Abstract
Enterotoxigenic Escherichia coli (ETEC) strains harbor multiple fimbriae and pili to mediate host colonization, including the type IVb pilus, colonization factor antigen III (CFA/III). Not all colonization factors are well characterized or known in toxin positive ETEC isolates, which may have an impact identifying ETEC isolates based on molecular screening of these biomarkers. We describe a novel coli surface antigen (CS) 8 subtype B (CS8B), a family of CFA/III pilus, in a toxin producing ETEC isolate from a Kenyan collection. In highlighting the existence of this putative CS, we provide the sequence and specific primers, which can be used alongside other ETEC primers previously described.
Keywords: Enterotoxigenic Escherichia coli, type IVb pilins, coli surface antigen 8 subtype B, Kenya, colonization factors
The authors report on a new adhesin, that they have named coli surface antigen 8 subtype B (CS8B); to date, there are five variants of CS8 undetectable using current primers.
Graphical Abstract Figure.
The authors report on a new adhesin, that they have named coli surface antigen 8 subtype B (CS8B); to date, there are five variants of CS8 undetectable using current primers.
Enterotoxigenic Escherichia coli (ETEC) pathotypes are a major cause of infantile watery diarrhea in developing countries as well as in travellers globally (Qadri et al. 2005). They are defined by the presence of a heat-labile toxin and/or a heat-stable toxin (Nataro and Kaper 1998). In addition, ETEC is known to carry an array of colonization factor antigens (CFAs) or adhesive fimbriae that promote adherence to and colonization of the host intestinal epithelial cells thus playing an important role in disease instigation and pathogenesis (Turner et al. 2006).
To date, 20 distinct colonization factors (CFs) have been identified in ETECs causing diarrhea in humans namely CFAI, CS1–8 and CS12–23 (Rodas et al. 2009; Mazariego-espinosa et al. 2010). ETECs may express one of two-type IVb pilli, CFA/III of coli surface antigen 8 (CS8) or Longus of coli surface antigen 21 (CS21). The CFA/III consists of a cluster of 14 member genes that make up the cof operon (Taniguchi et al. 2001; Yuen et al. 2013).
We describe a novel type IVb, CFA/III colonization factor designated CS8B, carried by an ETEC strain ESEI_111 obtained in September 2013 from a child under 5 years of age presenting with watery diarrhea. Escherichia coli strain ESEI_111 was sequenced at the Wellcome Trust Sanger Institute as part of a pilot global ETEC project. Genomic DNA was isolated by QIAamp® DNA mini Kit (Eigen, Courtaboeuf, France) and sequenced using the Illumina HiSeq2000 platform. Paired-end reads of 100 bp were assembled using Velvet (Zerbino 2011). For future epidemiological screening, primers for selected genes: cofJ-B and cofP-B of CS8B were designed using SeqBuilder software (Table 1) and were tested on the ESEI_111 strain. All the PCR reactions (single or multiplex) were performed in 25 μl final volume containing 1 μl of the template DNA, 22 μl of water and 0.5 μl of each primer mixed with the Ready-To- Go PCR Beads (GE healthcare, Buckinghamshire, UK) according to manufacturer's specifications. The thermocycling conditions were as follows: 95°C for 2 min, 95°C for 30 s, 60°C for 30 s and 30 s at 72°C for 35 cycles, with a final 10 min extension at 72°C, and the PCRs were performed in the DNA Engine PTC-0221 Dyad Cycler. Multilocus sequence typing (MLST) was performed as previously described (Aanensen and Spratt 2005). Plasmid typing was carried out in silico using an in-house script and primer sequences described previously (Carattoli et al. 2005). Manual annotation was carried out using the genome viewer Artemis (Rutherford et al. 2000). Comparative genomics of CS8B to available complete CS8 operon coding sequences from European Nucleotide Archive (ENA) was performed using GenomeMatcher (Ohtsubo et al. 2008). To determine the phylogenetic relationship between nucleotide sequences of 33 cofJ, 17 lngJ and one tcpJ, a tree was inferred by using the maximum likelihood method based on the Tamura–Nei model using the poisson model of amino acid substitution, uniform rates among sites, with a 95% site coverage cut-off and 1000 bootstrap replicates (Tamura and Nei 1993). Percentage nucleotide identities were calculated using ClustalW Omega (Sievers et al. 2011). The KEMRI/National Ethics Review Committee SCC No 2507 approved all procedures.
Table 1.
Primers designed for CS8B.
| Primer name | 5′ -3′ Sequence | Annealing Tm | Product size |
|---|---|---|---|
| CS8-JB_F | GCCGGGGATGGCATCACCA | 64 | 968 bp |
| CS8-JB_R | GCGTTTATAAGAGGCTATCTG | 60 | |
| CS8-PB_F | CACTGGTATGTGTGTTGGTAGC | 64 | 730 bp |
| CS8-PB_R | GGAAATTGCCGGGCCAAAAG | 62 |
ESEI_111, by MLST, belongs to sequence type 410, encodes a heat labile toxin (eltA and eltB) and harbors a multidrug-resistant IncI1 plasmid encoding tetB, blaTEM-1, sul2, strA, ampC and dfrA8 resistance genes.
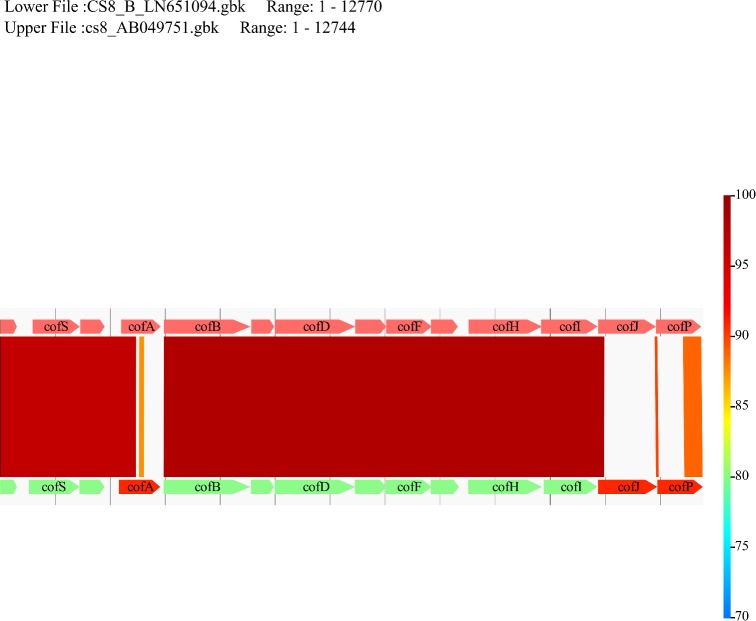
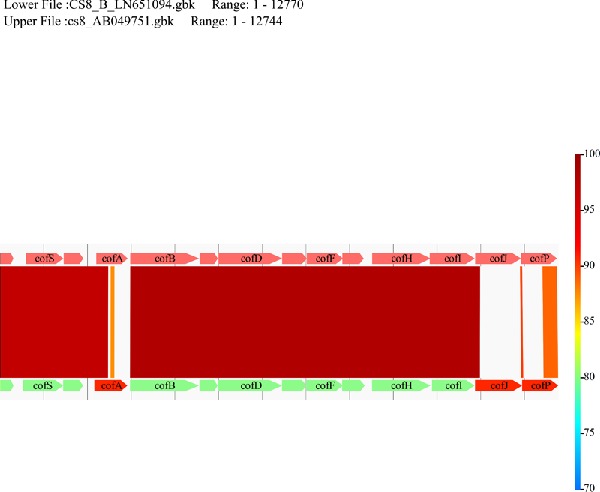
Although our understanding of the pathogenesis of ETEC has been accelerated by data from whole genome sequencing (WGS), not all CFs have been identified. Current moelcular screening of ETECs involves PCR amplification of the known toxin genes and CFs (Rodas et al. 2009). However, some ETECs are classed as CF negative either because they lack a CF or they may harbor unknown or divergent CFs. ESEI_111 is one such isolate lacking all known CFs, as defined by PCR, but haboring a CS8B operon. WGS of ESEI_111, followed by mapping of the resultant short reads to all known CFs using SMALT software version 0.6.4 and a 90% identity cut-off, showed that this isolate did in fact harbor a CF which was related, but distinct from the well-characterized CF CS8 operon. Figure 1 shows the comparative genomic analysis of cofR, cofS, cofT, cofA-J and cofP which are predicted to encode the CS8B CF protein with the orthologous cluster encoding CS8 (Taniguchi et al. 2001). These data showed that CS8B was a chimeric operon with cofR, cofS, cofT, cofB, cofC, cofD, cofE, cofF, cofG, cofH and cofI showing more than 96% sequence identity to their orthologs in the CS8 operon. However, cofA, cofJ and cofP, which encode the major subunit pilin, the secreted CF and the prepilin peptidase, respectively, were highly divergent showing less than 88% identity with their CS8 orthologs.
Figure 1.
Comparative genomics for CS8 and CS8B from this study. Comparative genomics with CS8B cof operon from this study at the bottom and CS8 of accession AB049751 at the top using GenomeMatcher (Ohtsubo et al. 2008). cofR, cofS, cofT, cofB, cofC, cofD, cofE, cofF, cofG, cofH and cofI remain conserved with more than 96% similarity upstream. CFA/III gene products are cofA- major pilin, cofB- minor pilin, cofD- secretin, cofE, cofF and cofG- IMAPs (inner membrane accessory proteins), cofH- assembly ATPase, cofI- IMCP (inner membrane core protein), cofJ- secreted colonization factor, cofP- prepilin peptidase. Comparing CS8B cof operon to CS8 reveals divergence in cofA, cofP and cofJ, which encode the major subunit pilin, the prepilin peptidase and the secreted colonization factor, respectively. Colored scale on the right-hand side indicates percent nucleotide identity. Off-white areas between cofA, cofJ and cofP represent regions of no synteny.
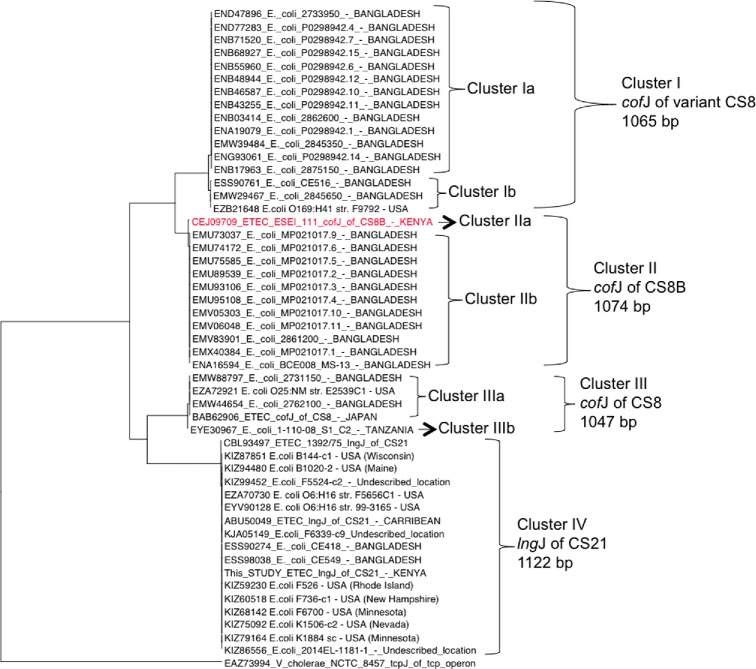
Phylogenetic analysis of all known prepilin peptidases related to cofJ showed that CS8B, clusters distinctly from all other cofJ genes (Fig. 2), and homolog tcpJ of Vibrio cholerae (EAZ73994), which has 44% identity to the CS8B cofJ gene identified in this study. We observed three distinct clusters of variant cofJ genes: Cluster I (1065 bp), Cluster II (1074 bp), III (1047 bp), IV (1122 bp). Cluster IIb share 99% nucleotide identity to cofJ described in this study, which we hypothesize to be CS8B. The cofJs from this clade have 16 SNPs, resulting in 10 non-synonymous amino acid substitutions relative to accession CEJ09709 of CS8B. The CS8-JB primers described in this study amplify the cofJ of ESEI_111 (970 bp product) in addition to cofJ from Cluster IIb producing a 968 bp product by in silico PCR, and the five variants of CS8 (Cluster Ia/b, IIa/b, IIIb) currently undetectable using the current primers that amplify a region in the cofA gene.
Figure 2.
Phylogeny of CS8, CS8B from this study and CS21. Phylogenetic dendrogram constructed by the maximum likelihood method based on the Tamura–Nei model with MEGA 6.05, based on the complete major subunits of Type 4b pili gene sequences of cofJ, tcpJ and lngJ obtained from ENA. cofJ of CS8B from this study is shown in red. Most of the isolates originate from Bangladesh and those from other regions and their respective clusters are also indicated.
It is of note that unlike CS8, which is occasionally coexpressed with CS6 (Shaheen et al. 2003), CS8B was the only detected CF in the genome sequence of ETEC strain ESEI_111, novel or otherwise. Although other strains of CS8 variants were not available for in vitro testing, variants belonging to Cluster II can be covered by primers described in this study. Therefore, including CS8B primers that target cofJ and cofP alongside previously described primers (Rodas et al. 2009) when screening for CFs is recommended.
Considering that ETEC cause approximately 400 million diarrheal cases and almost 400 000 deaths per year in children under 5 years of age extending our ability to detect and monitor novel CFs found in low-/middle-income countries like Kenya will aid ETEC screening efforts and increase our ability to determine how the diversity of these genes may influence host–pathogen interactions. Importantly, this in turn may have future implications for vaccine development (Fleckenstein et al. 2010; Nada et al. 2011; Begum et al. 2014).
Although cofJ of CS8B have been detected before, we believe this is the first whole CS8B operon report, a variant of CS8. The annotated complete coding sequence reference for the CS8B cof operon is LN651094 and respective gene sequences have been deposited in the Genbank under accession numbers; cofR- CEJ09697, cofS- CEJ09698, cofT- CEJ09699, cofA- CEJ09700, cofB- CEJ09701, cofC- CEJ09702, cofD- CEJ09703, cofE- CEJ09704, cofF- CEJ09705, cofG- CEJ09706, cofH- CEJ09707, cofI- CEJ09708, cofJ- CEJ09709 and cofP- CEJ09710.
Acknowledgments
We appreciate the efforts of ESEI project staff especially the KEMRI team; Joyce Arua, RoseTabby Kiiru, Winnie Kitali, Christitne Juma, Purity Karimi and Hannah Njeri at the Enterics WT lab, KNH grounds.
This work is published with permission from Director, KEMRI.
FUNDING
This work was supported by the Medical Research Council; Natural Environment Research Council; Economic and Social Research Council; Biotechnology and Biosciences Research Council [grant numberG1100783/1]. CJB was supported by the Medical Research Council [grant number G1100100/1]. Sequencing was supported by the Wellcome Trust [grant number 098051].
Conflict of interest. None declared.
REFERENCES
- Aanensen DM, Spratt BG. The multilocus sequence typing network: mlst.net. Nucleic Acids Res. 2005;33:728–33. doi: 10.1093/nar/gki415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum YA, Baby NI, Faruque ASG, et al. Shift in phenotypic characteristics of enterotoxigenic Escherichia coli (ETEC) isolated from diarrheal patients in Bangladesh. PLoS Neglect Trop D. 2014;8:1–7. doi: 10.1371/journal.pntd.0003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A, Bertini A, Villa L, et al. Identification of plasmids by PCR-based replicon typing. J Microbiol Meth. 2005;63:219–28. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Fleckenstein JM, Hardwidge PR, Munson GP, et al. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010;12:89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazariego-espinosa K, Cruz A, Maria A, et al. Longus, a type IV pilus of enterotoxigenic Escherichia coli, is involved in adherence to intestinal epithelial cells. J Bacteriol. 2010;192:2791–800. doi: 10.1128/JB.01595-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada RA, Shaheen HI, Khalil SB, et al. Discovery and phylogenetic analysis of novel members of class b enterotoxigenic Escherichia coli adhesive fimbriae. J Clin Microbiol. 2011;49:1403–10. doi: 10.1128/JCM.02006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Micobiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo Y, Ikeda-ohtsubo W, Nagata Y, et al. GenomeMatcher: a graphical user interface for DNA sequence comparison. BMC Bioinformatics. 2008;9:1–9. doi: 10.1186/1471-2105-9-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F, Svennerholm A, Faruque ASG, et al. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Micobiol Rev. 2005;18:465–83. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodas C, Iniguez V, Qadri F, et al. Development of multiplex PCR assays for detection of enterotoxigenic Escherichia coli colonization factors and toxins. J Clin Microbiol. 2009;47:1218–20. doi: 10.1128/JCM.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford K, Parkhill J, Crook J, et al. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16:944–5. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- Shaheen HI, Kamal KA, Wasfy MO, et al. Phenotypic diversity of enterotoxigenic Escherichia coli (ETEC) isolated from cases of travelers’ diarrhea in Kenya. Int J Infect Dis. 2003;7:35–8. doi: 10.1016/s1201-9712(03)90040-3. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:1–6. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–26. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Akeda Y, Haba A, et al. Gene cluster for assembly of pilus colonization factor antigen III of enterotoxigenic Escherichia coli. Infect Immun. 2001;69:5864–73. doi: 10.1128/IAI.69.9.5864-5873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SM, Scott-Tucker A, Cooper LM, et al. Weapons of mass destruction: virulence factors of the global killer enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 2006;263:10–20. doi: 10.1111/j.1574-6968.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Yuen ASW, Kolappan S, Ng D, et al. Structure and secretion of CofJ, a putative colonization factor of enterotoxigenic Escherichia coli. Mol Microbiol. 2013;90:898–918. doi: 10.1111/mmi.12407. [DOI] [PubMed] [Google Scholar]
- Zerbino DR. Using the Velvet de novo assembler for short-read sequencing technologies. Curr Protoc Bioinformatics. 2011 doi: 10.1002/0471250953.bi1105s31. Chapter 11:Unit 11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]