Abstract
β1 integrin adhesion is believed to require binding of talins and kindlins to the membrane proximal and distal NPxY motifs of the β1 cytoplasmic tail, respectively. To test this hypothesis we substituted the membrane proximal and distal tyrosines (Y) of the β1 tail with alanine (A) residues (β1 Y783A; β1 Y795A) in the germline of mice. We report that β1 Y783A or β1 Y795A substitutions blocked talin or kindlin binding, respectively, and led to β1 null-like peri-implantation lethality. Expression of β1 Y783A or β1 Y795A in the epidermis, however, resulted in skin blister and hair follicle phenotypes that were considerably milder than those observed with β1 integrin gene deletion or a β1 double Y-to-A substitution (β1 YY783/795AA). In culture, defects in adhesion, spreading and migration were more severe with the β1 Y783A than with the β1 Y795A substitution despite markedly reduced β1 Y795A integrin surface levels due to diminished protein stability. We conclude that regulation of β1 integrin adhesion through talins and kindlins may differ substantially between stably adherent keratinocytes and cells of the developing embryo and that β1 cytoplasmic NPxY motifs contribute individually and independent of each other to β1 function in keratinocytes.
INTRODUCTION
Integrins are adhesion receptors that bind extracellular matrix proteins and counter receptors. When integrins bind their ligand they cluster and recruit a large number of adaptor and signaling proteins to their cytoplasmic domain to finally form cell-extracellular matrix anchoring structures called focal adhesions (FAs). FAs provide a large signaling platform, which decodes the physical and chemical qualities of the extracellular environment critically required for orchestrating tissue development and homeostasis (Legate et al., 2009; Moser et al., 2009). All integrins are composed of an α and a β subunit. The β1 integrin subunit is ubiquitously expressed and dimerizes with 12 integrin α chains (Meves et al., 2009). Not surprisingly, deletion of the β1 integrin gene in mice is embryonic lethal at peri-implantation (Fassler and Meyer, 1995).
The β1 integrin cytoplasmic tail contains two key adaptor protein binding sites; the membrane proximal W(x)4NPIY motif that binds talins, and the membrane distal TT(x)2NPKY motif that binds kindlins (Meves et al., 2011; Moser et al., 2008). Binding of talins to the membrane proximal motif induces conformational changes in the extracellular portion of β1 integrin that increases its affinity for extracellular matrix (Wegener et al., 2007; Ye et al., 2010). It is therefore believed that the interaction of the β1 tail with talin preceeds integrin binding to extracellular matrix in a process referred to as integrin activation or inside-out signaling. More recently, we reported that integrin activation in platelets, leukocytes and epithelial cells such as primitive endoderm and intestinal epithelial cells requires not only the binding of talins but also of kindlins to the membrane distal NPxY motif (Moser et al., 2008). These findings were in contrast to reports attributing no or only a minor role to kindlins in integrin inside-out signaling (Ye et al., 2010).
To directly test the role of talin and kindlin binding to the β1 integrin tail in vivo, we compared phenotypes in mice and cells carrying a Y-to-A substitution in the membrane proximal (β1 Y783A) or distal (β1 Y795A) β1 NPxY motif. Both mutations lead to peri-implantation lethality resembling the β1 null phenotype. Interestingly, however, targeted expression of single Y-to-A mutations in the epidermis leads to a phenotype that is significantly milder than the β1-null phenotype, indicating residual activity of the β1 Y783A and β1 Y795A integrins. A β1 null-like phenotype is only achieved with the simultaneous mutation of both tyrosine residues (β1 YY783/Y795AA). These findings indicate that the functional consequences of perturbed talin and kindlin binding to β1 integrin NPxY motifs differ between cell types.
RESULTS
Single β1 Y-to-A mutations result in peri-implantation lethality
Mice lacking β1 integrin expression or carrying homozygous β1 YYAA mutations die at the peri-implantation stage (Chen et al., 2006; Czuchra et al., 2006; Fassler and Meyer, 1995). To assess the in vivo phenotype of single β1 cytoplasmic Y-to-A mutations and to analyze the role of talin and kindlin binding to the β1 integrin cytoplasmic tail we generated mice with non-conservative tyrosine (Y) to alanine (A) mutations in the membrane proximal and distal β1 integrin NPxY motifs (β1 Y783A; β1 Y795A). The mutant knock-in (KI) alleles (KIneo+; Figure 1a) were comfirmed by Southern blotting and PCR on genomic DNA derived from tail biopsies (Figure 1b, c). Deletor-Cre-mediated removal of the neomycin cassette yielded mice with heterozygous β1 Y783A or Y795A mutations, respectively. They were healthy and fertile. Progeny of heterozygous crossings yielded wild-type and heterozygous but no homozygous offspring (Figure 1d).
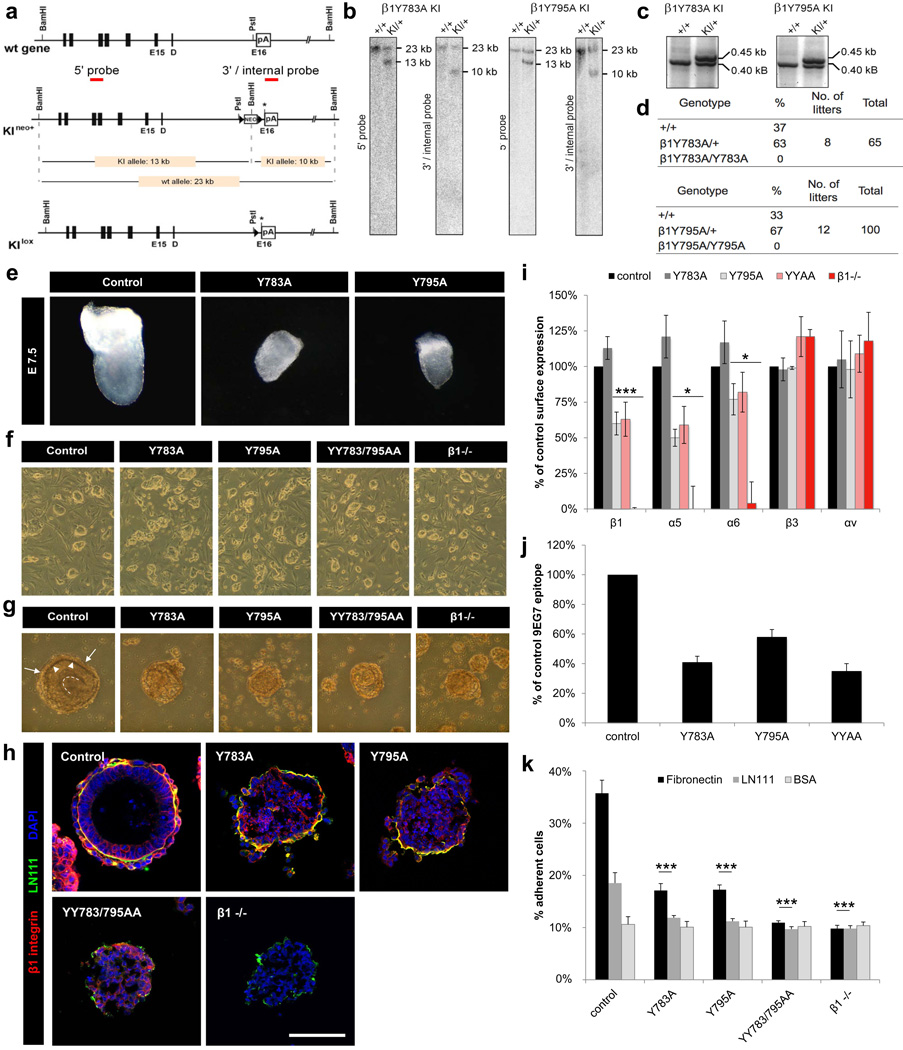
Figure 1. β1 Y-to-A mutations cause a β1-null-like phenotype in ES cells.
(a) Partial map of β1 wild-type (wt) and knock-in (KI) alleles before (KIneo+) and after (KIlox) neo deletion through Cre. Asterisk: site of point mutagenesis. Triangles: loxP sites. (b) Southern blotting identified homologous recombination. (c) PCR genotyping of KIlox mice using loxP site flanking primers. (d) Numbers and genotypes of offspring from heterozygous crossings. (e) Bright field images of E7.5 embryos. (f) ES cell colonies on feeder cells. (g–h) Bright field and immunofluorescence images of EBs on day 5 of suspension culture. (g) Arrows: endoderm cells. Arrowheads: BM. Dotted line: central cavity lining. (h) EBs were stained with antibodies against β1 integrin (red) and Laminin 111 (green) and nuclei were counterstained with DAPI (blue). Scale bar=100µm. (i) Expression of integrin subunits on ES cells by FACS (mean ± SD; n=4; *P<0.05, ***P<0.0001 vs. control). (j) Quantification of the 9EG7 epitope by FACS (mean ± SD; n=4). (k) Quantification of cell adhesion (mean ± SD; n=4; ***P<0.0001 vs. control). LN111, Laminin 111; BSA, bovine serum albumin.
To assess the embryonic defects of mice with single β1 cytoplasmic Y-to-A mutations, we performed timed heterozygous matings and analyzed the gross morphology of embryos at embryonic day (E) 7.5. Compared to wild-type embryos, β1 Y783A and Y795A embryos were severely malformed indicating that the embryos die at the peri-implantation stage (Figure 1e). To better define the cause of death during early development, we established ES cell cultures from homozygous β1 Y783A and Y795A blastocysts. Similar to β1-null and β1 YY783/795AA (YYAA) ES cells, single β1 Y-to-A ES cells adhered less to feeder cells (Figure 1f). We then generated embryoid bodies (EBs) from wild-type or mutant ES cells to further study peri-implantation development (Montanez et al., 2007). The formation of wild-type EBs from an aggregate of ES cells followed a series of events starting with the formation of an outer layer of endoderm cells which secret and assemble a laminin-rich basement membrane, followed by the conversion of the undifferentiated core into a layer of pseudo-stratified primitive ectoderm and a central cavity on day 4 to 6 (Figure 1g) (Böttcher et al., 2012). In contrast, single β1 Y-to-A and β1 YYAA EBs formed compact aggregates with significant detachment of the endoderm cell layer, abnormally assembled and often discontinuous basement membrane and absent cavity formation, which resembled the defects of β1-null EBs (Figure 1g, h) (Li et al., 2002).
Fluorescence-activated cell sorting (FACS) analysis revealed an apparently normal expression of β1 integrin on ES cells carrying the membrane proximal β1 Y783A mutation (Figure 1i). As expected, β1 integrin surface levels on ES cells carrying the membrane distal Y795A mutation were significantly reduced (Figure 1i) due to the impaired recruitment of SNX17 to the β1 Y795A tails in early and recycling endosomes, which prevents lysosomal β1 degradation (Böttcher et al., 2012). The β1 heterodimerization partners α5 and α6 integrin were also reduced in β1 Y795A ES cells, while αvβ3 integrin surface levels were normal (Figure 1i). As expected, all β1 Y-to-A mutated integrins had reduced extracellular ligand binding activity as assessed by the reduced availability of 9EG7 epitope during FACS (Figure 1j) and reduced adhesion to fibronectin and laminin (LN) 111 (Figure 1k).
Compound heterozygous β1Y783A/Y795A mice die at peri-implantation
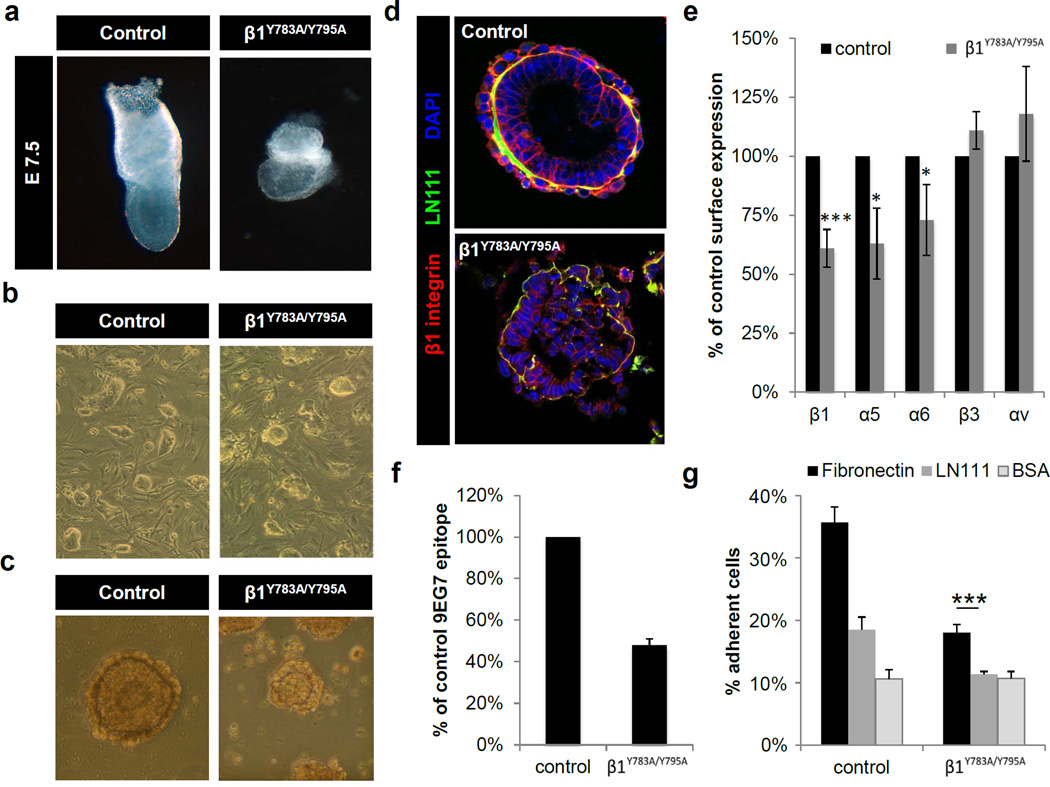
The availability of the β1 Y783A and Y795A mouse strains allowed the generation of compound heterozygous (β1Y783A/Y795A) animals that enable kindlin binding to the β1 Y783A and talin binding to the β1 Y795A allele and thus, testing whether talin and kindlin cooperate with each other in a trans configuration (Moser et al., 2009). Intercrosses of the β1 Y783A strain with the Y795A strain revealed no live offspring. The dissection of decidua chambers at different stages of pregnancy showed that the β1Y783A/Y795A compound mice died at the peri-implantation stage with a phenotype that resembled the homozygous β1 Y783A or β1 Y795A mutations (Figure 2a). β1Y783A/Y795A ES cells were less adhesive compare to wild-type (Figure 2b) and EBs derived from β1Y783A/Y795A ES cells showed defects similar to β1-null EBs (Figure 2c,d). β1 integrin surface levels (Figure 2e), 9EG7 epitope availability (Figure 2f) and cell adhesion to fibronectin and LN111 (Figure 2g) were reduced to a similar extent as observed with the homozygous β1 Y795A mutation.
Figure 2. Absence of β1 integrin trans activation in compound heterozygous β1Y783A/Y795A embryos.
(a) Bright field images of E7.5 embryos. (b) ES cell colonies on feeder cells. (c–d) Bright field (c) and immunofluorescence images (d) of embryoid bodies on the 5th day of suspension culture. EBs were stained with antibodies against β1 integrin (red) and Laminin 111 (green) and nuclei were counterstained with DAPI (blue). Scale bar=100µm. (e) Expression of integrin subunits on ES cells determined by FACS (mean ± SD; n=4; *P<0.05, ***P<0.0001 vs. control). (f) Integrin activation on ES cells measured by 9EG7 binding (mean ± SD; n=4). (g) Quantification of cell adhesion (***P<0.0001 vs. control). LN111, Laminin 111; BSA, bovine serum albumin.
Single β1 Y-to-A integrin is partially active in keratinocytes
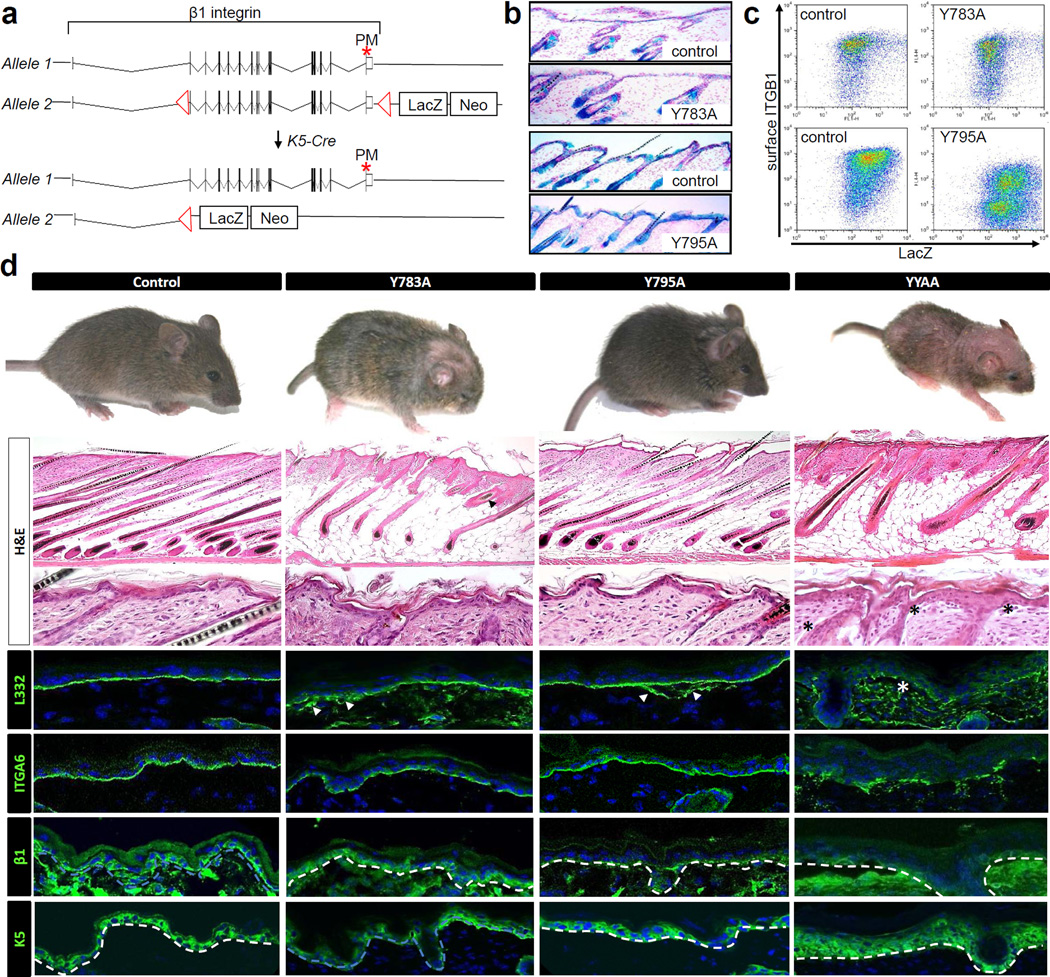
To test whether the β1 Y-to-A mutations also lead to β1-null-like phenotypes in other cell types we decided to express the β1 Y783A or β1 Y795A mutations in the epidermal keratinocytes. Epidermal keratinocytes were chosen because they lack β3 integrin, express low levels of αv integrin and predominantly express β1 integrin. To achieve hemizygous targeted expression of the knock-in alleles in epidermis, we intercrossed a floxed (fl) β1 allele with the β1 Y-to-A alleles or with a non-floxed wild-type β1 gene (control) and ablated the floxed β1 gene using a keratin (K) 5 promoter-driven Cre recombinase (Figure 3a). Since the floxed β1 integrin allele was designed to couple genomic β1 integrin deletion to the activation of a lacZ gene (Brakebusch et al., 2000), successful Cre activity can be readily detected in the epidermis and hair follicles of hemizygous wild-type and β1 Y-to-A skin by immunohistochemistry (Figure 3b) and flow cytometry (Figure 3c). Deletion of homozygous β1fl/fl integrin alleles by K5-Cre (Brakebusch et al., 2000) or hemizygous expression of β1 YYAA in epidermis (Czuchra et al., 2006) leads to a near complete loss of terminal hair in mice and reduced weight gain. In contrast to this marked phenotype, β1 Y783A or β1 Y795A epidermis showed patchy hair loss over the dorsal midline of the skull in β1 Y783A epidermis (Figure 3d) but otherwise no additional defects on gross inspection. This finding suggests that the activities of the mutant integrins are not or only marginally affected by impaired talin or kindlin binding.
Figure 3. Single β1 Y-to-A integrin is partially active in keratinocytes.
(a) Approach for achieving hemizygous β1 Y-to-A integrin expression in epidermis. Exon structure of β1 integrin is depicted. Site of point mutation is highlighted by an asterisk. LoxP sites are shown as triangles. Recombination of β1fl/fl loxP sites by Cre results in LacZ transcription (b and c) Detection of LacZ activity by immunohistochemistry (b) or flow cytometry (c). (d) Gross morphology and histological analysis of mice and epidermis with single or double β1 Y-to-A mutations at postnatal day 14. Dotted lines: dermatoepidermal junction. Asterisks: subepidermal split. White arrow heads: non-linear sub-epidermal LN332 deposition.
To further test whether the β1 Y783A or the β1 Y795A integrins are indeed partially active in epidermis, we analyzed skin histologically (Figure 3d). β1-null epidermis is characterized by subepidermal blistering, patchy deposition of laminin 332 in the papillary dermis, impaired hemidesmosome assembly and dermal inflammatory infiltrates that induce hyperproliferation of basal keratinocytes (Brakebusch et al., 2000). The same phenotype was also seen in β1 YYAA epidermis (Figure 3d) (Czuchra et al., 2006). In contrast, single β1 Y-to-A epidermis was less affected. We observed linear deposition of α6 integrin at the dermato-epidermal junction, demonstrating proper localization of hemidesmosomes, and normal proliferation in the skin as indicated by the single layer of K5 expressing basal keratinocytes and normal numbers of Ki67-positive cells (wild-type: 6 ± 3; β1 Y783A: 8 ± 3; β1 Y795A: 8 ± 3; β1 YYAA: 18 ± 1 Ki67 positive cells per high-powered field) Laminin 332 deposition was focally shaggy and abnormal, however, not to the extent observed with β1 YYAA epidermis. Interestingly, we failed to observe subepidermal blistering indicating the ability of basal keratinocytes to firmly adhere to the underlying basement membrane. As expected from the focal hair loss in vivo, we saw impaired hair follicle morphogenesis in β1 Y783A but not β1 Y795A skin; enlarged hair follicle stumps were seen intermixed with normally shaped thin hair follicles extending deep into the subcutaneous fat. In β1 YYAA as well as β1-null epidermis, all hair follicles were enlarged and abnormal. In summary these findings suggest that keratinocytes expressing either the β1 Y783A or the β1 Y795A substitution must possess residual β1 activity.
Distinct consequences of β1 Y783A and β1 Y795A mutations on β1 function in keratinocytes
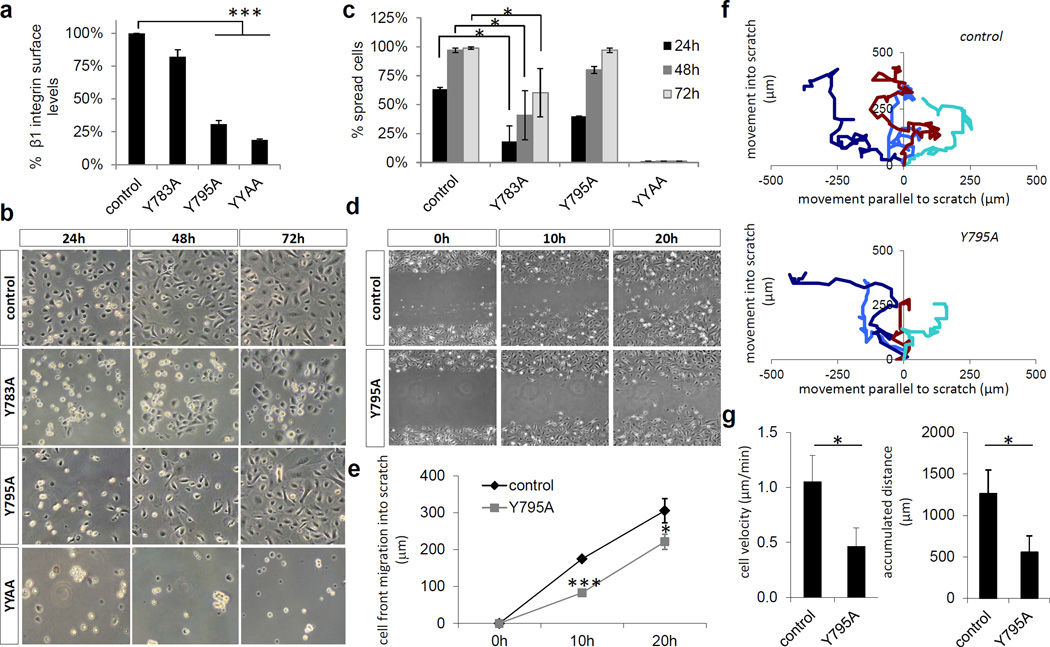
The subtle defects of β1 Y783A or the β1 Y795A epidermis in vivo prompted us to analyze β1 integrin functions ex vivo. In line with the findings from the mutant ES cells, flow cytometry revealed normal expression levels of β1 Y783A and markedly reduced levels of β1 Y795A and β1 YYAA on freshly isolated primary keratinocytes (Figure 4a). Despite the normal β1 Y783A levels keratinocytes exhibited defects in adhesion (Figure 4b) and spreading that were more pronounced compared with β1 Y795A keratinocytes (Figure 4c). β1 YYAA keratinocytes did not adhere (Figure 4b) (Czuchra et al., 2006). β1 Y783A keratinocytes did not grow into confluent monolayers but instead become large and flat and rapidly initiated terminal differentiation (Figure 4b). In contrast, β1 Y795A keratinocytes underwent normal spreading, proliferated and consistently formed confluent monolayer in culture. Scratch wounding assays demonstrated that β1 Y795A keratinocytes were able to close the in vitro wound, although slower than wild-type (Figure 4d and e). Single-cell tracking revealed that β1 Y795A keratinocytes migrated directionally (Figure 4f) but with reduced speed when compared to wild-type (Figure 4g). Since β1 Y783A keratinocytes were unable to form monolayers in vitro we were unable to perform scratch assays.
Figure 4. Adhesion and migration of β1 Y-to-A keratinocytes.
(a) Epidermis-derived single cell suspensions of normal and β1 Y-to-A epidermis were assayed for β1 integrin surface expression (mean ± SD; n=4; ***P<0.0001 vs. control). (b) Adhesion of normal and β1 Y-to-A keratinocytes to fibronectin and collagen coated tissue culture plastic over the indicated time period. (c) Spreading of normal and β1 Y-to-A keratinocytes on fibronectin and collagen coated tissue culture plastic (mean ± SD; n=3; *P<0.05 vs. control). (d) Scratch wounding of a confluent monolayer of normal and β1 Y795A keratinocytes. (e) Quantification of cell front migration into a scratch wound (mean ± SD; n=3; *P<0.05, ***P<0.0001 vs. control). (f) Assessment of migratory directionality. (g) Quantification of keratinocyte migratory velocity and accumulated distance (mean ± SD; n=3; *P<0.05 vs. control).
In summary, the β1 Y783A mutation allows normal surface expression but impairs keratinocyte adhesion, spreading and proliferation. The β1 Y795A mutation reduces β1 integrin surface expression and migration speed but allows normal adhesion, spreading and proliferation of keratinocyte. The β1 YYAA mutation abrogates all β1 integrin functions.
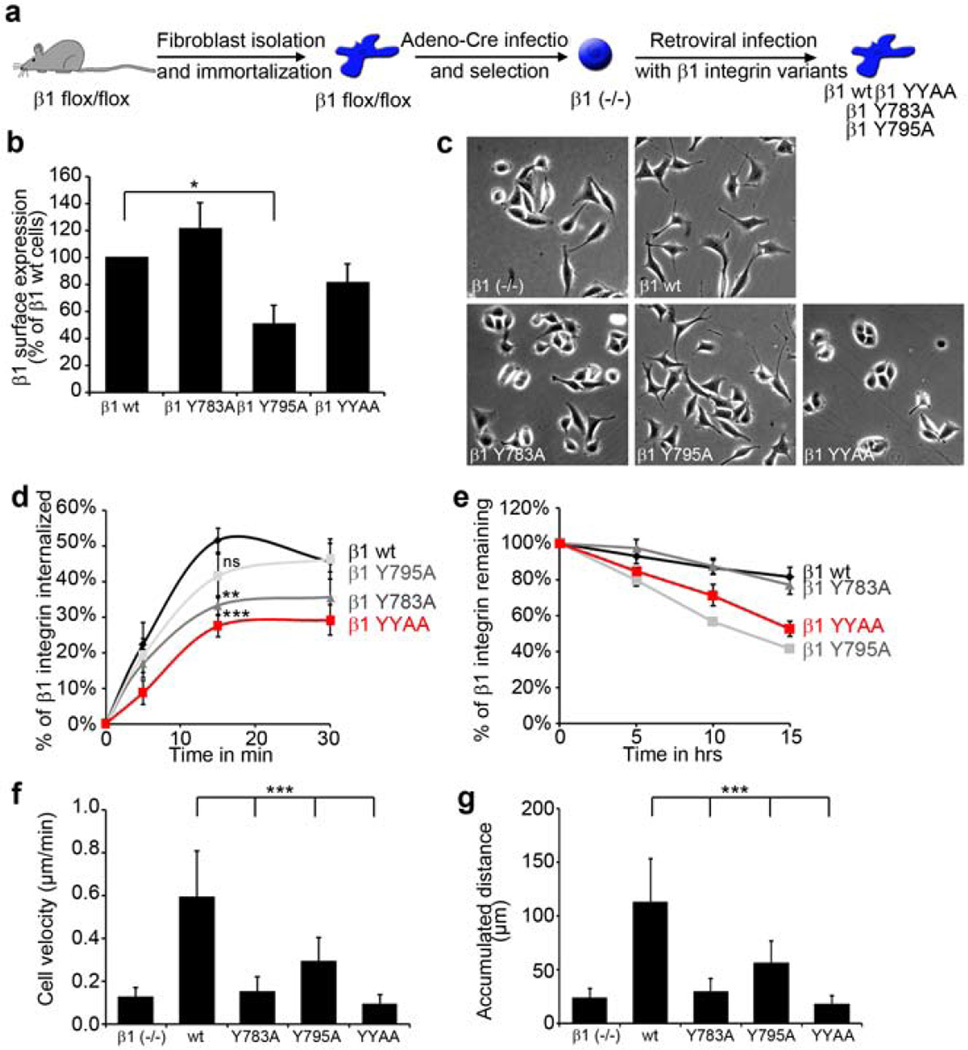
Distinct consequences of β1 Y783A and β1 Y795A mutations on β1 function in fibroblasts
β1 Y783A and β1 YYAA keratinocytes poorly adhered and did not grow in culture due to terminal differentiation. To further characterize the cellular phenotype of single and double β1 Y-to-A mutations, we decided to reconstitute β1-null fibroblasts with wild-type, β1 Y783A, β1 Y795A or β1 YYAA integrins by retroviral cDNA transduction (Figure 5a) (Böttcher et al., 2012). In contrast to keratinocytes, fibroblasts express β3 integrin, which partially compensates for the loss in β1 integrin-mediated adhesion. FACS analysis showed reduced β1 integrin surface levels in β1 Y795A cells while the levels of other β1 Y-to-A variants were comparable to wild-type β1 integrin surface levels (Figure 5b). Similar to primary keratinocytes, β1 Y795A and wild-type fibroblasts were well-spread whereas β1 Y783A and β1 YYAA fibroblasts resembled the more rounded β1-null cells (Figure 5c). Interestingly, Y-to-A mutation in the Y783A motif significantly impaired internalization of cell surface β1 integrin (Figure 5d), while β1 integrin stability was only reduced after Y-to-A mutation of the Y795A motif (Figure 5e). In single-cell migration assays on fibronectin all β1 Y-to-A mutations exhibited reduced cell velocity (Figure 5f and g). However, the velocity defect was least pronounced with the membrane distal β1 Y795A mutation.
Figure 5. Effect of β1 Y-to-A mutations on β1 integrin stability, internalization and migration.
(a) Scheme depicting the generation of wild-type (wt) and β1 Y-to-A expressing fibroblasts. (b) β1 integrin surface levels as determined by FACS (mean ± SD; n=3; *P<0.05). (c) Phase contrast images of β1 integrin reconstituted fibroblasts. (d) Quantification of cell surface β1 integrin internalization (mean ± s.e.m.; n=5; **P<0.005; ***P<0.001 vs. control). ns; not significant. (e) Quantification of cell surface β1 integrin stability (mean ± SD; n=3). (f–g) Quantification of fibroblast velocity (f) and accumulated distance (g) (mean ± SD; n=30 cells from three movies; ***P<0.0001 vs. control).
In summary, the phenotypes of the β1 Y-to-A mutations were similar in fibroblast and keratinocytes. Specifically, β1 Y783A mutations had a greater impact on spreading and migration than β1 Y795A mutations. The latter however significantly impaired integrin half-life.
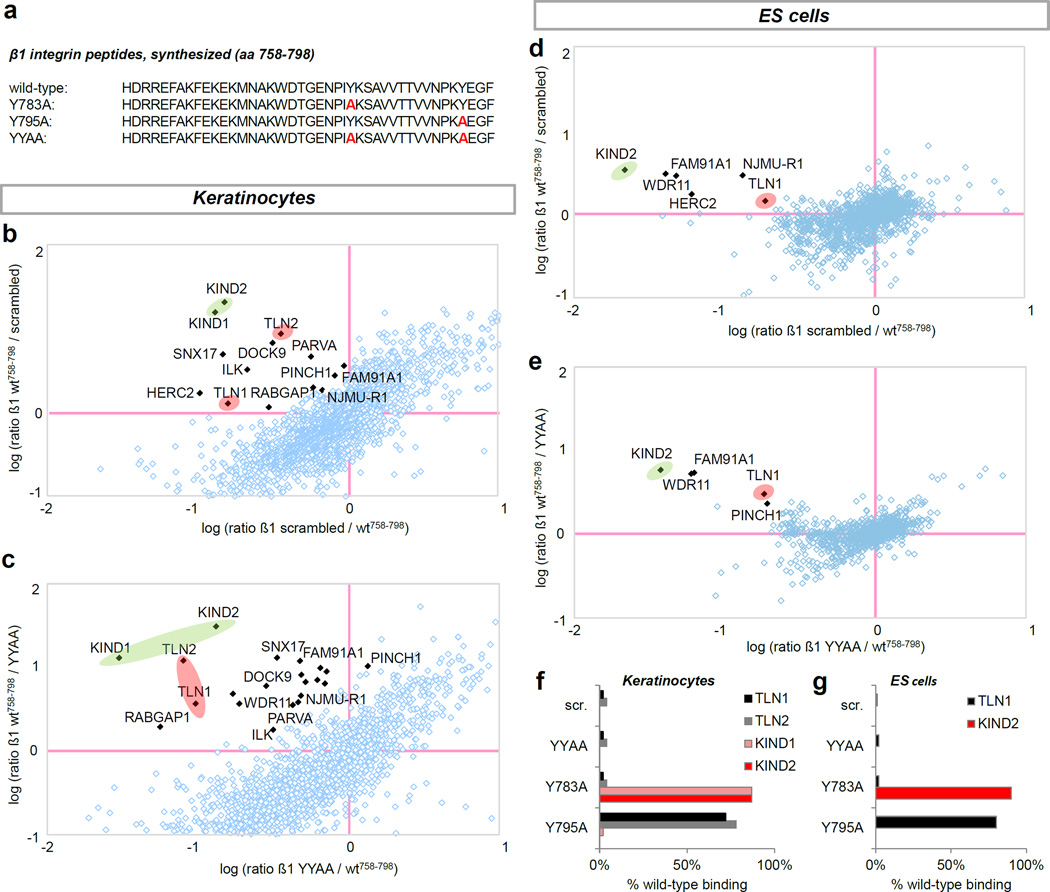
Talin and kindlin recruitment is affected by the β1 cytoplasmic Y-to-A mutations
The subtle defects of the of the β1 Y783A or the β1 Y795A epidermis in vivo suggest a less prominent role for talin and kindlin binding to the β1 tails than in ES cells (Montanez et al., 2008). Therefore, we tested to which degree β1 NPxY motifs were disrupted by single (β1 Y783A, β1 Y795A) or double (β1 YYAA) β1 Y-to-A mutations in keratinocytes versus ES cells by performing pulldown experiments with synthesized peptides corresponding to wild-type and β1 Y-to-A mutated β1 integrin cytoplasmic tails using SILAC-labeled ES cell and keratinocyte protein lysates followed by quantification of precipitated proteins using mass spectrometry (Meves et al., 2011) (Figure 6a). We found that the β1 YYAA peptide completely lost the ability to recruit proteins from both keratinocyte and ES cell lysates when compared to a scrambled peptide sequence (Figure 6b–e). Specifically, binding of talins and kindlins was completely abolished in keratinocytes (Figure 6c) and ES cells (Figure 6e). Selective disruption of single NPxY motifs by β1 Y-to-A substitutions only prevented binding of a specific subset of proteins, most notably talins or kindlins to the membrane proximal or distal NPxY motifs, respectively (Figure 6f and g). This observation was made with keratinocyte (Mathew et al., 2012) and ES cell lysates.
Figure 6. β1 integrin cytoplasmic Y-to-A mutations impair protein recruitment in keratinocytes and ES cells.
(a) Amino acid sequence of synthesized β1 integrin peptides. (b–e) Loss in protein binding vs. wild-type peptide in keratinocytes using scrambled (b) and β1 YYAA peptides (c). Loss in protein binding vs. wild-type peptide in ES cells using scrambled (d) and β1 YYAA peptides (e). Scatter plots highlights kindlins (red circles) and talins (green circles). (f–g) Quantification of talin and kindlin binding to β1 Y-to-A peptides relative to wild-type using keratinocyte (f) or ES cell lysates (g).
DISCUSSION
Single disrupted β1 cytoplasmic NPxY motifs have been predicted to prevent talin and kindlin binding to β1 tails and therefore, to result in β1 integrin loss-of-function (Moser et al., 2009). Consistent with established models of integrin activation (Ye et al., 2012), we found that both single β1 Y-to-A mutations were early embryonic lethal and resulted in β1-null-like in vivo phenotypes and ex vivo EB defects. The same observations were made for compound heterozygous β1Y783A/Y795A mice, supporting a role for both adaptor proteins on the same β1 integrin tail and thus, lack of β1 integrin trans activation through talins and kindlins (Moser et al., 2009). Unexpectedly, the epidermis exhibited a β1-null phenotype only when both β1 NPxY motifs were disrupted, while single Y-to-A mutations resulted in subtle defects. Mice harboring single β1 Y-to-A mutations in either the talin or kindlin binding sites retained a fully attached and normally differentiated epidermis with minor abnormalities in basement membrane structure, indicating only minor impairment of β1 integrin function.
Talin binding has long been viewed as the ‘on-switch’ for integrins by inducing a high affinity state for extracellular ligand (Tadokoro et al., 2003; Ye et al., 2010). Absence of this interaction was thought to yield integrins unable to adhere and signal. Our study, however, shows that models of β1 integrin regulation that predict activity based on talin recruitment are oversimplified. It becomes increasingly clear that the regulation of integrin activity is multi-facetted. In addition to talins, kindlins are required for affinity regulation in cells that need rapid integrin adhesion (Montanez et al., 2008; Moser et al., 2008; Ussar et al., 2008). Other mechanisms such as mechanical force (Friedland et al., 2009; Schiller et al., 2011; Shi and Boettiger, 2003), clustering (Roca-Cusachs et al., 2009), chemical modifications (Isaji et al., 2010), compartmentalization (Larkin et al., 2009), trafficking (Margadant et al., 2011) and stability (Böttcher et al., 2012) exert important additional spatiotemporal control of integrin functions. It appears likely that the latter become particularly important to cells that do not require rapid regulation of adhesion. In contrast to platelets or leukocytes, basal keratinocytes are stably exposed to ligand of the basement membrane, and integrin-extracellular matrix bonds may form and dissolve continuously without affinity modulation by adaptor proteins (Boettiger, 2012). Stable adhesions may form primarily as a consequence of adhesion strengthening, a process that involves mechanical force (Schiller et al., 2011) or clustering of integrins (Paszek et al., 2009). These processes also require adaptor protein binding to β1 integrin NPxY motifs (Feng et al., 2012; Zhang et al., 2008). However, in contrast to affinity modulation, disruption of either β1 NPxY is insufficient to block their roles completely.
While effective affinity modulation requires binding of talins and kindlins, e.g. in platelets (Moser et al., 2008) or ES cells (Montanez et al., 2008), stabilization of the β1 integrin-ligand interaction in cells that are continuously adhere to the extracellular matrix such as keratinocytes occurs, at least to a certain extent, even in the absence of direct talin or kindlin binding to the β1 integrin cytoplasmic domain. While both β1 NPxY motives have unique properties such as the regulation of β1 protein stability via the distal NPxY, each β1 NPxY motif couples to the actin cytoskeleton through its respective focal adhesion-based adaptor protein talin or kindlin, and thus may be used independently to strengthen the integrin-ligand interaction through catch bonds, i.e. integrin-ligand bonds that increase in stability and lifetime through acto-myosin-mediated pulling (Boettiger, 2012). Hence, deletion of a single NPxY motif would not eliminate but only reduce the ability of a cell to regulate integrin adhesion through force. In contrast, deletion of both NPxY motifs would eliminate all β1 integrin adhesion, as was found in this study.
In summary, β1 integrin adhesion in the absence of either talin or kindlin binding is possible but significantly delayed and functionally compromised. While epidermis is well equipped to compensate for some of the consequences of deficient β1 integrin function, e.g. through the hemidesmosomal α6β4 integrin, other types of adhesion receptors such as dystroglycans, or other yet unknown cell-type specific adaptor proteins, this may not be the case for the developing embryo, which depends on the mechanical and signaling support of β1 integrin. In addition, the developing embryo is a rapidly evolving organism that likely needs to regulate adhesion quickly and may therefore depend on β1 integrin affinity regulation.
MATERIALS AND METHODS
Generation of mice
Mice with β1 YYAA and β1 Y795A mutations were reported previously (Böttcher et al., 2012; Czuchra et al., 2006). Mouse genomic DNA used to generate the targeting vector for the β1 Y783A knock-in and the targeting strategy were as previously described (Czuchra et al., 2006; Fassler and Meyer, 1995). Upon germline transmission, mutant mice were intercrossed with deleter-Cre transgenic mice (Czuchra et al., 2006) to remove the neomycin cassette. Compound heterozygous β1Y783A/Y795A ES cells and embryos were generated by inter-crossing heterozygous β1 Y783A with β1 Y795A animals. All animal studies were approved by the Regierung von Oberbayern.
Flow cytometry
Flow cytometry of freshly isolated keratinocytes and ES cells was as previously described (Böttcher et al., 2012; Czuchra et al., 2006). The following antibodies were used: β1 integrin PE (102207, HMB1-1, BioLegend; 1:400), β1 integrin 9EG7 (550531, 9EG7, BD Pharmingen; 1:100), β3 integrin PE (12-0611, 2C9.G3, eBioscience; 1:400), α5-integrin PE (557447, 5H10-27, BD Pharmingen; 1:400), α6-integrin PE (555736, GoH3, BD Pharmingen) and αv-integrin PE (551187, RMV-7, BD; 1:400). Dilutions were as previously described (Czuchra et al., 2006).
Immunohistochemistry
The following antibodies were used for immunohistochemistry: β1 integrin (MAB1997, Millipore), laminin 111 (ab30320, Abcam), laminin 332 (a kind gift from M. Aumailley, University of Cologne, Cologne, Germany), α6 integrin–FITC (BD Biosciences) and keratin 5 (ab24647, Abcam). β-Galactosidase activity was determined as previously described (Czuchra et al., 2006).
SILAC-based peptide pulldowns
Pulldowns were performed as previously described (Meves et al., 2011).
ES cells and embryoid bodies
ES cells were isolated and cultured as previously described (Böttcher et al., 2012). Embryoid bodies were generated as described previously (Böttcher et al., 2012).
Isolation of primary keratinocytes, adhesion, spreading, and migration assays
Primary keratinocytes were isolated from P21 mice and grown to confluence as previously described (Czuchra et al., 2006; Meves et al., 2011). Adhesion of ES cells and primary keratinocytes to fibronectin, laminin and collagen I and cell spreading were performed and analyzed as described previously (Böttcher et al., 2012; Czuchra et al., 2006). Cell wounding was performed as previously described (Lorenz et al., 2007). Live-cell recordings were performed immediately after wounding for 12 h at 37°C and 5% CO2. At least four independent scratch-wound experiments were used for calculations. Single-cell tracking of cells within the leading edge was performed using MetaMorph software, choosing 15 cells each in at least three independent experiments.
β1 Y-to-A fibroblast lines
Point mutations of β1 integrin cDNA (β1 Y783A, β1 Y795A, β1 YY783/795AA) were introduced by site-directed mutagenesis. For stable expression in fibroblasts, β1 integrin cDNA was cloned into the retroviral expression vectors pCLMFG or pLZRS. Viral particles were concentrated from cell culture supernatant as described previously (Pfeifer et al., 2000) and used for infection.
Integrin stability and internalization
This was determined as previously described (Böttcher et al., 2012).
ACKNOWLEDGMENTS
This work was funded by the Max Planck Society, the DFG (SFB 914) and the Mayo Clinic.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Boettiger D. Mechanical control of integrin-mediated adhesion and signaling. Curr Opin Cell Biol. 2012;24:592–599. doi: 10.1016/j.ceb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Böttcher RT, Stremmel C, Meves A, Meyer H, Widmaier M, Tseng HY, Fässler R. Sorting nexin 17 prevents lysosomal degradation of [beta] 1 integrins by binding to the [beta] 1-integrin tail. Nat Cell Biol. 2012;14:584–592. doi: 10.1038/ncb2501. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zou Z, Sarratt KL, Zhou D, Zhang M, Sebzda E, Hammer DA, Kahn ML. In vivo beta1 integrin function requires phosphorylation-independent regulation by cytoplasmic tyrosines. Genes Dev. 2006;20:927–932. doi: 10.1101/gad.1408306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Czuchra A, Meyer H, Legate KR, Brakebusch C, Fassler R. Genetic analysis of beta1 integrin "activation motifs" in mice. J Cell Biol. 2006;174:889–899. doi: 10.1083/jcb.200604060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Feng C, Li YF, Yau YH, Lee HS, Tang XY, Xue ZH, Zhou YC, Lim WM, Cornvik TC, Ruedl C. Kindlin-3 Mediates Integrin αLβ2 Outside-in Signaling, and It Interacts with Scaffold Protein Receptor for Activated-C Kinase 1 (RACK1) J Biol Chem. 2012;287:10714. doi: 10.1074/jbc.M111.299594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Isaji T, Kariya Y, Xu Q, Fukuda T, Taniguchi N, Gu J. Functional roles of the bisecting GlcNAc in integrin-mediated cell adhesion. Methods Enzymol. 2010;480:445–459. doi: 10.1016/S0076-6879(10)80019-9. [DOI] [PubMed] [Google Scholar]
- Larkin D, Treumann A, Murphy D, DeChaumont C, Kiernan A, Moran N. Compartmentalization regulates the interaction between the platelet integrin αIIbβ3 and ICln. Br J Haematol. 2009;144:580–590. doi: 10.1111/j.1365-2141.2008.07483.x. [DOI] [PubMed] [Google Scholar]
- Legate KR, Wickström SA, Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fässler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157:1279–1290. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K, Grashoff C, Torka R, Sakai T, Langbein L, Bloch W, Aumailley M, Fassler R. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol. 2007;177:501–513. doi: 10.1083/jcb.200608125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23:607–614. doi: 10.1016/j.ceb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Mathew S, Lu Z, Palamuttam RJ, Mernaugh G, Hadziselimovic A, Chen J, Bulus N, Gewin LS, Voehler M, Meves A, et al. β1 Integrin NPXY Motifs Regulate Kidney Collecting-Duct Development and Maintenance by Induced-Fit Interactions with Cytosolic Proteins. Mol Cell Biol. 2012;32:4080–4091. doi: 10.1128/MCB.00568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves A, Geiger T, Zanivan S, DiGiovanni J, Mann M, Fassler R. [beta]1 integrin cytoplasmic tyrosines promote skin tumorigenesis independent of their phosphorylation. Proc Natl Acad Sci U S A. 2011;108:15213–15218. doi: 10.1073/pnas.1105689108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves A, Stremmel C, Gottschalk K, Fassler R. The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 2009;19:504–513. doi: 10.1016/j.tcb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Montanez E, Piwko-Czuchra A, Bauer M, Li S, Yurchenco P, Fässler R. Analysis of Integrin Functions in Peri-Implantation Embryos, Hematopoietic System, and Skin. Methods Enzymol. 2007;426:239–289. doi: 10.1016/S0076-6879(07)26012-4. [DOI] [PubMed] [Google Scholar]
- Montanez E, Ussar S, Schifferer M, Bosl M, Zent R, Moser M, Fassler R. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Boettiger D, Weaver VM, Hammer DA. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput Biol. 2009;5:e1000604. doi: 10.1371/journal.pcbi.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer A, Kessler T, Silletti S, Cheresh DA, Verma IM. Suppression of angiogenesis by lentiviral delivery of PEX, a noncatalytic fragment of matrix metalloproteinase 2. Proc Natl Acad of Sci U S A. 2000;97:12227–12232. doi: 10.1073/pnas.220399597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of αvβ3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11:1392–1402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of α5β1 integrins determines adhesion strength whereas αvβ3 and talin enable mechanotransduction. Proc Natl Acad of Sci U S A. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller HB, Friedel CC, Boulegue C, Fassler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011;12:259–266. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Boettiger D. A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397. Mol Biol Cell. 2003;14:4306–4315. doi: 10.1091/mbc.E03-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Ussar S, Moser M, Widmaier M, Rognoni E, Harrer C, Genzel-Boroviczeny O, Fassler R. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 2008;4:e1000289. doi: 10.1371/journal.pgen.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Ye F, Hu G, Taylor D, Ratnikov B, Bobkov AA, McLean MA, Sligar SG, Taylor KA, Ginsberg MH. Recreation of the terminal events in physiological integrin activation. J Cell Biol. 2010;188:157–173. doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Kim C, Ginsberg MH. Reconstruction of integrin activation. Blood. 2012;119:26–33. doi: 10.1182/blood-2011-04-292128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10:1062–1068. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]