to disease risk.
Muscatell, K. A., & Eisenberger, N. I. (2012). (See References). A recent review of the neuroimaging literature on stressor-evoked changes in autonomic, neuroendocrine, and immune systems.
Pruessner, J. C., Dedovic, K., Pruessner, M., Lord, C., Buss, C., Collins, L., Dagher, A., Lupien, S. J. (2010). Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations. Psychoneuroendocrinology, 35, 179–91. Provides a conceptual and methodological review of neuroimaging research on the functional and structural neural correlates of acute and chronic stress, as well as an overview of work on the central regulation of the HPA axis, which was not covered here.
Roy, M., Shohamy, D., Wager, T. D. (2012). (See References). A review and meta-analysis focusing on the role of the ventromedial prefrontal cortex as a hub that links meaningful and self-relevant conceptual information about situations, contexts, and other constructs with brainstem areas important for visceral control.
Acknowledgments
We thank J. Richard Jennings, Natasha Tokowicz, Anna Marsland, and J. David Creswell for their comments. This work was supported by National Institutes of Health R01 grants HL089850 to Peter Gianaros and MH076136 to Tor Wager.
Footnotes
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to their authorship or the publication of this article.
Contributor Information
Peter J. Gianaros, Department of Psychology, Center for the Neural Basis of Cognition, University of Pittsburgh
Tor D. Wager, Departments of Psychology and Neuroscience, University of Colorado
References
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Research Bulletin. 2000;53(1):95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: The basal forebrain cholinergic link. Behavioral Brain Research. 1998;94(2):225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. European Journal of Neuroscience. 2003;18(8):2103–2109. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- Brindle RC, Ginty AT, Phillips AC, Carroll D. A tale of two mechanisms: A meta-analytic approach toward understanding the autonomic basis of cardiovascular reactivity to acute psychological stress. Psychophysiology. 2014;51(10):964–976. doi: 10.1111/psyp.12248. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The wisdome of the body. New York: WW Norton & Company; 1932. [Google Scholar]
- Carroll D, Ginty AT, Der G, Hunt K, Benzeval M, Phillips AC. Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology. 2012;49(10):1444–1448. doi: 10.1111/j.1469-8986.2012.01463.x. [DOI] [PubMed] [Google Scholar]
- Charvat J, Dell P, Folkow B. Mental Factors and Cardiovascular Diseases. Cardiologia. 1964;44:124–141. doi: 10.1159/000167892. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77(4):624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126(Pt 10):2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Nagai Y, Gray MA, Mathias CJ. Dissecting axes of autonomic control in humans: Insights from neuroimaging. Autonomic Neuroscience: Casic & Clinical. 2011;161(1–2):34–42. doi: 10.1016/j.autneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology. 2005;492(2):145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Human Brain Mapping. 2012;33:1700–1716. doi: 10.1002/hbm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Salomon K, Zhou F, Owens JF, Edmundowicz D, Kuller LH, et al. A greater reduction in high-frequency heart rate variability to a psychological stressor is associated with subclinical coronary and aortic calcification in postmenopausal women. Psychosomatic Medicine. 2005;67:553–560. doi: 10.1097/01.psy.0000170335.92770.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK. A review of neuroimaing studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. NeuroImage. 2009;47:922–936. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarck TW, Shiffman S, Sutton-Tyrrell K, Muldoon MF, Tepper P. Daily psychological demands are associated with 6-year progression of carotid artery atherosclerosis: the Pittsburgh Healthy Heart Project. Psychosomatic Medicine. 2012;74(4):432–439. doi: 10.1097/PSY.0b013e3182572599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Waldstein SR, Chesney MA, Jennings JR, Lovallo WR, Kozel PJ, et al. The rebirth of neuroscience in psychosomatic medicine, part I: historical context, methods and relevant basic science. Psychosomatic Medicine. 2009;71:117–134. doi: 10.1097/PSY.0b013e31819783be. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Psychological stress and the coping process. New York: McGraw-Hill; 1966. [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosomatic Medicine. 2003;65(1):36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trends in Neuroscience. 2011;34(6):283–292. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology. 2008;4:33–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Eisenberger NI. A Social Neuroscience Perspective on Stress and Health. Social and Personality Psychology Compass. 2012;6(12):890–904. doi: 10.1111/j.1751-9004.2012.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular Psychophysiology: a Perspective. New York, NY: Plenum Press; 1981. [Google Scholar]
- Öngür D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opininon in Neurobiology. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Correa FM. Involvement of the medial prefrontal cortex in central cardiovascular modulation in the rat. Autonomic Neuroscience. 2006;126–127:130–138. doi: 10.1016/j.autneu.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Science. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne AJM, Owens NC. Cortical modulation of the cardiovascular system. Progress in Neurobiology. 1998;54(2):149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wager TD, Van Ast V, Hughes B, Davidson M, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, Part II: prefrontal- subcortical pathways and relationship with anxiety. NeuroImage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist MA, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat, Part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


