Abstract
The whitebacked planthopper Sogatella furcifera (Horváth) (Hemiptera: Delphacidae) is one of the most destructive pests of rice in East and Southeast Asia. It is also a long-distance migratory insect and population size fluctuates frequently in these rice regions along the middle and lower Yangtze River. We analyzed the population seasonality of S. furcifera based on field surveys, light trap catching, and meteorological factors. We found that many S. furcifera were retained in local late rice in 2012, due to continuous rain and slightly windy weather conditions during the migration period. These results suggest that a new pattern of population fluctuation may occur where resident S. furcifera are dispersed into a single medium rice during harvest period, then rebound and thrive in late rice when there are suitable temperatures in September. Although the residency of S. furcifera in late rice fields in 2012 seems to be a special case, our findings suggest that S. furcifera exhibit a type of facultative migration. Our research also illuminates studies of the migration events of S. furcifera and benefits our understanding of the dynamics of S. furcifera in Hunan Province.
Keywords: Sogatella furcifera (Horváth), population seasonality, facultative migration, residency
Rice (Oryza sativa) is the main cereal crop that provides the primary source of calories for around half population of the world, especially in Asia. However, various pests in paddy fields restrict rice yield and quality. The whitebacked planthoppers, Sogatella furcifera (Horváth) (Hemiptera: Delphacidae), cause main yield loss of rice in China and the other East- and southeast-Asian countries (Riley et al. 1991). Moreover, S. furcifera is a vector of viral diseases in rice, such as southern rice black-streak dwarf virus which led to rice failures across Hunan province, China in 2009 (Zhang et al. 2008, Ma et al. 2012). S. furcifera was first considered as a sporadic pest in rice fields during the early 20th century in Hunan Province. Since the Green Revolution and the large-scale use of chemical pesticides and improved cultivars (Jennings 1974), S. furcifera has evolved an increasing resistance to insecticides, adapted to resistant rice varieties and became one of major insect pests of rice that severely threaten rice security in Hunan Province (Xiao and Tang 2007).
S. furcifera can migrate a long distance (Kisimoto 1971, Bottrell and Schoenly 2012) and it overwintering region is restricted to an area with a 10°C of average temperature in January. Hunan Province is located at a critical juncture of the migration route in spring–summer and the return migrant route in autumn. Therefore, rice was often infested severely with S. furcifera since the early 2000s. Recent studies showed that resistances of rice planthoppers to insecticides are still in debate (Otuka et al. 2008, 2009; Bass et al. 2011; Peng et al. 2012). Studies on the migration of rice planthoppers have revealed the mechanisms of take-off, cruising, and landing, as well as weather/climate conditions that affect migratory patterns (Riley et al. 1991; Otuka et al. 2006, 2009; Shen et al. 2011). However, little were known about S. furcifera that fails to migrate and resides in a particular location.
In this study, S. furcifera population data were collected from field surveys, and light trap catches were compared with meteorological data from 2010 to 2012. This data were collected to address the following questions: 1) What were the population dynamics of S. furcifera in late rice fields of 2012? 2) Why did some S. furcifera reside in late rice, in Hunan 2012? 3) Which was the better choice for S. furcifera: a long-distance migration or reside in a particular location? Our results provide evidence that S. furcifera exhibits a type of facultative migration.
Materials and Methods
Host Plants and Field Management
From 2010 to 2012, late rice (Fengyuanyong No 299, hybrid rice) was sowed in mid-June and then transplanted to fields with a 20 cm by 18 cm spacing in Changsha (E112.08°, N28.49°), where the late rice was planted more than 1,000 ha and single cropping medium rice was about 10 ha. The single cropping medium rice (Y Liangyou No 1, hybrid rice), which was sporadically distributed around the late rice, was transplanted with a 24 cm by 20 cm spacing in mid-May. 1,300 m2 late rice and 1,300 m2 single cropping medium rice were used as the system survey fields in this study. The fields were supplemented with 600 kg of 14-5-6 (N-P-K) and 120 kg of potassium chlorate as the base fertilizer, and 60 kg of urea as topdressing fertilizer. The 14-5-6 fertilizer was applied 2 d before the transplanting and the urea was applied 7 d after transplanting. During the experiment, Armure (Syngenta Crop Protection Co., Ltd.) was used to control rice disease at rice late tillering-stage and initial heading-stage in late rice and single rice system survey fields. No pesticides were applied to the late rice system survey fields, but Dinotefuran (Mitsui Chemicals Inc.) was sprayed on the single cropping medium rice, due to an outbreak of planthoppers at booting-stage and heading-stage.
Field Surveys
A continuous 3 years of population sampling of S. furcifera was carried out in paddy fields once a week between June and October from 2010 to 2012. In each survey, 10 samples (two rice hills per sample, and hills means clump, which a hectare can accommodate about 27,000 hills rice) were investigated uniformly using plant- shaking method with a white porcelain plate (40 cm by 30 cm by 2 cm) that was used to flush out the polymorphisms planthoppers (Hu et al. 2011). The numbers of nymphs and macropterous and brachypterous adults were recorded. Fifty macropterous S. furcifera females collected randomly from paddy fields in 2011 and 2012 and were dissected to observe the developmental stage of ovarian (grades 1–5) referencing the method by Chen et al. (1979) and Dong et al. (2011). The grades 1 and 2 were considered as a low-level developmental stage of ovary and the grades 3–5 were considered as the high-level stage. The daily low and high temperatures from the paddy field were recorded by a WeatherLink Datalogger (Davis, CA, USA).
Light Trap Survey
A 30-W black-light lamp (JDB1-III, Jiaduo Science, Industry and Trade Co. Ltd., China) was used to trap rice planthoppers near the system survey field. The black light lamp (wavelength 365 nm) was shielded with a top cover (diameter 60 cm) to protect the lamp from rain and used three crash screens (594 mm by 212 mm by 5 mm) at 120° angles, an ultrared rays device and a funnel-shaped entrance with eight collection bags. The trap was operated every night automatically by light intensity (from 10:30 to 22:30, UTC) from early March to late October, and the number of S. furcifera trapped were recorded.
Meteorological Data
The amount of rainfall at the study site was obtained from the China meteorological data sharing service system data (nationwide coverage, including real-time acquisition of 2,419 stations, 24-h, 0.25° grid data, http://cdc.cma.gov.cn/). Data on wind speed at 850 hPa level obtained from the NCEP/NCAR (National Center for Environmental Prediction/National Center for Atmospheric Research). Re-analysis data (global coverage from 1948 to present, 6-h, 1.0° grid data, http://rda.ucar.edu/) was obtained for 2010–2012.
Data Analysis
Population dynamics of S. furcifera were mapped with the software Origin 8.0. Wind maps were based on 850 hPa re-analysis data of wind fields in the GrADS (Environmental Systems Research Institute, USA) software. Rainfall maps were made with ArcGIS (Environmental Systems Research Institute) software.
Results
Planthopper Population Dynamics in Rice Fields
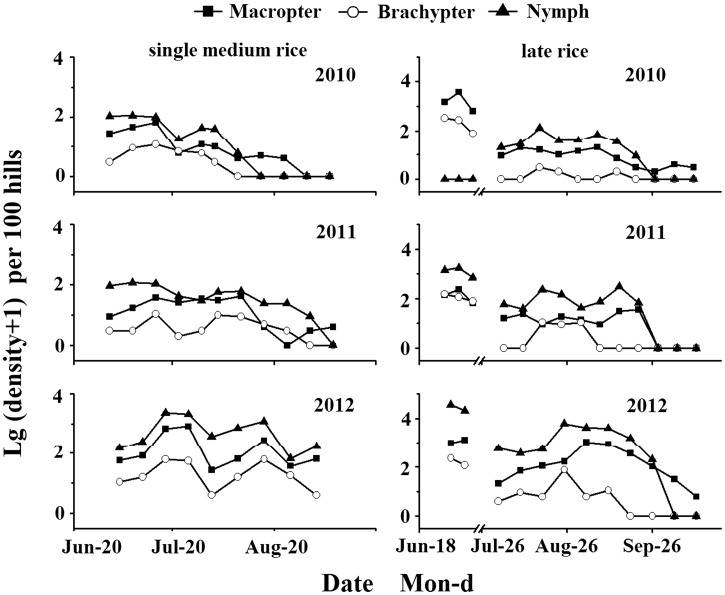
The S. furcifera population fluctuated with a tendency to increase during the booting stage, followed by a sharp decrease to almost 0 at the mature stage of rice (Fig. 1). Compared with the levels of S. furcifera populations in 2010 and 2011, the population in 2012 was elevated from August until mid-September. The peak of macropterous adults and nymphs occurred on 2 September 2012, with up to 1,070 macropterous adults and 4,125 nymphs per 100 hills, while the population size was less than 350 per 100 hills in 2010 and 2011. In 2012, a surge in the population densities of S. furcifera was recorded in single cropping medium rice in mid July (Fig. 1) and the populations increased from 249 nymphs and 83 macropterous adults on July 11 to 2,304 nymphs and 694 macropterous adults on July 18, followed by a sharp reduction in mid August.
Fig. 1.
Log density of S. furcifera in single medium rice and late rice fields from June to October between 2010 and 2012. The breaks represent the harvest period. Three charts at left show population density of S. furcifera in single medium rice, and the right show that in late rice.
Population Dynamics in Light Trap Catches of Planthoppers
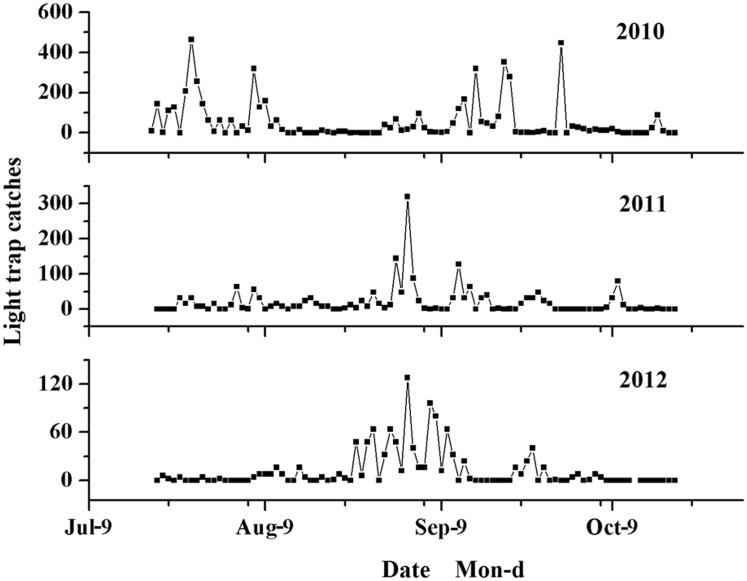
The daily catches of S. furcifera were highly variable over 3 years (Fig. 2). There were more S. furcifera caught in 2010 with discrepancies in the temporal distribution of high catch days. A serial of peaks that the daily catches of S. furcifera was more than 100 in the whole late rice were observed in 2010, but in 2011 and 2012 only two and one peak were recorded, respectively. The main peak in 2010 occurred in mid September, but in 2011 and 2012 both occurred in early September. During the late rice seasons of 2010, 2011, and 2012, there were a total of 5,060, 1,811, and 1,068 S. furcifera collected by light trap, respectively.
Fig. 2.
Daily catches of S. furcifera in a light trap from 2010 to 2012.
Ovary Development of S. furcifera
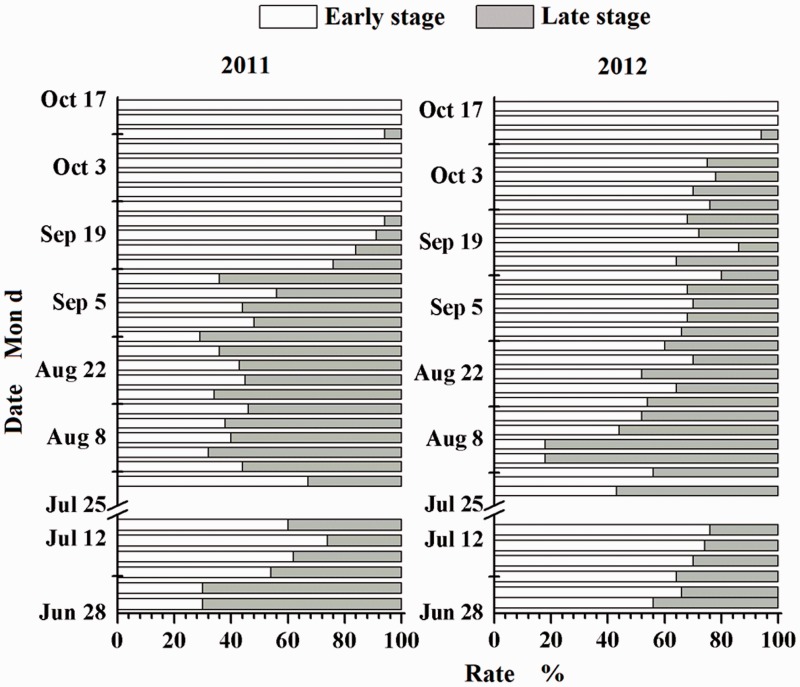
During early August and early September, 2011, ∼60% of S. furcifera macropterous female adult’s ovaries had developed into a high-level stage (Fig. 3), while at mid-September, S. furcifera females with a high-level stage ovary were less. However, the ovary development of 92% and 14% females still maintained a high level on 22 August and 18 September in 2012, respectively, which was significantly different from 2011.
Fig. 3.
The development of ovaries in macropterous females of S. furcifera in 2011 and 2012. The breaks represent the harvest period.
Temperature Monitoring
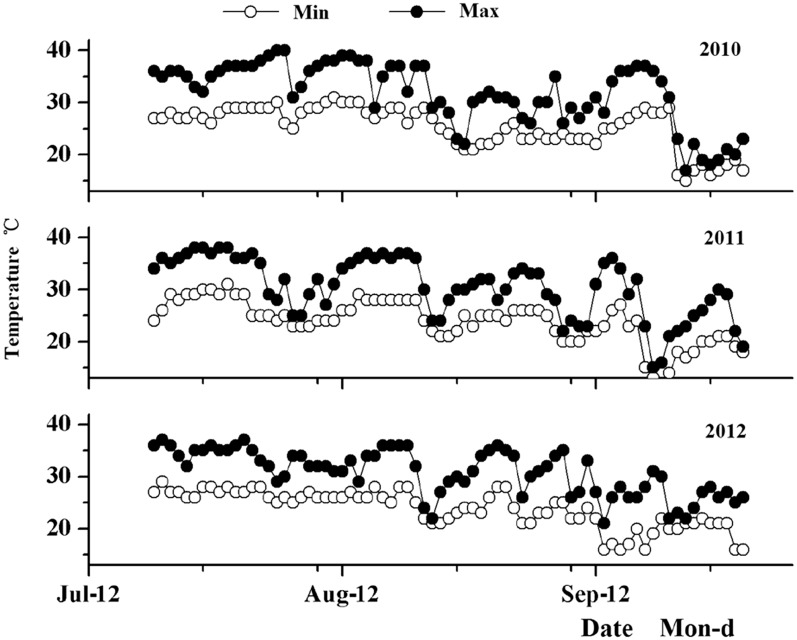
From 2010 to 2012, the highest temperature was recorded on 1 and 2 August 2010 (Fig. 4) during which the temperature reached 40°C on both days. The lowest temperature was 12°C on 20 September 2011. The temperature exceeded 35°C for 31 days in 2010, 24 days in 2011, and 19 days in 2012. Compared with 2010 and 2011, the daily temperature maximum and minimum curves were relatively stable (around 27°C) during September 2012.
Fig. 4.
The maximum and minimum daily temperature in rice fields from 2010 to 2012.
General Weather Conditions
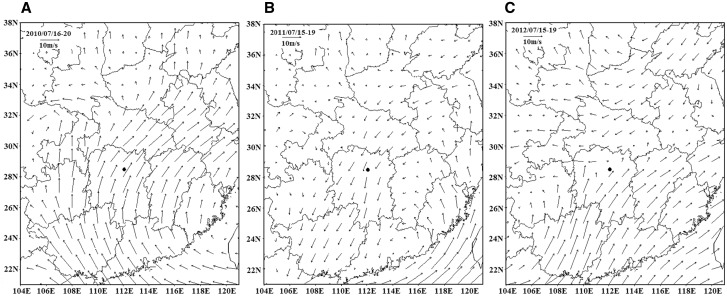
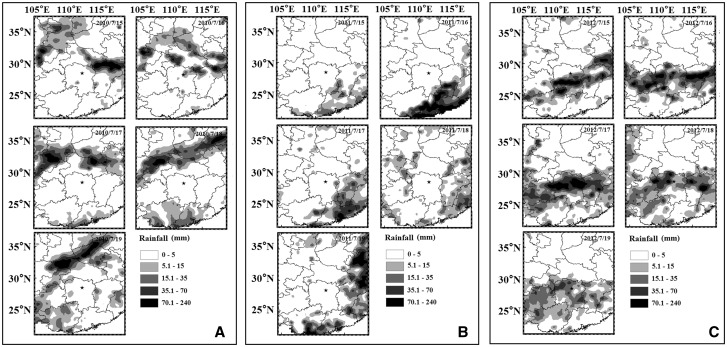
In mid-July of 2010, the middle-lower reaches of the Yangtze River were affected by a strong Western Pacific subtropical high and the Northeast cold vortex was weaker than in it had been in the past. These particular changes in Western Pacific subtropical high and northeast cold vortex contributed to the relatively strong and stable southwest current that ran through East China and South China. Winds swept over the Changsha at speeds to 12–15 m/s at 850 hPa (Fig. 5). However, a deep cold vortex developed over northeast China and east-central China was dominated by an upper-level trough during mid-July in 2011. This resulted in a southwest current that followed the coastline and contributed to the Jianghuai cyclone over Changsha. The north-easterly wind speed was 8–10 m/s at the level of 850 hPa. In comparison with 2010 and 2011, 2012 was characterized by a light wind at 850 hPa over Changsha, and can attributed to the formation of a convergence zone between a southwest current and a cold air mass. Moreover, this weather situation brought heavy rainfall in 2012 (Fig. 6) in comparison to the rainfall in 2010 and 2011.
Fig. 5.
The horizontal wind field at a level of 850 hPa during the harvest period 2010–2012. (A) Horizontal wind field in 2010; (B) horizontal wind field in 2011; and (C) horizontal wind field in 2012. Solid circle shows the location of observation.
Fig. 6.
The rainfall in Changsha during the harvest period from 2010 to 2012. (A) Rainfall in 2010. (B) Rainfall in 2011. (C) Rainfall in 2012. The solid pentagram shows the location of observation.
Discussion
It is widely accepted that S. furcifera is migratory species, and this aspect of their life histories has been considered an important adaptive strategy (Otuka et al. 2006, 2008). For many years the population size of S. furcifera was small, with 350 individuals or fewer per 100 hills. However, in 2012 an abnormally large number of S. furcifera was recorded in late rice and the population continued to grow until mid-September (the milky stage). The peak density of S. furcifera occurred in the g2 generation (immigration was g0) in Changsha, which was inconsistent with Matsumura’s (1996) finding that the peak density of S. furcifera was in g1. A surge in population density of S. furcifera occurred in mid-July, 2012 in single medium rice when the early rice was being harvested and the late rice was being transplanted. However, it was inconceivable that the numbers of 3–5th nymph rose suddenly to 1,623 individuals per 100 hills on July 18, which was inconsistent with the normal growth patterns based field investigations on July 11. Therefore, the most possible reason for the surge in population growth of S. furcifera in 2012 July in single medium rice was the diffusion of S. furcifera 3–5th nymphs and adults that unsuccessfully migrated and lost the habitat during the early rice harvesting period. However, many farmers applied chemical pesticides to control the growth of S. furcifera in single medium rice fields in early August 2012 (at heading-stage), but the first pesticides for the late rice by most farmers were used between 18 and 22 August. The surviving S. furcifera and their offspring were forced back to the late rice fields from the surrounding single medium rice fields. Therefore, it was reasonable to believe that the single medium rice was a refuge for S. furcifera during the harvest period and large number of S. furcifera was over the hump safely.
Early immigration played a crucial role in establishing populations in the destination and population seasonal dynamics were positively correlated with the amount of early immigrants. Light trap is a useful and most widely used method to monitor the immigration of S. furcifera. In 2012, 1,068 S. furcifera were collected by light trap in late rice, which was only 20% in 2010. However, the S. furcifera population density in 2012 was much larger than that in 2010. Compared with 2010, therefore, there was little possibility for long-distance immigration S. furcifera in the late rice as the constructive species that resulted in sharply increase in population density in mid-September.
The widespread occurrence of migratory insects is mostly consistent with the hypothesis of a trade-off between flight and fecundity (Johnson 1963). This hypothesis explains that large amounts of energy are necessary to maintain flight capability and this energy expenditure is negatively associated with reproductive potential (Roff 1984, Crnolrak and Roff 2002). The effects of this trade-off have been identified in planthoppers, in that the migration mostly occurs at a particular time that is called the adult teneral or post-teneral stage (ovarian development stage I or II) and the presence of stronger flight muscles (Chen et al. 1979, Dong et al. 2011). After migration and feeding at appropriate destination, ovariole and lateral oviduct of S. furcifera can develop rapidly in preparation for copulation and reproduction. In this article, until to early October in 2012, there were still about 20% high-level stage ovary of S. furcifera macropterous adults to be found in the paddy field, which indicated that more than 20% of the macropterous adults in Changsha had remained to lay eggs and maintain a high population density on local rice from August to October. The resident macropterous adults of S. furcifera and their reproductive success positively influenced the thriving populations of S. furcifera in 2012 late rice. S. furcifera are highly fecund when temperatures are suitable (Ammaro et al. 1980). Temperature stress is not conducive for planthopper survival or reproduction (Liu and Zhang 2013) and this partly accounts for the long-distance migration strategy. The average temperature in September 2012 was ∼27–30°C. The period and magnitude of extreme heat days was feebler in 2012 than in 2010 or 2011. Therefore, the weather conditions in September 2012 were amenable to S. furcifera survival and reproduction, and favored the formation of brachyptery.
There is a close relationship between migratory insect and weather conditions: wind is thought to be the dispersal vehicle for many migratory organisms, and wind plays an important role in migration (Johnson 1963; Munoz et al. 2004; Feng et al. 2006, 2007, Syobu et al. 2011). During the Bai-u rainy season, rice planthoppers migrate to north-eastern China, Korea and Japan from Myanmar, Laos and Vietnam via strong southerly, south-westerly winds or typhoons (Otuka et al. 2006, 2008, 2009, 2012; Shen et al. 2011). The average wind speed at the level of 850 hPa over Changsha in 2010 and 2011 were both more than 8 m/s. But in 2012, the wind speed was only 3 m/s, which indicated that there was not enough air flow to transport S. furcifera on their migration (Feng et al. 2006, 2007). Furthermore, the northwestern Pacific subtropical high was weaker in 2012 than in 2010 and 2011, which led to an unfavorable weather condition that Changsha was the convergence zone for the southwest current and cold air masses. These compositive weather conditions contributed to strong downdrafts and heavy rainfall in 2012. The weather conditions were not favorable for S. furcifera to take-off, climb up, or migration. Moreover, the possibility of a forced landing for S. furcifera by rainfall was very high, even if they had taken off (Riley et al. 1991, Otuka et al. 2006). Therefore, because of continuous rain and low wind levels, most S. furcifera macropterous adults could not have emigrated successfully and were locally stranded.
Migration is a successful evolutionary strategy for insect populations to increase their chances of survival and reproduction when they are affected by variations in nutrients of host plants and abiotic factors (Holland et al. 2006). Migration is also considered as a ‘multigenerational bet-hedging strategy’ that allows offspring to avoid deteriorating environmental conditions and being deprived of habitat (Holland et al. 2006). However, due to the flexibility and uncertainty in environmental factors, S. furcifera also faced many challenges during the migration, such as a low-temperature threshold (Shen et al. 2011), rainfall stress, and subsiding airflow (Otuka et al. 2008, 2009). Most importantly, S. furcifera bet their offspring on migration, because conditions at the destination were not always favorable to establishing populations (Wada et al. 1987). Therefore, S. furcifera may remain or emigrate wholly based on conditions and environmental factors in their habitats.
In conclusion, many S. furcifera resided in Changsha rice in 2012 and this can be attributed to continuous rain and low wind levels. The S. furcifera dispersed into single medium rice during the harvest period, but then re-invaded and thrived in late rice with suitable temperatures in September. Although the residence of S. furcifera in late rice fields in 2012 is a special case, it gave us the opportunity to study migration events of S. furcifera during these unusual environmental conditions. It was also beneficial to help us understand the dynamics of S. furcifera in Hunan Province.
Acknowledgments
We thank Prof. Zhai BP (Nanjing Agricultural University), Prof. Zhao ZM (Southwest University) and Prof. Liu XD (Nanjing Agricultural University) for their valuable suggestions for the data analysis and manuscript composition. This work was supported by the National Sci-Tech R&D Project of China (Grant 2012BAD19B03), the Natural Science Foundation of Hunan Province, China (Grant 14JJ6063), the National “973” Program of China (Grant 2010CB126200) and the Agro-Industry R&D Special Fund of China (Grant 200903051).
References Cited
- Ammaroe D., Lamie I., Khodeir I. 1980. Biology of the planthopper Sogatella furcifera HORV in Egypt (Horn., Delphacidae). Deutsche Entomologische Zeitschrift 27: 21–27. [Google Scholar]
- Bass C., Carvalho R. A., Oliphant L., Puinean A. M., Field L. M., Nauen R., Williamson M. S., Moores G., Gorman K. 2011. Overexpression of a cytochrome P450 monooxygenase, CYP6ER1, is associated with resistance to imidacloprid in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 20: 763–773. [DOI] [PubMed] [Google Scholar]
- Bottrell D. G., Schoenly K. G. 2012. Resurrecting the ghost of green revolutions past: the brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia-Pac. Entomol. 15: 122–140. [Google Scholar]
- Chen J. C., Cheng X. N., Yang L. M., Yin H. T. 1979. The ovarian development of the brown planthopper (Nilaparvata lugens) and its relation to migration. Acta Ecologica Sinica 22: 280–287 (in Chinese). [Google Scholar]
- Crnolrak P., Roff D. A. 2002. Trade-offs to flight capability in Gryllus firmus: the influence of whole-organism respiration rate on fitness. J. Evol. Biol. 15: 388–398. [Google Scholar]
- Dong S. Z., Ma Y., Hou Y., Yu X. P., Ye G. Y. 2011. Development of an ELISA for evaluating the reproductive status of female brown planthopper, Nilaparvata lugens, by measuring vitellogenin and vitellin levels. Entomologia Experimentalis et Applicata 139: 103–110. [Google Scholar]
- Feng H. Q., Wu K. M., Ni Y. X., Cheng D. F., Guo Y. Y. 2006. Nocturnal migration of dragonflies over the Bohai Sea in northern China. Ecol. Entomol. 31: 511–520. [Google Scholar]
- Feng H. Q., Zhang Y. H., Wu K. M., Cheng D. F., Guo Y. Y. 2007. Nocturnal windborne migration of ground beetles, particularly Pseudoophonus griseus (Coleoptera: Carabidae), in China. Agric. For. Entomol. 9: 103–113. [Google Scholar]
- Holland R. A., Wikelski M., Wilcove D. S. 2006. How and why do insects migrate?. Science 313: 794–796. [DOI] [PubMed] [Google Scholar]
- Hu G., Cheng X. N., Qi G. J., Wang F. Y., Lu F., Zhang X. X., Zhai B. P. 2011. Rice planting systems, global warming, and outbreaks of Nilaparvata lugens (Stål). Bull. Entomol. Res. 101: 187–199. [DOI] [PubMed] [Google Scholar]
- Jennings P. R. 1974. Rice breeding and world food production. Science 186: 1085–1088. [DOI] [PubMed] [Google Scholar]
- Johnson C. G. 1963. Physiological factors in insect migration by flight. Nature 198: 423–427. [Google Scholar]
- Kisimoto R. 1971. Long-distance migration of planthoppers, Sogatella furcifera and Nilaparvata lugens, pp. 201–216. In Rice Insects, Tropical Agricultural Research Centre, Tokyo, Japan. [Google Scholar]
- Liu X. D., Zhang A. M. 2013. High temperature determines the ups and downs of small brown planthopper Laodelphax striatellus population. Insect Sci. 20: 385–392. [DOI] [PubMed] [Google Scholar]
- Ma M. Y., Peng Z. P., He Y. 2012. Effects of temperature on functional response of Anagrus nilaparvatae Pang et Wang (Hymenoptera: Mymaridae) on the eggs of whitebacked planthopper, Sogatella furcifera Horváth and brown planthopper, Nilaparvata lugens Stål. J. Integ. Agric. 8: 1313–1320. [Google Scholar]
- Matsumura M. 1996. Population dynamics of the whitebacked Planthopper, Sogatella furcifera (Hemiptera: Delphacidae) with special reference to the relationship between its population growth and the growth stage of rice plants. Res. Popul. Ecol. 38: 19–25. [Google Scholar]
- Munoz J., Felicisimo A. M., Cabezas F., Burgaz A. R., Martinez I. 2004. Wind as a long-distance dispersal vehicle in the southern hemisphere. Science 304: 1144–1147. [DOI] [PubMed] [Google Scholar]
- Otuka A., Watanabe T., Suzuki Y., Matsumura M., Chino F. M., Kondo T., Kamimuro T. 2006. A migration analysis of Sogatella furcifera (Horváth) (Homoptera: Delphacidae) using hourly catches and a three-dimensional simulation model. Agric. For. Entomol. 8: 35–47. [Google Scholar]
- Otuka A., Matsumura M., Watanabe T., Dihn T. V. 2008. A migration analysis for rice planthoppers, Sogatella furcifera (Horváth) and Nilaparvata lugens (Stål) (Homoptera: Delphacidae), emigrating from northern Vietnam from April to May. Appl. Entomol. Zool. 43: 527–534. [Google Scholar]
- Otuka A., Matsumura M., Watanabe T. 2009. The search for domestic migration of the white-backed planthopper, Sogatella furcifera (Horváth) (Homoptera: Delphacidae), in Japan. Appl. Entomol. Zool. 44:379–386. [Google Scholar]
- Otuka A., Huang S. H., Sanada-Morimura S., Matsumura M. 2012. Migration analysis of Nilaparvata lugens (Hemiptera: Delphacidae) from the Philippines to Taiwan under typhoon-induced windy conditions. Appl. Entomol. Zool. 47: 263–271. [Google Scholar]
- Peng Q., Tang Q. Y., Wu J. L., Miao Q. L., Cheng J. A. 2012. Determining the geographic origin of the brown planthopper, Nilaparvata lugens, using trace element content. Insect Sci. 19: 21–29. [Google Scholar]
- Riley J. R., Cheng X. N., Zhang X. X., Reynolds D. R., Xu G. M., Smith A. D., Cheng J. Y., Bao A. D., Zhai B. P. 1991. The long-distance migration of Nilaparvata lugens (Stål) (Delphacidae) in China: radar observations of mass return flight in the autumn. Ecol. Entomol. 16: 471–489. [Google Scholar]
- Roff D. A. 1984. On the cost of being able to fly: a study of wing polymorphism in two species of crickets. Oecologia 63: 30–37. [DOI] [PubMed] [Google Scholar]
- Shen H. M., Lu J. P., Zhou J. Y., Zhang X. X., Cheng X. N., Zhai B. P. 2011. Source areas and landing mechanism of early immigration of white-backed planthoppers Sogatella furcifera (Horváth) in Yunnan, 2009. Acta Ecologica Sinica 31: 4350–4364 (in Chinese). [Google Scholar]
- Syobu S., Otuka A., Matsumura M. 2011. Trap catches of the small brown planthopper, Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae), in northern Kyushu district, Japan in relation to weather conditions. Appl. Entomol. Zool. 46: 41–50. [Google Scholar]
- Wada T., Seino H., Ogawa Y., Nakasuga T. 1987. Evidence of autumn overseas migration in the rice planthoppers, Nilaparvata lugens and Sogatella furcifera: analysis of light trap catches and associated weather patterns. Ecol. Entomol. 12: 321–330. [Google Scholar]
- Xiao T. G., Tang J. X. 2007. Effects of the susceptibility of rice varieties to Sogatella furcifera on nymphal development and reproduction of Microvelia horvathi through a food chain. Insect Sci. 14: 317–321. [Google Scholar]
- Zhang H. M., Yang J., Chen J. P., Adams M. J. 2008. A black-streaked dwarf disease on rice in China is caused by a novel fijivirus. Arch. Virol. 153: 1893–1898. [DOI] [PubMed] [Google Scholar]