Abstract
The aqueous outflow system is unique because nowhere else can the pattern of flow of an extravascular fluid be directly observed as it returns to the vascular system. Such observations reveal that aqueous flow both from Schlemm’s canal into the aqueous veins and from the aqueous veins into the episcleral veins is pulsatile. Pulsatile aqueous flow mechanisms are observable in vivo not only in normal and but also in glaucomatous eyes. A series of specific patterns accompany the pulsatile mixing of aqueous with blood in the episcleral veins. These directly observable patterns of pulsatile flow are synchronous with intraocular pressure (IOP) transients induced by the cardiac pulse, blinking and eye movement. Patterns of pulsatile flow are altered by events that increase IOP such as pressure on the side of the eye, tonography and water drinking. Pulsatile flow stops when IOP is reduced below its resting level, but begins again when IOP returns to the resting level. Pulsatile flow reduction probably results from the intrinsic reduction of pulse amplitude at a lower IOP, and may thus provide a passive mechanism to maintain short-term homeostasis. Thus modulation of the pulsatile flow phenomenon appears to maintain a homeostatic IOP setpoint. Visible pulsatile flow abnormalities develop in glaucoma patients. Medications that reduce IOP through improvement in outflow do so through pulsatile flow mechanisms. Laboratory studies have demonstrated that cyclic stresses in outflow tissues alter signaling pathways, cytoskeletal responses, extracellular matrix composition and cytokine secretion. How physiologic pulse transients orchestrate cellular responses and how cellular responses identified in the laboratory may in turn regulate pulsatile aqueous outflow is unknown. Linkage of laboratory and in vivo observations await an improved understanding of how cellular and extracellular structures within the outflow system are able to generate an aqueous pulse wave. The purpose of the current report is to provide a summary of in vivo IOP-induced patterns of cyclic flow that can be used as part of a framework for interpretation of responses to cyclic stresses identified in the laboratory.
Keywords: aqueous vein, pulsatile, flow, cyclic, oscillatory, glaucoma, trabecular meshwork, Schlemm’s canal
1. Introduction
1.1. Overview
Aqueous humor circulation through the anterior segment of the eye involves one of the vascular circulatory loops that are driven down a continuous pressure gradient initially set up by the heart (Forrester, 2002). Aqueous is secreted into the eye from the general circulation via the ciliary processes. Aqueous then exits the eye by passing through the trabecular meshwork to Schlemm’s canal. After entering Schlemm’s canal, aqueous enters collector channels that have a lumen in communication with the aqueous veins. The aqueous vein lumen in turn communicates with episcleral veins providing the final pathway for return of aqueous to the general circulation. Aqueous and episcleral veins on the surface of the eye are clearly visible providing a unique opportunity for study of flow patterns (Johnstone, 2010). Normal aqueous flow, abnormalities of flow in glaucoma and responses to medications can all be directly monitored (Ascher, 1961).
In the initial reports of the aqueous veins, pulsatile flow was one of the salient features and its manifestations described in remarkable detail (Ascher, 1942a,b,c,d; Goldmann, 1946a; De Vries, 1947). Those seminal studies demonstrated that pulsatile flow occurs in response to oscillatory or cyclic IOP transients (Fig. 1) such as those that occur with the ocular pulse (Figs. 1 and 2), blinking and eye movements (Fig. 2) (Coleman and Trokel, 1969). Stepanik demonstrated that flow through the aqueous veins is amenable to direct measurement, a measurement made possible by the pulsatile nature of aqueous flow (Stepanik, 1954b).
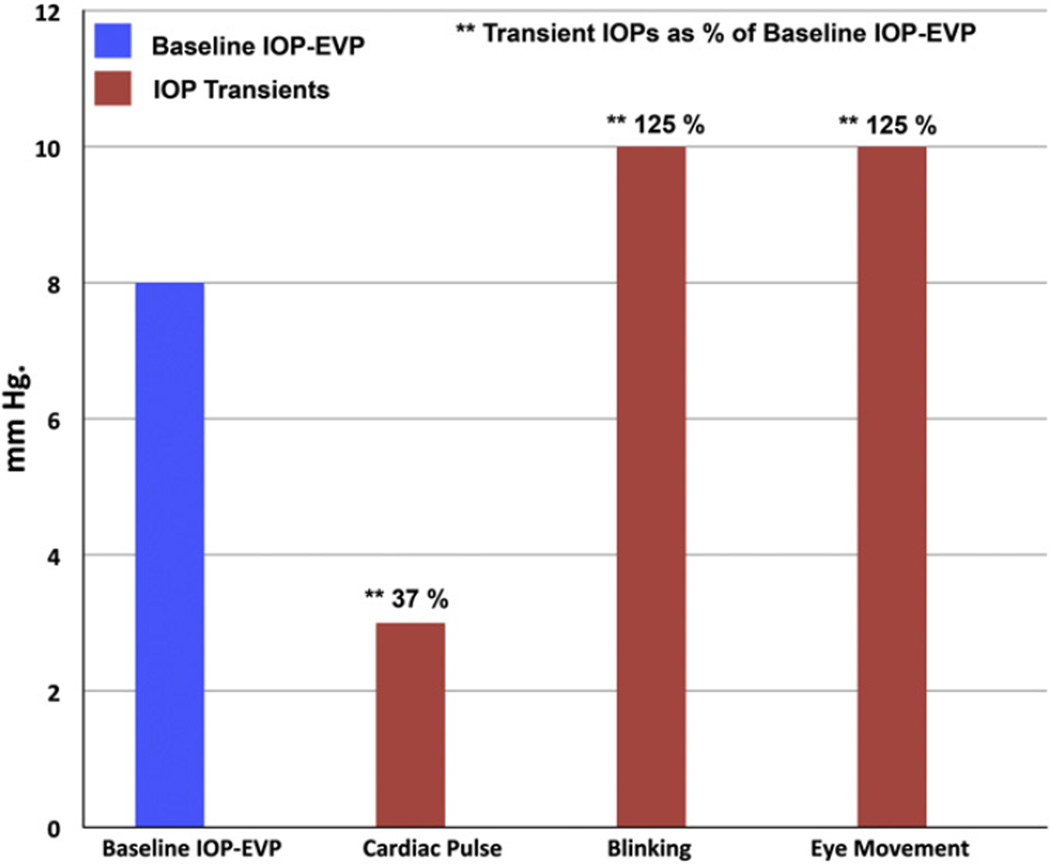
Fig. 1.
Comparison between (IOP–EVP) and ocular transients. Mean intraocular pressure (IOP) in the population is ~16 mm Hg and episcleral venous pressure (EVP) is ~8 mm Hg. The difference between IOP and EVP (IOP–EVP) is thus ~8 mm Hg and represents the baseline hydrostatic pressure gradient driving aqueous flow from the eye. Ocular transients of the cardiac pulse, blinking and eye movements superimpose relatively large cyclic IOP gradients on the underlying baseline IOP–EVP gradient. Ocular transient IOP amplitudes from data reported by Coleman and Trokel (1969).
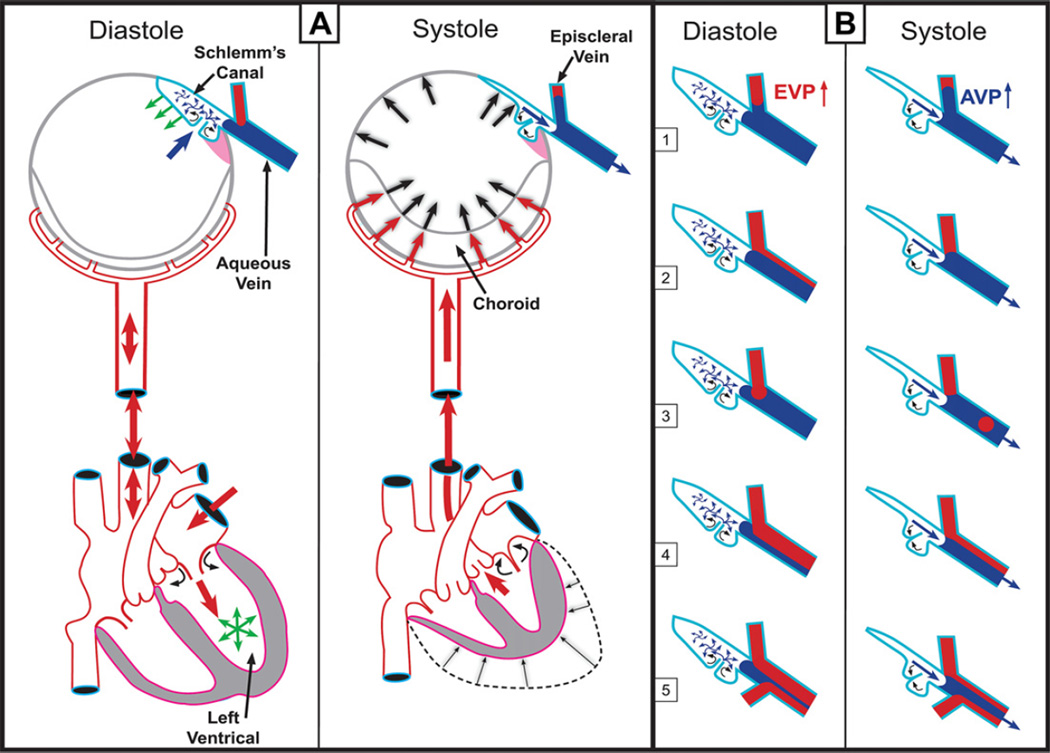
Fig. 2.
(Panel A – Systole) Cardiac source of pulsatile flow. Systole-induced choroidal vasculature expansion (red arrows). Transient IOP increase (large black arrows). Aqueous pulse wave distends the trabecular meshwork (TM) forcing it outward into Schlemm’s canal (SC). One-way channels into SC prevent backflow (small curved arrows). Distention of the TM into SC reduces SC volume. SC pressure increases. Small black arrow denotes aqueous discharge from SC. Aqueous pulse wave then enters the aqueous vein. (Panel A – Diastole) Blood enters the left ventricle (green circle of arrows). Double red arrows indicate absence of a pressure wave in diastole. TM moves inward during diastole (green arrows). Aqueous enters SC (large blue arrow). (Panel B) During diastole episcleral venous pressure (EVP) is slightly higher than aqueous vein pressure (AVP), resulting in a relative EVP ↑). The EVP ↑ causes episcleral vein blood to move toward (B 1) or into (B 2–5) the aqueous mixing vein. The next systole causes a transient AVP ↑. The oscillations result in pulsatile flow manifestations in the aqueous veins. The AVP ↑ causes transient movement of a standing aqueous wave into a tributary episcleral vein (B 1), transient elimination of a lamina of blood (B 2), a bolus of blood swept into the increased aqueous stream (B 3), an oscillating increase in diameter of the aqueous component of a persistent laminar (B 4) or trilaminar (B 5) aqueous flow wave. Panel A is adapted from data of Phillips et al. (1992). Panel B adapted from observations of De Vries (1947) and Ascher (1961). (With kind permission from Springer: The Glaucoma Book, Aqueous Veins and Open Angle Glaucoma, 2010, pg. 68, Johnstone et al., Fig. 7.4.). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
A bolus of blood often enters an aqueous vein during each cardiac cycle to be followed by an aqueous pulse wave that sweeps the blood bolus along the vessel (Fig. 2, Panel B 3). By in vivo measurement of vessel diameter and velocity of flow of the blood bolus, the volume of flow can be determined. Stepanik found flow through a single aqueous vein to average 1.08 µl/min (Stepanik, 1954b). Ascher observed that two aqueous veins could account for all of aqueous outflow (Ascher, 1961). Since pulsatile aqueous outflow can account for a majority of aqueous discharge, laboratory studies of cyclic stresses at the cellular level (WuDunn, 2009) may provide important insights into homeostatic mechanisms of flow control.
1.2. Aqueous and cardiovascular pulsatile flow correlates
Many decades have passed since the initial documentation that aqueous outflow is pulsatile. Flow through the outflow system is generally discussed as a passive phenomenon without reference to transient ocular pressure changes as a driving force (Johnstone, 2009). One might question whether simple observations of a dynamic process made so long ago have any current relevance.
However, studies leading to recognition of pulsatile aqueous flow and its manifestations involved careful observation as well as measurements. The studies demonstrated that aqueous flow was pulsatile, unidirectional, and could account for all of normal aqueous flow. Numerous studies support the observations (Ascher, 1961), which have neither been refuted nor even challenged (Johnstone, 2010). Such observations and measurements are remarkably similar to those of Harvey (Harvey, 1995) whose demonstration that blood circulates through a pulsatile flow mechanism was initially not well accepted (Harvey, 1995). Harvey’s observations and measurements ultimately established a set of principles that have persisted not for decades but rather for centuries.
Harvey’s work is now widely recognized as the classic early study using the principles of biomechanics (Fung, 1993, 1996; Humphrey, 2002). The principles of physiology revealed through his observations continue to become more relevant with time as implications of his principles are studied in ever greater depth (Fung, 1996; Humphrey, 2002; Levick, 2003; Nichols and O’Rourke, 2005). Similar pulsatile flow evidence in the aqueous outflow system may reasonably be expected to have physiologic importance that is not diminished but rather enhanced by time, as mechanisms underlying the phenomenon of pulsatile aqueous outflow also become more clearly understood.
1.3. Aqueous vein observations – contributions to current understanding of glaucoma
The modern treatment of glaucoma owes much to the discovery of the aqueous veins. Although currently not a subject of debate, the concept of aqueous as a stagnant fluid held sway in many quarters and was not eliminated entirely from discussion until the 1970s (Bill, 1977). Prior to discovery of the aqueous veins, some laboratory evidence suggested that aqueous circulated (Kinsey and Reddy, 1964) but such studies involved indirect measurements that were inadequately convincing to permit a consensus view. Much more definitive evidence that aqueous circulated was provided by the direct observation of aqueous flow into the aqueous veins.
In the absence of a clear consensus that aqueous flowed, the concepts of pupillary block and the value of peripheral iridectomy were difficult to advocate. Efforts to improve aqueous outflow in open angle glaucoma did not have a solid rationale. The ability of physicians to directly observe pulsatile aqueous discharge into the aqueous veins eliminated uncertainty and ushered in a new era (Ascher, 1961). Gonioscopy became relevant, peripheral iridectomy had a clear rationale and was rapidly accepted worldwide. A logical framework could be developed for the use of outflow drugs. Microsurgical procedures involving the trabecular meshwork and SC could be tied to a clear goal of restoring normal flow.
1.4. Laboratory studies: simulation of IOP-induced cyclic stresses
Small pressure gradient changes systematically controlled in the laboratory in living and enucleated eyes demonstrate marked pressure-dependent changes in tissue and cellular morphology (Johnstone and Grant, 1973; Grierson and Lee, 1975a, 1975b; Johnstone, 1979). Periodic and transient stresses that alter both biaxial and linear stretch have also been studied in the laboratory at the cellular level (WuDunn, 2009). Physical changes that induce stretch designed to simulate pulsatile transients alter signaling pathways and cytoskeletal responses (Tumminia et al., 1998; Sato et al., 1999; Tamm et al., 1999; Chow et al., 2007) extracellular matrix composition (Bradley et al., 2001, 2003; WuDunn, 2001) and cytokine secretion (Liton et al., 2009).
1.5. The current search for homeostatic mechanisms associated with cyclic stresses
Identification of homeostatic mechanisms associated with periodic and short-term cyclic IOP stresses has been the goal of a number of laboratory studies. Acott’s group has emphasized the importance of understanding cyclic stresses that may modulate extracellular matrix composition to maintain IOP homeostasis (Bradley et al., 2001, 2003; Vittal et al., 2005; Keller et al., 2007; Acott and Kelley, 2008).
A variety of other cyclic and periodic stress responses have also been identified as candidates for maintaining IOP homeostasis (Tumminia et al., 1998; Liton et al., 2005, 2005; Ramos and Stamer, 2008; Baetz et al., 2009, 2009; Ramos et al., 2009). The importance of such laboratory studies is made apparent by in vivo evidence that pulsatile flow transients change under physiologic conditions, that pulsatile flow is reduced in glaucoma and that pulsatile flow improves in response to glaucoma medications.
Regulation of pulsatile flow is likely to result from interplay of a number of the suggested mechanisms. Currently the details of mechanisms by which cellular responses identified in the laboratory may regulate pulsatile aqueous outflow are unknown. The current report summarizes manifestations of in vivo IOP-induced cyclic flow that may serve as a framework for interpretation of responses to cyclic stresses seen in the laboratory.
2. Aqueous vein characteristics
2.1. Location and frequency
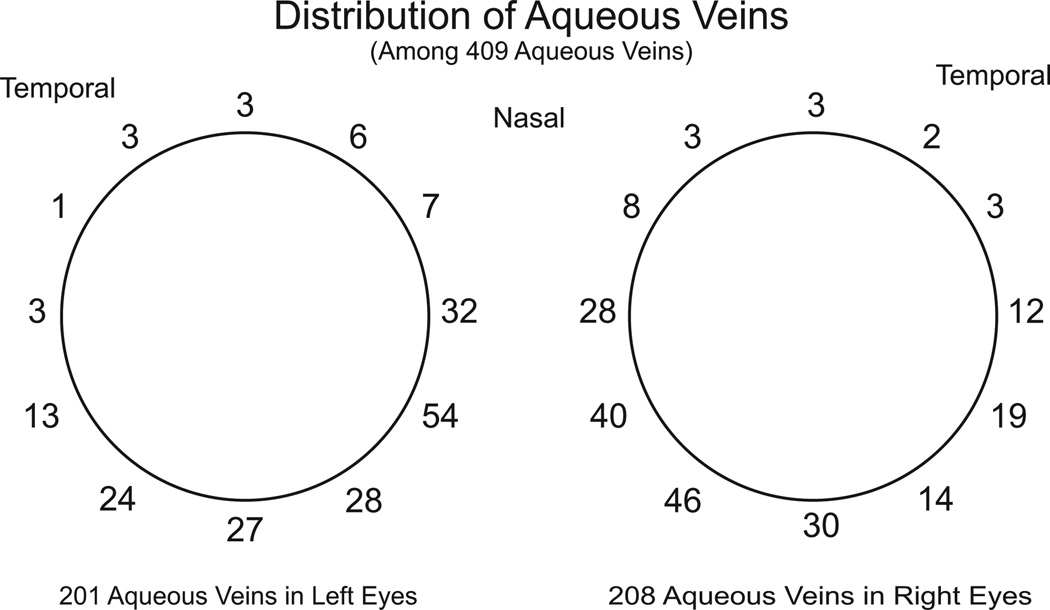
In about one-half of cases aqueous veins originate from the depths of the limbus. The rest of the aqueous veins originate from more anterior limbal loops and from more posterior scleral emissaries. Before joining episcleral veins the aqueous veins range in length from fractions of a millimeter to as much as a centimeter (Ascher, 1942a,c, 1961). Four to five aqueous veins are visible in a few eyes while there may be a maximum of six (De Vries, 1947). However, two to three aqueous veins are typically visible in an eye (De Vries, 1947). The aqueous veins are not distributed symmetrically around the limbus. The inferior nasal quadrants are where the aqueous veins are most commonly found (285 of 409 in one study) with most of the rest in the inferior temporal quadrant (De Vries, 1947) (Fig. 3).
Fig. 3.
Distribution of visible aqueous veins. Two to three aqueous veins are typically visible in an eye although there may be a maximum of four to six. Distribution is highly assymetric with the majority of visible aqueous veins at or below the horizontal midline, the greatest number being present in the nasal quadrant. Derived from data of De Vries (1947).
In some eyes intrascleral mixing of aqueous and blood occurs (Ascher, 1946), preventing easy identification of clear aqueous veins on the surface of such eyes. Although Ascher (1961) observed, “With high power magnification they can be seen in almost every eye.” the reported occurrence of visible aqueous veins in patients was reported to be ~75% by Goldmann (1946B). Aqueous veins have an average diameter of about 50 microns with a range of 20–100 microns (Goldmann, 1946b; De Vries, 1947; Stepanik, 1954b). Histologically, aqueous vein appearance is indistinguishable from that of regular conjunctival and episcleral veins (Ashton, 1951, 1952; Thomassen and Bakken, 1951). When present, aqueous flow is subject to direct observation providing an effective tool for analysis of outflow mechanisms (Stepanik, 1954b).
2.2. Aqueous veins, mixing veins and episcleral veins: a changing continuum
At their origins aqueous veins generally contain clear aqueous discharged from Schlemm’s canal (Figs. 4 and 5). Near their origins, aqueous veins often have small episcleral tributaries that intermittently discharge small amounts of blood into the stream of clear aqueous (Fig. 5 Panel A). Aqueous veins eventually join recipient episcleral vessels that contain blood (Figs. 2 and 5). In these transitional regions of mixing the vessels may be more appropriately called mixing veins since they contain a spectrum of aqueous and blood in varying proportions (De Vries, 1947; Johnstone, 2004).
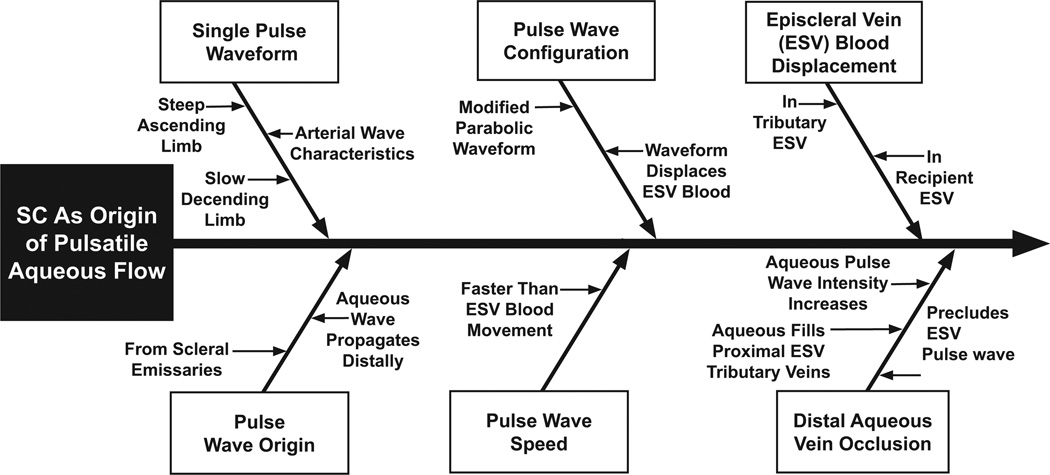
Fig. 4.
Evidence of Schlemm’s canal origin of pulsatile flow summarized in Ascher’s treatise (Ascher, 1961). Episcleral vein (ESV).
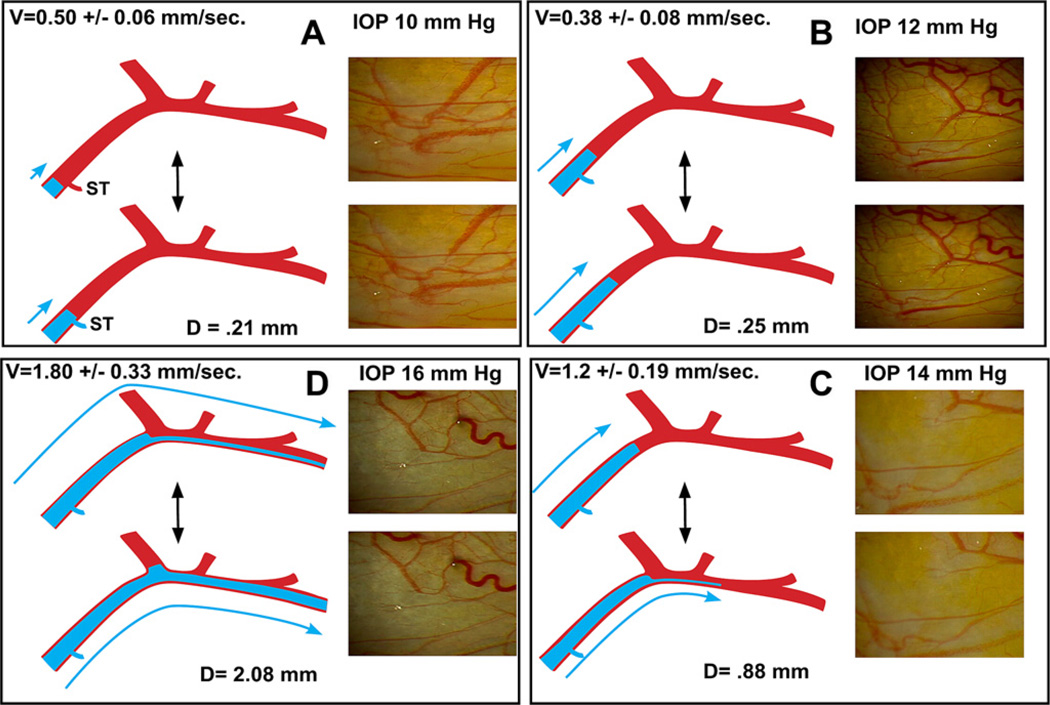
Fig. 5.
Illustration of characteristic pulsatile flow changes caused by increasing IOP or addition of medications. Still frames and illustrations derived from video images of 59-year-old male. Increase in intraocular pressure (IOP) followed a water-drinking test but typifies pulsatile flow increase from other causes such as medications. (Panel A) Baseline IOP, velocity (V) is low and aqueous pulse wave travel (D) with each stroke is small. A standing transverse interface of aqueous and blood oscillates resulting in systolic discharge of aqueous into a small venous tributary (ST). (Panel B) An increased distance of travel of the oscillatory aqueous fluid wave. (Panel C) Increased velocity and travel of the aqueous fluid wave. At each systole a lamina of clear aqueous discharges into an episcleral vein. (Panel D) Velocity and travel of the fluid wave increase further. Continuous oscillating laminar flow is present in a more distal episcleral vein. Two hours after drinking water, IOP was again 10 mm Hg and stroke volume returned to appearance seen in Panel A. (With kind permission from Springer: The Glaucoma Book, Aqueous Veins and Open Angle Glaucoma, 2010, pg. 67, Johnstone et al., Fig. 7.2.)
In the same vein complex in an eye, the transitional area of mixing varies and is dependent on the vigor of aqueous discharge (De Vries, 1947; Johnstone, 2004). For example, when the amplitude of the pulsatile flow wave is modest, the vascular region involved in mixing of aqueous and blood may be near the aqueous vein origin. With increasing amplitude and speed of the pulsatile flow wave, for example after administration of an outflow improving medication, a much longer region of the recipient vein may fill with aqueous (Ascher, 1942b).
With more distal movement of the aqueous-filled portion of the vessel, the region of mixing also moves distally in the same vessel complex. When intraocular pressure (IOP) increases for example with diurnal changes (Ascher, 1942b; Thomassen, 1947b, Thomassen et al., 1950) or water drinking (Johnstone, 2004) aqueous mixing with blood occurs at a more distal location within the vascular complex. The anatomic features of an aqueous, mixing and episcleral vein complex remain stable for many years and probably a lifetime (Ascher, 1961). However, the pulsatile aqueous column advances and retreats along the stable anatomic vascular complex of aqueous and recipient episcleral veins depending on the vigor of pulsatile flow.
Stratification or lamination occurs in the joined vessel (Ascher, 1942a, 1961; De Vries, 1947) when an aqueous vein joins an episcleral vein of comparable size to create a single vessel (Fig. 5). A clear layer of aqueous from the aqueous vein streams alongside a layer of blood from the episcleral vein within the single joined vessel. Ascher suggests that because of the differences of specific gravity, surface tension and viscosity between blood and aqueous (Ascher, 1961) stratification can persist for a long distance in the vessel. However, the lack of mixing is a defining characteristic of laminar flow and may have little to do with different fluid properties. Trilaminar flow results when an aqueous vein empties into the mid portion of the junction of two episcleral veins creating a central aqueous column and two peripheral columns of blood.
2.3. Recognition of pulsatile aqueous outflow
Two of the most effective tools for identifying aqueous veins are stratification of lamina of aqueous and blood as discussed above and pulsatile aqueous flow into the veins to be discussed below. Veins may be primarily carrying blood at the time of examination preventing identification as aqueous veins even though they may intermittently carry aqueous. Gentle digital pressure on the eye through the lower lid to raise IOP slightly can provide a useful identification tool in such vessels. A transient bolus of aqueous will enter and at times completely fill the recipient vessel as a result of the transient elevation of IOP. A change from a blood-filled lumen like that seen in Fig. 5 Panel A to that of aqueous-filled lumen like that seen in Fig. 5 Panel D occurs in a matter of seconds (Johnstone, 2010).
Vessels containing clear aqueous can be similarly difficult to identify. Intraocular pressure can be elevated transiently by again applying pressure to the eye by gentle digital pressure through the lower lid transiently forcing extra aqueous into the aqueous vein. There is a brief interval when IOP is reduced below the homeostatic setpoint after release of pressure on the eye. Aqueous transiently fails to flow into the aqueous veins because pressure is below the equilibrium point normally associated with oscillatory flow (Johnstone, 2004, 2010).
The resulting reduced pressure in the aqueous vein very briefly favors flow of episcleral blood into the vein previously containing clear aqueous thus revealing the presence of the previously transparent aqueous vein. Further confirmation is attained in a matter of seconds when aqueous reenters the vein forcing blood out as IOP returns to the homeostatic setpoint. Careful examination generally demonstrates the origin of the aqueous vein emissary once these vessels are identified. Further recognition is often made possible by identifying small episcleral venous tributaries just distal to the aqueous vein scleral emissary that are discharging blood into the clear aqueous vein. Continued examination of the region will often then permit identification of a well-defined network of aqueous veins (Ascher, 1961; Johnstone, 2010).
The ability of light digital pressure lasting a few seconds to cause pulsatile aqueous flow to stop suggests that very little IOP reduction is necessary to achieve the effect, but the issue has not been systematically studied. Miotics and adrenergics both have been observed to markedly increase pulsatile flow in glaucomatous eyes with high pressure. The increase in pulsatile flow is followed by IOP reduction to a new medication-induced equilibrium IOP that remains considerably above episcleral venous pressure. At the new lower equilibrium pressure, pulsatile flow slows or stops prior to a recurrent rise in IOP, a finding indicating that a lack of pulsatile flow does not occur because of a lack of a positive hydrostatic pressure gradient.
3. Pulsatile aqueous flow mechanisms
3.1. Origin of pulsatile aqueous outflow
A body of evidence indicates that SC rather than the episcleral veins is the origin of pulsatile aqueous flow (Ascher, 1961; Johnstone, 2004, 2010) (Fig. 4). Episcleral venous pulse waves are not implicated for the following reasons. Propagation of the aqueous wave moves distally from its origin in the aqueous vein emissary on the surface of the eye. The waveform arising from the emissary area has a steep ascending but a less steep descending limb characteristic of an arterial rather than a venous pulse wave. The propagating aqueous wave moves distally at a faster speed than episcleral blood in the same vessel. Furthermore, aqueous also displaces episcleral blood both within the primary vessel and within tributary vessels as it moves forward. No pulsatile behavior is observed in adjacent episcleral veins. Continuous rather than pulsatile flow is observed in adjacent episcleral vessels. The rate of propagation of the aqueous pulse wave is more rapid than blood flow seen in the episcleral veins they enter.
When potential venous pressure waves are prevented, pulsatile aqueous flow increases (Ascher, 1942a,c; De Vries, 1947; Ascher and Spurgeon, 1949; Weinstein, 1950). Temporary occlusion of a distal recipient episcleral vein with an instrument precludes any episcleral vein pulse wave from entering the aqueous vein. The temporary distal episcleral vein occlusion prevents aqueous flow causing the amplitude and velocity of the aqueous pulse wave in the aqueous vein to increase. Blood previously present in areas of laminar flow is displaced by aqueous. Pulsating columns of aqueous also fill small proximal episcleral vein tributaries. The aqueous veins become distended and even tense taking on a “glass rod” appearance (Ascher, 1961).
Stegmann has directly observed pulsatile flow in the collector channels just distal to Schlemm’s canal in 3 human eyes using gonioscopy and an 80-power microscope (Johnstone, 2004, 2006). Pulsatile flow in the collector channels provides additional convincing evidence of Schlemm’s canal as the origin of pulsatile flow.
3.2. Causes of pulsatile aqueous outflow transients
Pulsatile flow transients are a salient feature of aqueous movement through the aqueous veins; such descriptions of pulsatile flow were strongly emphasized in the original independent aqueous vein descriptions of Ascher (1942a) and Goldmann (1946a,b) as well as many later investigators (De Vries, 1947; Kleinert, 1951; Stepanik, 1954b). Pulsatile flow in the aqueous veins occurs in synchrony with small IOP transients such as those induced by the ocular pulse, blinking and eye movements (Ascher, 1961; Johnstone, 2004) (Fig. 1).
The choroidal vasculature contributes 85–95% of the intraocular blood volume (Bill, 1975). The systolic cardiac pulse wave induces a rapid expansion of choroidal volume (Fig. 2, Panel A) thus causing choroidal pulsations (Phillips et al., 1992). The increase in choroidal blood volume “acts like the piston of a fluid displacement pump in relation to the outflow of aqueous humor” (Phillips et al., 1992). The expanding choroidal volume in the closed space of the eye causes transient oscillatory changes in IOP of about 3 mm Hg that are synchronous with the cardiac pulse (Coleman and Trokel, 1969) (Fig. 1). Similarly blinking and eye movements cause transient IOP elevations by inducing pressure on the surface of the eye (Coleman and Trokel, 1969).
4. Pulsatile aqueous outflow: directly observable with multiple distinct presentations
4.1. Pulsatile oscillations with little or no flow
Pulsatile aqueous outflow has several discrete manifestations, each very well documented (Ascher, 1942a, 1942b, 1942c, 1942d; Goldmann, 1946a; Goldmann, 1946b; De Vries, 1947; Goldmann, 1948; Ascher, 1949a; Kleinert, 1951; Stepanik, 1954b; Ascher, 1961). A standing aqueous wave in an episcleral vein tributary oscillates during systole and diastole in one manifestation (Fig. 2 Panel B 1, Fig. 5, Panel A). If an increase in pulse amplitude occurred alone, the actual flow rate would not necessarily increase. Similarly, the speed of flow may increase without necessarily increasing flow, for example when the diameter of a vessel decreases with an associated increase in flow. However, as described below, reports regularly describe an increase in the amplitude of the aqueous lamina of the flow wave that is simultaneous with an increase in speed of the flow wave.
4.2. Pulsatile flow causes blood entering aqueous veins in diastole to move forward in systole
A second manifestation (Fig. 2 Panel B 2) involves a proximal tributary of the aqueous vein having sufficiently high pressure to cause entry of a laminar column of blood into an aqueous vein during diastole. There is sufficient pressure driving the aqueous wave during the next systole to completely eliminate blood entry into the aqueous vein. However, the pulse wave of aqueous moving forward in the vein sweeps the lamina of blood forward to clear it from the vessel. A third manifestation (Fig. 2, Panel B 3) involves a proximal tributary of an episcleral vein having sufficient pressure during diastole to force a discrete bolus of blood into the aqueous stream. Pressure in the aqueous vein during the next systole is high enough to again stop blood flow into the aqueous vein. In this case the bolus of blood deposited in the aqueous vein in diastole is swept along and cleared from the aqueous vein during the next systolic pulse wave.
4.3. Pulsatile flow results in oscillating Thicknesses of aqueous and blood lamina
Oscillations of persisting laminar components in the same vessel result in the fourth and fifth manifestations of pulsatile flow. The fourth presentation (Fig. 2, Panel B 4) results from an oscillating increase in diameter of the aqueous component of a persistent lamina of aqueous and blood. Trilaminar flow is the fifth presentation (Fig. 2, Panel B 5); the pattern of flow results from the rather common entry of an aqueous vein at the apex of the junction of two episcleral veins. Aqueous then forms a central clear column. A laminar column of blood is then present on each side of a clear column of aqueous. Oscillatory trilaminar flow results from oscillating increases in pressure in the central aqueous column.
As the aqueous wave propagates through a single vessel complex, several and at times all of the above manifestations of pulsatile flow are seen (Fig. 2 Panel B 1–5). Proximally near the aqueous emissary, for example, small proximal episcleral vein tributaries experience oscillatory movement within the vessel, causing a transient lamina of blood or a bolus of blood to discharge into the aqueous vein. More distally, where the aqueous vein joins a comparable sized recipient episcleral vein, oscillating laminar and then trilaminar flow are encountered along the course of the same vessel as the aqueous wave propagates forward.
5. Causes of increased pulsatile flow
Pulsatile flow increases prior to spontaneous diurnal reductions in IOP (Ascher, 1944; Thomassen, 1947b; Thomassen et al., 1950; Stepanik, 1954a). Pulsatile flow into the aqueous veins similarly slows or stops before a diurnal rise in IOP (Ascher, 1944; Thomassen, 1947b; Thomassen et al., 1950; Stepanik, 1954a). The observation that changes in pulsatile flow precede rather than occurring simultaneously or after changes in IOP suggest that the changes in pulsatile flow are responsible for the IOP changes. Because a temporal relationship does not prove a causal relationship, the increase in pulsatile flow that precedes the reduction in IOP cannot prove a cause and effect relationship in this setting.
A transient rise in IOP caused by pressure on the side of the eye (Goldmann, 1946b; Thomassen, 1947b), tonometry (Goldmann, 1946b; Thomassen, 1947b) and ophthalmodynamometry (De Vries, 1947; Kleinert, 1951) is accompanied by an increase in pulsatile aqueous flow. The artificially induced rise in pressure induces fluid flow out of the eye and results in an IOP lower than the prior baseline IOP once external pressure is eliminated. Until pressure rises to the baseline or homeostatic setpoint present prior to application of external pressure, pulsatile flow into the aqueous veins is absent (Thomassen, 1947a; Kleinert, 1951).
An elevation of IOP induced by water drinking also causes an increase in pulsatile aqueous outflow (Johnstone, 2004) (Fig. 5 Panel B–D). Until IOP returns to its baseline level, the increase in pulsatile flow caused by water drinking persists. An increase in pulsatile aqueous outflow precedes the reduction in IOP following instillation of three classes of medications. Distal occlusion of an episcleral vein results in more vigorous pulsatile aqueous inflow into the aqueous vein that would otherwise be able to discharge fluid to the episcleral vein. Pulsatile aqueous waves are more prominent proximal to the occlusion site. More complete aqueous filling of the proximal vessel is accomplished by elimination of any previously present lamina of blood. Pulsating columns of aqueous also enter into the more proximal tributary episcleral vessels forcing blood from them (Ascher, 1942a, 1942c; Ascher and Spurgeon, 1949; Kleinert, 1951).
6. Increases in pulsatile flow: visible manifestations
6.1. Low amplitude and speed of pulse wave
Pulsatile flow varies greatly (Fig. 2 Panel B, Fig. 5 Panels A–D) within the same group of aqueous veins, tributary veins, mixing veins and distal episcleral veins depending on physiologic status (Ascher, 1961) and pharmacologic exposure (Ascher, 1942b, 1942b, 1961). At a resting homeostatic setpoint, visible aqueous vein pulsatile waves are often of low amplitude and speed resulting in a low volume of aqueous movement. The low amplitude and speed lead to the appearance of an interface of blood and aqueous that generates an oscillating transverse wave within the recipient episcleral vein with a limited or even an apparent absence of a net movement of aqueous (Fig. 2 Panel B 1, Fig. 5 Panel A).
6.2. Increasing amplitude and speed of pulse wave
A progressive increase in amplitude and speed of the pulse wave results in an increase in volume of the aqueous wave that manifests itself through a spectrum of findings (Fig. 2 Panel B 2–5, Fig. 4 Panel B–D). An increased volume of the aqueous wave is induced by forces that raise IOP such as pressure on the side of the eye (Ascher, 1942a; Goldmann, 1946a, 1946b; De Vries, 1947), ophthalmodynamometry (De Vries, 1947) and water drinking (Johnstone, 2004). The same characteristic spectrum of changes associated with an increased aqueous volume is seen following pharmacologic exposure and precedes an IOP fall to a new homeostatic setpoint (Ascher, 1942a, 1942b; Goldmann, 1946a; De Vries, 1947; Kleinert, 1955; Cambiaggi, 1958; Ascher, 1961). An increasingly thick column of pulsatile clear aqueous regularly precedes a diurnal reduction in IOP, while an increasing blood content in the aqueous veins (Ascher, 1944; Thomassen et al., 1950) precedes a diurnal IOP rise.
6.3. Distal advance of oscillating pulse wave in vessel lumen
Appearance of an increased aqueous wave volume initially involves distal movement of an oscillating interface of aqueous and blood that is oriented transversely to the vein length (Fig. 5 Panel A–B). The oscillating aqueous-blood interface enters proximal tributary episcleral veins previously containing only blood (Johnstone, 2010). In the stratified aqueous veins a wider aqueous lamina develops. The rising phase of the axial aqueous wave has a greater velocity, greater amplitude and a steeper leading wave front than the falling phase.
Vessels previously containing lamina of aqueous and blood become completely filled with aqueous. The pulsatile aqueous lamina progresses distally into episcleral veins that previously contained only blood. Distal tributary episcleral vessels previously containing only blood now have an oscillatory discharge of aqueous into them during each systolic wave (Johnstone, 2010) (Fig. 5B–D).
Higher ocular pulse amplitude strongly correlated with a higher IOP in a group of normals and ocular hypertensives (Phillips et al., 1992). Such an increase in pulse amplitude in response to an increase in IOP may provide a simple mechanical coupling able to act as a short-term feedback mechanism to regulate the volume of pulsatile flow and thus regulate IOP.
6.4. Individual pulse waves characterized as a stroke volume
The concept of stroke volume is used to characterize pulsatile flow in the arterial system and serves as a surrogate for functional efficiency of the cardiac pump (Humphrey, 2002; Levick, 2003). Structural and functional parameters that determine stroke volume have placed study of cardiac output on a rational basis. In returning loops of the circulatory system such as the veins and lymphatics, the concept of “stroke volume” is also employed to describe and characterize pulsatile flow (Levick, 2003).
The aqueous outflow system represents a circulatory loop returning fluids to the heart somewhat analogous to the veins and lymphatics. The transient IOP increase associated with each systole causes an aqueous volume to discharge from Schlemm’s canal. The aqueous volume discharged with each pulse wave can also be described as a stroke volume. Structural and functional parameters that define stroke volume in the normal aqueous outflow system are the same parameters that must become abnormal in glaucoma. Examination of parameters identified in cardiac physiology as leading to abnormalities of stroke volume may provide useful tools for understanding aqueous outflow abnormalities (Johnstone, 2006, 2010).
7. Pulsatile flow abnormalities present in glaucoma
7.1. Compensation maximum
A number of studies document pulsatile flow abnormalities present in glaucoma (Table 1), abnormalities nicely described in Ascher’s aqueous vein treatise (Ascher, 1961). For example the “compensation maximum test” involves raising IOP by ophthalmodynamometry. When IOP is raised in normal eyes, pulsatile aqueous flow increases markedly but in glaucoma eyes, pulsatile flow is attenuated or stops (Kleinert, 1951; Ascher, 1961).
Table 1.
Aqueous outflow system pulsatile flow abnormalities observed in glaucoma.
| Finding | Details |
|---|---|
| Few aqueous veins with pulsatile flow identified. | Suggests diminished ability of outflow tissues to fill aqueous veins. |
| Pulsatile flow in visible veins decreased or absent. | Abnormality of tissues generating pulsatile flow. |
| Medication instillation increases pulsatile flow. | After new medication-induced lower homeostatic setpoint is reached, pulsatile flow slows. |
| Prior to instillation of next pilocarpine dosage pulsatile flow diminishes or stops and intraocular pressure rises. | Pilocarpine short duration of actions makes effect apparent. |
| Aqueous influx phenomenon in normals changes to blood influx phenomenon in glaucoma. | Follows occlusion of distal recipient aqueous vein. |
| “Compensation maximum” (Performed with ophthalmodynamometry) | Normal response of an increase of pulsatile aqueous vein flow caused by pressure on eye is reduced or absent. |
7.2. Aqueous influx phenomenon
An equilibrium is normally present in which a pulse wave of aqueous enters episcleral veins in systole while blood from the episcleral veins enters aqueous veins in diastole. The aqueous influx phenomenon occurs when a distal episcleral vein is intentionally occluded by external compression (Ascher, 1942a; Ascher, 1942c; De Vries, 1947; Ascher and Spurgeon, 1949; Weinstein, 1950). As a result of distal occlusion of a recipient aqueous vein there is more vigorous pulsatile flow in the proximal portion of the aqueous vein with increased velocity, increased amplitude of the aqueous lamina, and a steeper waveform. Proximal tributary episcleral veins that formerly contained blood become filled with aqueous. About one-half of all normals exhibit the aqueous influx phenomenon, while a blood influx phenomenon occurs in the rest (Ascher, 1961).
7.3. Blood influx phenomenon
By contrast, in almost all glaucoma eyes, a blood influx phenomenon is seen (Ascher, 1942d; De Vries, 1947; Goldmann, 1948). When the distal episcleral recipient vein is occluded, blood refluxes into the aqueous vein from tributary episcleral vessels proximal to the occlusion. Complete filling of the aqueous vein with blood results with blood even entering the scleral emissary of the aqueous vein. In one study of six patients with unilateral glaucoma, all showed aqueous reflux in the normal eye and blood reflux in the glaucoma eye (Ascher, 1942d). The lack of vigorous pulsatile aqueous flow and blood reflux into aqueous veins are thought to be manifestations of an abnormality of the outflow system in open angle glaucoma (Ascher, 1961). Administration of pilocarpine to enlarge Schlemm’s canal can cause the blood influx phenomenon to change into an aqueous influx phenomenon in glaucoma eyes (De Vries, 1947).
8. Medications that increase pulsatile flow in the aqueous veins
Three classes of medications are reported to cause an increase in pulsatile aqueous outflow (Table 2), miotics (Ascher, 1942a,, 1942b, 1942c; De Vries, 1947; Thomassen, 1947a, 1947b; Hodgson and MacDonald, 1954; Cambiaggi, 1958); adrenergics (Ascher, 1942b, 1942c; Goldmann, 1946a; De Vries, 1947; Kleinert, 1955; Johnstone et al., 2006) and prostaglandins (Johnstone et al., 2007, 2009). Following instillation of epinephrine and brimonidine the typical onset of a directly observable increase in pulsatile flow is about 5 min, while with miotics 5–20 min and with prostaglandins 20–30 min. Videography and a calibrating micrometer permit measurement of the aqueous stratum diameter. Measurement of the speed of movement of a bolus of blood provides a measurement of velocity (Fig. 2, Panel B3). The pulsatile aqueous flow increase following instillation of miotics has been observed to persist up to about 4 h and that with epinephrine for up to 2 h. The duration of the pulsatile flow response to brimonidine and prostaglandins persist for an hour but have not been studied beyond that time interval.
Table 2.
Medications reported to increase pulsatile aqueous outflow.
| Receptor class | Onset (minutes) |
Mechanisms of action suggested in literature. |
|---|---|---|
| Adrenergic | 5 | Aqueous pulse waves may face a reduced pressure head or “afterload” when precapillary arterioles constrict reducing volume of blood flow through capillary beds thus reducing flow and pressure head in episcleral veins. |
| Muscarinic | 5–20 | Ciliary muscle contraction rotates scleral spur, Schlemm’s canal enlarges, trabecular meshwork spaces enlarge, trabecular meshwork under greater tension may distend and recoil more forcefully in response to ocular transients. |
| Prostaglandin | 20–30 | Vasorelaxation of choroidal vasculature may result in an increased volume of blood entering the choroid during systole thus causing a larger pulse amplitude. |
These combined measurements provide the ability to measure volume of aqueous flow. Instillation of each of the above agents results in an increase in stroke volume and velocity like that illustrated in Fig. 5 B–D. The above studies indicate that more than one class of medication can act through a pulsatile flow mechanism. Although the final common pathway that causes an increase in pulsatile aqueous flow may be the same, mechanisms by which drug classes increase stroke volume may differ.
For example, the adrenergic agent brimonidine has been recently shown in the laboratory to rapidly reduce episcleral venous pressure (Reitsamer et al., 2006) The time course of episcleral venous pressure reduction corresponds to the onset of increased stroke volume of aqueous seen with adrenergic agents (Ascher, 1961; Johnstone et al., 2006). Veins are compliance vessels and are over two orders of magnitude more elastic than arteries. Adrenergic agents act primarily on precapillary arterioles to reduce the volume of flow to the capillary beds (Katzung, 2007). A reduction of blood flow to the capillary bed can reduce the volume of flow to the episcleral veins and thus the pressure within the veins. One adrenergic mechanism of action may be to reduce episcleral venous pressure that in turn reduces the pressure head or afterload (Levick, 2003) that the pulse waves of aqueous must exceed to permit aqueous discharge.
Pilocarpine causes ciliary muscle contraction, which increases tension on the scleral spur. Increased scleral spur tension causes a rotation that pulls the trabecular tissues further from the external wall of Schlemm’s canal and enlarges the canal lumen (Van Buskirk, 1982). The scleral spur movement causes lengthening of the distance between the trabecular meshwork attachments to Schwalbe’s line and scleral spur thus altering length–tension relationships. Tension on the ciliary muscle that rotates the scleral spur then enlarges Schlemm’s canal, juxtacanalicular space and spaces between trabecular lamellae (Van Buskirk, 1982). These altered structural relationships may permit greater movement of the trabecular tissues in response to ocular transients as well as greater flow through the tissues resulting in an increase in pulsatile flow.
An increase in the ocular pulse following prostaglandin instillation has been shown in four studies (McKibbin and Menage,1999; Geyer et al., 2001; Georgopoulos et al., 2002; Liu et al., 2002). The pulsatile flow increase has been suggested to result from latanoprost-induced vasorelaxation of the choroidal vasculature (Geyer et al., 2001; Georgopoulos et al., 2002). An increase in amplitude of the choroidal pulse wave induces an increase in the ocular pulse (Phillips et al., 1992).
All the prostanoid receptor genes are expressed in the trabecular meshwork (Kamphuis et al., 2001). Immunofluorescent labeling revealed expression of FP receptor protein in the outer portion of the trabecular meshwork, endothelial cells of SC, collector channels and aqueous veins (Schlotzer-Schrehardt et al., 2002). In addition, trabecular meshwork cells can produce PGE2 and PGF2alphs (Weinreb et al., 1983, 1988). An improvement of uveoscleral outflow has been found in most outflow studies of the mechanism of action of prostaglandins (Toris et al., 2008). However, the results of such studies are highly dependent on the methods used in measurement (Lim et al., 2008). Studies in mouse (Crowston et al., 2004), and human eyes (Ziai et al., 1993; Brubaker et al., 2001; Christiansen et al., 2004; Lim et al., 2008) have shown moderate to marked improvement in conventional aqueous outflow after exposure to prostaglandins.
Although multiple complex interactions are likely to be involved, the above discussed mechanisms illustrate that various classes of outflow drugs acting by different mechanisms may each result in an increase in volume of the aqueous pulse wave. Since multiple agents converge to increase pulsatile flow, each resulting in a reduction of IOP, cellular pathways that modulate pulsatile flow may be important in establishing homeostatic IOP setpoints in normal and glaucoma patients.
9. Conclusion
Aqueous outflow is pulsatile, the pulsatile flow becomes abnormal in glaucoma, and some medications that restore pulsatile flow to normal do so by increasing pulsatile aqueous outflow. Pulsatile flow studies in vivo provide a means to study normal mechanisms as well as abnormal mechanisms of aqueous outflow present in glaucoma. The effect of cellular responses to cyclic stresses that have been identified in the laboratory may be correlated with in vivo observations and provide a framework for interpretation of the laboratory observations. Modulation of cellular responses to cyclic stresses observed in the laboratory may provide a means to restore homeostasis in glaucoma.
Acknowledgements
Grant Support: NEI Core Grant EY01730, Research to Prevent Blindness, New York. Charles Applegate Glaucoma Research Fund.
References
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008 Apr;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher KW. Aqueous veins: preliminary note. Am. J. Ophthalmol. 1942a;25:31–38. [Google Scholar]
- Ascher KW. Local pharmacologic effects on aqueous veins. Am. J. Ophthalmol. 1942b;25:1301–1315. [Google Scholar]
- Ascher KW. Physiologic importance of the visible elimination of intraocular fluid. Am. J. Ophthalmol. 1942c;25:1174–1209. doi: 10.1016/j.ajo.2018.05.025. [DOI] [PubMed] [Google Scholar]
- Ascher KW. Glaucoma and the aqueous veins. Am. J. Ophthalmol. 1942d;25:1309–1315. [Google Scholar]
- Ascher KW. Backflow Phenomena in aqueous veins of normal and of glaucomatous eyes. Am. J. Ophthalmol. 1944;27:1074–1089. [Google Scholar]
- Ascher KW. Further observations on aqueous veins. Am. J. Ophthalmol. 1946;29:1373–1387. doi: 10.1016/0002-9394(46)92033-8. [DOI] [PubMed] [Google Scholar]
- Ascher KW. Aqueous veins and their significance for pathogenesis of glaucoma. Arch. Ophthalmol. 1949;42:66–76. doi: 10.1001/archopht.1949.00900050069006. [DOI] [PubMed] [Google Scholar]
- Ascher KW. The Aqueous Veins: Biomicroscopic Study of Aqueous Humor Elimination. Springfield, IL: Charles C Thomas; 1961. [Google Scholar]
- Ascher KW, Spurgeon WM. Compression tests on aqueous veins of glaucomatous eyes; application of hydrodynamic principles to the problem of intraocular-fluid elimination. Am. J. Ophthalmol. 1949;32:239–251. [PubMed] [Google Scholar]
- Ashton N. Anatomical study of Schlemm’s canal and aqueous veins by means of neoprene casts, part I. Brit. J. Ophthalmol. 1951;35:291. doi: 10.1136/bjo.35.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N. Anatomical study of Schlemm’s canal and aqueous veins by means of neoprene casts, part II, aqueous veins. Brit. J. Ophthalmol. 1952;36:265. doi: 10.1136/bjo.36.5.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz NW, Hoffman EA, Yool AJ, Stamer WD. Role of aquaporin-1 in trabecular meshwork cell homeostasis during mechanical strain. Exp. Eye Res. 2009 Jun 15;89:95–100. doi: 10.1016/j.exer.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill A. Blood circulation and fluid dynamics in the eye. Physiol. Rev. 1975;55:383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- Bill A. Basic physiology of the drainage of aqueous humor. Exp. Eye Res. 1977;25(Suppl.):291–304. doi: 10.1016/s0014-4835(77)80025-0. [DOI] [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest. Ophthalmol. Vis. Sci. 2001 Jun;42:1505–1513. [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest. Ophthalmol. Vis. Sci. 2003 Dec;44:5174–5181. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- Brubaker RF, Schoff EO, Nau CB, Carpenter SP, Chen K, Vandenburgh AM. Effects of AGN 192024, a new ocular hypotensive agent, on aqueous dynamics. Am. J. Ophthalmol. 2001 Jan;131:19–24. doi: 10.1016/s0002-9394(00)00843-6. [DOI] [PubMed] [Google Scholar]
- Cambiaggi A. Effeto della jaluronidasi sulla pressone intraocular e sull’asetto della vene dell’accqueo. Boll. Soc. di Biologia Sperimentale. 1958;34:1–7. [PubMed] [Google Scholar]
- Chow J, Liton PB, Luna C, Wong F, Gonzalez P. Effect of cellular senescence on the P2Y-receptor mediated calcium response in trabecular meshwork cells. Mol. Vis. 2007;13:1926–1933. [PMC free article] [PubMed] [Google Scholar]
- Christiansen GA, Nau CB, McLaren JW, Johnson DH. Mechanism of ocular hypotensive action of bimatoprost (Lumigan) in patients with ocular hypertension or glaucoma. Ophthalmology. 2004 Sep;111:1658–1662. doi: 10.1016/j.ophtha.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch. Ophthalmol. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- Crowston JG, Aihara M, Lindsey JD, Weinreb RN. Effect of latanoprost on outflow facility in the mouse. Invest. Ophthalmol. Vis. Sci. 2004 Jul;45:2240–2245. doi: 10.1167/iovs.03-0990. [DOI] [PubMed] [Google Scholar]
- De Vries S. Zichtbare Afvoer Van Het Kamerwater. Amsterdam: Drukkerij Kinsbergen; 1947. [Google Scholar]
- Forrester JV. The Eye: Basic Sciences in Practice. Edinburgh: WB Saunders Co; 2002. [Google Scholar]
- Fung Y. Biomechanics: Mechanical Properties of Living Tissues. New York: Springer; 1993. [Google Scholar]
- Fung YC. Biomechanics: Circulation. New York: Springer-Verlag; 1996. [Google Scholar]
- Georgopoulos GT, Diestelhorst M, Fisher R, Ruokonen P, Krieglstein GK. The short-term effect of latanoprost on intraocular pressure and pulsatile ocular blood flow. Acta Ophthalmol. Scand. 2002 Feb;80:54–58. doi: 10.1034/j.1600-0420.2002.800111.x. [DOI] [PubMed] [Google Scholar]
- Geyer O, Man O, Weintraub M, Silver DM. Acute effect of latanoprost on pulsatile ocular blood flow in normal eyes. Am. J. Ophthalmol. 2001 Feb;131:198–202. doi: 10.1016/s0002-9394(00)00797-2. [DOI] [PubMed] [Google Scholar]
- Goldmann H. Abfluss des Kammerwassers beim Menschen. Ophthalmologica. 1946a;111:146–152. doi: 10.1159/000300317. [DOI] [PubMed] [Google Scholar]
- Goldmann H. Weitere Mitteilung über den Abfluss des Kammerwassers beim Menschen. Ophthalmologica. 1946b;112:344–346. doi: 10.1159/000300402. [DOI] [PubMed] [Google Scholar]
- Goldmann H. Uber Abflussdruck und Glasstab-phanomen. Pathogenese des einfachen Glaukoms. Ophthalmologica. 1948;116:195–198. doi: 10.1159/000300593. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (1) Pressure effects within the near-physiological range (8–30 mmHg) Exp. Eye Res. 1975a;20:505–521. doi: 10.1016/0014-4835(75)90218-3. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (2) Pressures outside the physiological range (0 and 50 mmHg) Exp. Eye Res. 1975b;20:523–530. doi: 10.1016/0014-4835(75)90219-5. [DOI] [PubMed] [Google Scholar]
- Harvey W. The Anatomical Excercises: De Motu Cordis and De Circulatione Sanquinis in English Translation. New York: Dover Publications, Inc; 1995. [Google Scholar]
- Hodgson TH, MacDonald RK. Slitlamp studies on the flow of aqueous humor. Brit. J. Ophthalmol. 1954;38:266–272. doi: 10.1136/bjo.38.5.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. New York: Springer-Verlag; 2002. [Google Scholar]
- Johnstone MA. Pressure-dependent changes in nuclei and the process origins of the endothelial cells lining Schlemm’s canal. Invest. Ophthalmol. Vis. Sci. 1979;18:44–51. [PubMed] [Google Scholar]
- Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J. Glaucoma. 2004;13:421–438. doi: 10.1097/01.ijg.0000131757.63542.24. [DOI] [PubMed] [Google Scholar]
- Johnstone MA. A new model describes an aqueous outflow pump and explores causes of pump failure in glaucoma. In: Grehn H, Stamper R, editors. Glaucoma. Essentials in Ophthalmology. II. Heidelberg: Springer; 2006. pp. 3–34. [Google Scholar]
- Johnstone MA. Aqueous humor outflow. In: Stamper R, Lieberman MF, Drake MV, editors. Diagnosis and Therapy of the Glaucomas. St. Louis: Mosby; 2009. pp. 25–46. [Google Scholar]
- Johnstone MA. The importance of the aqueous veins in the diagnosis of glaucoma. In: Samples S, editor. The Glaucoma Book. New York: Springer; 2010. pp. 65–78. [Google Scholar]
- Johnstone MA, Grant WM. Pressure-dependent changes in structure of the aqueous outflow system in human and monkey eyes. Am. J. Ophthalmol. 1973;75:365–383. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- Johnstone MA, Martin E, Mills R. Brimonidine-dependent pulsatile aqueous discharge to the episcleral veins. Invest. Ophthalmol. Vis. Sci. 2006;47S:253. [Google Scholar]
- Johnstone MA, Martin E, Jamil A. Latanoprost instillation results in a rapid directly measurable increase in conventional aqueous outflow. Invest. Ophthalmol. 2007;48:76. [Google Scholar]
- Johnstone MA, Martin E, Jamil A. Travaprost instillation results in a rapid directly observable increase in conventional aqueous outflow in normal subjects. Invest. Ophthalmol. Vis. Sci. 2009;49S:28. [Google Scholar]
- Kamphuis W, Schneemann A, van Beek LM, Smit AB, Hoyng PF, Koya E. Prostanoid receptor gene expression profile in human trabecular meshwork: a quantitative real-time PCR approach. Invest. Ophthalmol. Vis. Sci. 2001 Dec;42:3209–3215. [PubMed] [Google Scholar]
- Katzung BG. Basic and Clinical Pharmacology. New York: McGraw Hill; 2007. [Google Scholar]
- Keller KE, Kelley MJ, Acott TS. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Invest. Ophthalmol. Vis. Sci. 2007 Mar;48:1164–1172. doi: 10.1167/iovs.06-0875. [DOI] [PubMed] [Google Scholar]
- Kinsey VE, Reddy DV. Chemistry and dynamics of aqueous humor. In: Prince JH, editor. The Rabbit in Eye Research. Springfield: Thomas; 1964. pp. 218–219. [Google Scholar]
- Kleinert H. The compensation maximum: a new glaucoma sign in aqueous veins. Arch. Ophthalmol. 1951;46:618–624. [PubMed] [Google Scholar]
- Kleinert H. Uber das Zustandekommen der augendrucksenkenden Wirkung des Adrenalins und anderer gefassverengender Pharmaka. von Graefes Arch. Ophthalmol. 1955;157:24–30. [PubMed] [Google Scholar]
- Levick JR. Cardiovascular Physiology. London: Arnold; 2003. [Google Scholar]
- Lim KS, Nau CB, O’Byrne MM, et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008 May;115:790–795. e4. doi: 10.1016/j.ophtha.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Luna C, Bodman M, Hong A, Epstein DL, Gonzalez P. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem. Biophys. Res. Commun. 2005 Dec 2;337:1229–1236. doi: 10.1016/j.bbrc.2005.09.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Li G, Luna C, Gonzalez P, Epstein DL. Cross-talk between TGF-beta1 and IL-6 in human trabecular meshwork cells. Mol. Vis. 2009;15:326–334. [PMC free article] [PubMed] [Google Scholar]
- Liu CJ, Ko YC, Cheng CY, Chou JC, Hsu WM, Liu JH. Effect of latanoprost 0.005% and brimonidine tartrate 0.2% on pulsatile ocular blood flow in normal tension glaucoma. Br. J. Ophthalmol. 2002 Nov;86:1236–1239. doi: 10.1136/bjo.86.11.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin M, Menage MJ. The effect of once-daily latanoprost on intraocular pressure and pulsatile ocular blood flow in normal tension glaucoma. Eye. 1999;13:31–34. doi: 10.1038/eye.1999.6. [DOI] [PubMed] [Google Scholar]
- Nichols WM, O’Rourke MF. McDonald’s Blood Flow in Arteries. London: Hodder Arnold; 2005. [Google Scholar]
- Phillips CI, Tsukahara S, Hosaka O, Adams W. Ocular pulsation correlates with ocular tension: the choroid as piston for an aqueous pump? Ophthalmic Res. 1992;24:338–343. doi: 10.1159/000267190. [DOI] [PubMed] [Google Scholar]
- Ramos RF, Stamer WD. Effects of cyclic intraocular pressure on conventional outflow facility. Invest. Ophthalmol. Vis. Sci. 2008 Jan;49:275–281. doi: 10.1167/iovs.07-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos RF, Sumida GM, Stamer WD. Cyclic mechanical stress and trabecular meshwork cell contractility. Invest. Ophthalmol. Vis. Sci. 2009 Aug;50:3826–3832. doi: 10.1167/iovs.08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsamer HA, Posey M, Kiel JW. Effects of a topical alpha2 adrenergic agonist on ciliary blood flow and aqueous production in rabbits. Exp. Eye Res. 2006 Mar;82:405–415. doi: 10.1016/j.exer.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Sato Y, Matsuo T, Ohtsuki H. A novel gene (oculomedin) induced by mechanical stretching in human trabecular cells of the eye. Biochem. Biophys. Res. Commun. 1999 Jun 7;259:349–351. doi: 10.1006/bbrc.1999.0797. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Zenkel M, Nusing RM. Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Invest. Ophthalmol. Vis. Sci. 2002 May;43:1475–1487. [PubMed] [Google Scholar]
- Stepanik J. Diurnal tonographic variations and their relation to visible aqueous outflow. Am. J. Ophthalmol. 1954a;38:629–645. doi: 10.1016/0002-9394(54)90287-1. [DOI] [PubMed] [Google Scholar]
- Stepanik J. Measuring velocity of flow in aqueous veins. Am. J. Ophthalmol. 1954b;37:918–922. doi: 10.1016/0002-9394(54)91933-9. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 1999 Oct;40:2577–2582. [PubMed] [Google Scholar]
- Thomassen TL. The venous tension of eyes suffering from simple glaucoma. Acta Ophthalmol. 1947a;25:221. [Google Scholar]
- Thomassen TL. On aqueous veins. Acta Ophthalmol. 1947b;25:369–378. [Google Scholar]
- Thomassen TL, Bakken K. Anatomical investigations into the exit canals of aqueous humor. Acta Ophthalmol. 1951;29:257–268. [Google Scholar]
- Thomassen TL, Perkins ES, Dobree JH. Aqueous veins in glaucomatous eyes. Brit. J. Ophthalmol. 1950;34:221–227. doi: 10.1136/bjo.34.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv. Ophthalmol. 2008 Nov;53(Suppl. 1):S107–S120. doi: 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 1998 Jul;39:1361–1371. [PubMed] [Google Scholar]
- Van Buskirk EM. Anatomic correlates of changing aqueous outflow facility in excised human eyes. Invest. Ophthalmol. Vis. Sci. 1982;22:625–632. [PubMed] [Google Scholar]
- Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest. Ophthalmol. Vis. Sci. 2005;46:2857–2868. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Mitchell MD, Polansky JR. Prostaglandin production by human trabecular cells: in vitro inhibition by dexamethasone. Invest. Ophthalmol. Vis. Sci. 1983;24:1541–1545. [PubMed] [Google Scholar]
- Weinreb RN, Polansky JR, Alvarado JA, Mitchell MD. Arachidonic acid metabolism in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 1988;29:1708–1712. [PubMed] [Google Scholar]
- Weinstein P. New concepts regarding anterior drainage of the eye. Brit. J. Ophthalmol. 1950;34:161–168. doi: 10.1136/bjo.34.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuDunn D. The effect of mechanical strain on matrix metalloproteinase production by bovine trabecular meshwork cells. Curr. Eye Res. 2001 May;22:394–397. doi: 10.1076/ceyr.22.5.394.5500. [DOI] [PubMed] [Google Scholar]
- WuDunn D. Mechanobiology of trabecular meshwork cells. Exp. Eye Res. 2009 Apr;88:718–723. doi: 10.1016/j.exer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Ziai N, Dolan JW, Kacere RD, Brubaker RF. The effects on aqueous dynamics of PhXA41, a new prostaglandin F2 alpha analogue, after topical application in normal and ocular hypertensive human eyes. Arch. Ophthalmol. 1993 Oct;111:1351–1358. doi: 10.1001/archopht.1993.01090100059027. [DOI] [PubMed] [Google Scholar]