Abstract
Demotispa neivai Bondar (Coleoptera: Chrysomelidae) damage oil palm fruits, which makes it necessary to develop products to control this insect. The mortality, repellency, and antifeeding effects on adults of D. neivai of six plant extracts of Azadirachta indica A. Juss. (Sapindales: Meliaceae), Ricinus communis (L.) (Malpighiaes: Euphorbiaceae), Citrus sinensis Oesbek (Sapindales: Rutaceae), Nicotiana tabacum (L.) (Slanales: Solanaceae), Capsicum annuum (L.) (Solanales: Solanaceae), and Artemisia absinthium (L.) (Asterales: Asteraceae) were determined: 1) the lethal concentration LC50-90, lethal time of D. neivai was evaluated after spraying the fruits of oil palm; 2) repellent effects of each ingredient were evaluated by calculating the index of repellency; 3) antifeeding effects with the rate of inhibition calculated between doses of 20 and 24 g/liter. The mortality of D. neivai was higher with the extracts Ci. sinensis, R. communis, N. tabacum, and Ca. annuum. The mortality of D. neivai increased in the first 72 hr in all treatments. The extracts of N. tabacum, Ca. annuum, and A. indica were more repellent to D. neivai that those of Ci. sinensis, Ar. Absinthium, and R. communis. Antifeeding effect was higher with Ci. sinensis and R. communis. The increased mortality of D. neivai by Ci. sinensis can be explained by the effect of this compound on the respiratory system of insects. Extracts of Ci. sinensis, R. communis, N. tabacum, and Ca. annuum repelled and caused mortality of D. neivai and, thus, can be used in integrate pest management programs of this pest in oil palm plantations.
Keywords: antifeeding effect, Coleoptera, insect pest management, mortality, repellency
Among the factors that can limit production of oil palm Elaeis guineensis (Jacquin) (Arecales: Arecaceae), there is Demotispa neivai (Bondar) (Coleoptera: Chrysomelidae), a pest in Brazil, Colombia, Ecuador, Panama, Suriname, and Venezuela (Genty et al. 1978, Martínez et al. 2013). This insect damages the exocarp of oil palm fruits that become tenement and with ash color drying and tissue from the first week of formation (Aldana et al. 2004, Martínez et al. 2013). D. neivai was also reported on Bactris gasipaes Kunth (Arecales: Arecaceae), Cocos botryophora Mart. (Arecales: Arecaceae), Cocos nucifera(L.) (Arecales: Arecaceae), and Desmoncus polyacantha Mart. (Arecales: Arecaceae) (Staines 1996, 2002). Furthermore, damage by D. neivai prevents the maturity of the racim with losses estimated in 7% of extraction oil from E. guineensis (Genty et al. 1978, Martínez et al. 2008).
Pesticides such as acephate, methamidophos, and monocrotophos are used in oil palm plantations by the control of D. neivai (Genty et al. 1978); however, recent studies demonstrated the presence of pesticide traces in crude palm oil (Yeoh et al. 2006). Conventional insecticides are expensive and can cause collateral effects such as pest resistance, environmental pollution, toxic waste, emergence of new pests, and reducing insect fauna (Simmonds et al. 2002, Haouas et al. 2011).
In this sense, plant extracts represent an alternative for pest control as repellents, deterrents of oviposition and feeding, growth regulators, and toxicity to larvae and adults with low pollution and quick degradation in the environment (Bourguet et al. 2000, Tavares et al. 2009, Chermenskaya et al. 2010). Plant secondary metabolites such as lignans, neolignans, alkaloids, chalcones, kawapirones, flavones, essential oils, and amides are important in plant–insect relationships (Parmar et al. 1997, Abou-Fakhr et al. 2001, Martín et al. 2011). Repellent from plants are obtained from compounds with unpleasant odors or irritants (Parmar et al. 1997, Bourguet et al. 2000, Abou-Fakhr et al. 2001) and with phagoinhibiting is and biocide activity (Akhtar and Isman 2004, Abbassy et al. 2007). Preliminary studies showed lethal and sublethal effects of aqueous plant extracts on oil palm pests, especially on Rhynchophorus palmarum (L.) larvae (Coleoptera: Curculionidae) and D. neivai adults (Pérez and Iannacone 2006, Martínez et al. 2008).
There are a variety of plants that have insecticidal properties, deterrents, and repellents used in agriculture for pest control; however, these plants could be an alternative in the control of oil palm pest. This study evaluated the lethal concentration LC50-90, lethal time, and sublethal effects of six plants extracts on D. neivai adults in the laboratory and semi-controlled field experiments, in order to contribute for the development of new strategies for controlling this insect pest affecting an important source of food.
Materials and Methods
Insects
Adults of D. neivai were collected in commercial plantations of oil palm with 10 years old in the Municipality of Puerto Wilches, Santander, Colombia (07°20′ N, 73°54′ W) with 28.46°C mean temperature, 76–92% relative humidity, 145–225 h of sunshine per year, and 2,168 mm annual rainfall. Insects were daily collected by hand and transferred to the laboratory of the Entomology of the Research Center in Oil Palm (Cenipalma) in Barrancabermeja, Santander, Colombia, in plastic containers (25 by 40 cm), with perforated lid to allow air flow and fed on palm exocarp. Healthy males and females without amputations or apparent malformations were used in the bioassays.
Plant Extracts
Six species of plants were used as sources of natural products in this study (Table 1 ). Azadirachta indica A Juss (Sapindales: Meliaceae) is a herb native to Asia and introduced in America, Ricinus communis (L.) (Malpighiales: Euphorbiaceae) also from India, Citrus sinensis Oesbek (Sapindales: Rutaceae) of Central Asia and distributed throughout the Americas, Nicotiana tabacum (L.) (Solanales: Solanaceae) native to tropical America, Capsicum annuum (L.) (Solanales: Solanaceae) native to America, and Artemisia absinthium (L.) (Asterales: Asteraceae) distributed in Europe and introduced in America. Tissues of these plants were collected from experimental field agrobiology Safer-Agrisave (Medellin, Colombia) and dried in an oven at 35°C for 3 wk in darkness. Subsequently, tissues were ground and stored in glass jars (1,000 ml) at 18 ± 2°C in the dark until extraction. Preparations of extracts were made with 10 g of a sample from each plant in an Erlenmeyer flask of 100 ml with 50 ml of methanol. Flasks were covered with aluminum foil and placed in agitation at 100 oscillations per minute during 24 h (OS-300 Allsheng, China). Suspensions were obtained, filtered through meshes of tissue and transferred to a flask of 250 ml for evaporation in a rotary evaporator (Büchi R-114 AG, Switzeland) at 30 ± 2°C. The resulting residue was weighed and dissolved in acetone to produce the primary solution of 400 g per plant. The primary solution per plant extract was diluted with distilled water to obtain concentrations of six series adjusted to 4, 8, 12, 16, 20, and 24 g/liter.
Table 1.
Plant material used to prepare extracts for studies of their bioactivity against adults of D. neivai (Coleoptera: Chrysomelidae)
| Family name | Scientific name | Tissue used | Date of collection |
|---|---|---|---|
| Asteraceae | Ar. absinthium | Leaves | Jan./2010 |
| Euphorbiaceae | R. communis | Leaves | Jan./2010 |
| Meliaceae | Az. indica | Seeds | Jan./2010 |
| Rutaceae | C. sinensis | Fruits | Jan./2010 |
| Solanaceae | C. annuum | Fruits | Feb./2010 |
| Solanaceae | N. tabacum | Leaves | Feb./2010 |
Determination of LC50-90 and Semi-Controlled Conditions Test
Six concentrations besides the control (solvent control/liter distilled water) were used per plant extracts: 4, 8, 12, 16, 20, and 24 g/liter of distilled water. Concentrations of the extracts were applied in 5 μl of topical solution in the body of each individual of D. neivai. One hundred and twenty insects were used per dose in polystyrene containers (10 by 10 cm) and fed on exocarp palm. Mortality was recorded every 72 h during 15 days. In the field controlled test, 50 insects were caged in bags wrapping a racim of palm oil. Treatments consisted of plant extract with five replications per treatment. The lethal concentration LC50-90 was applied directly on each racim of fruits and the control had distilled water applied with manual pump spray Royal Condor at 40 psi pressure and a volume of 200 cc. Mortality was corrected in the laboratory and semi-controlled field bioassays (Abbott 1925).
Repellency Test
Four Petri dishes were used as an arena, connected to a central board with plastic pipes and diagonally at an angle of 45°. The others dishes were distributed around them in equidistant distances and two plates were put together symmetrically opposed. Fifty adults of D. neivai were released into the central board and the control group received exocarp palm. The LC90 was applied in the two opposite plates and non-exposed ones represented the control. Four replications per concentration of extract and a control were evaluated by the number of individuals per plate after 24 hr and calculating the repellency index RI: RI = 2 G/(G + P), where G is the percentage of insects in the treatment and P is the percentage of insects in control. The extract was classified as neutral if the index was equal to one (1); attractive, higher than one (1), and repellent; lower than one (1).
Antifeeding Effect
The area of exocarp of oil palm consumed by D. neivai was calculated with a millimeter mesh per fruits during 24 hr according to LC50-90 extract. After every 24 hr, the percentage of area consumed (estimated visually with the mesh) was achieved by obtaining food and inhibition index FII: FII = [(1 − T/C) × 100], where T is the food consumption per extract and C is the control.
Statistical Analysis
The parameters LC50-90 and its confidence limits were determined by logistic regression based on the concentration probit-mortality with the program XLSTAT-PRO v.7.5 for Windows (XLSTAT 2004). Mortality was evaluated under the semi-controlled conditions and repellency by ANOVA with the test HSD with a significance level (P ≤ 0.05) (Tukey 1949). The antifeeding effect was evaluated using the paired t test or Wilcoxon. Data from the bioassay mortality, repellency, and antifeeding effect on D. neivai in semi-controlled conditions were analyzed with SAS User v. 9.0 for Windows (SAS 2002).
Results
Mortality
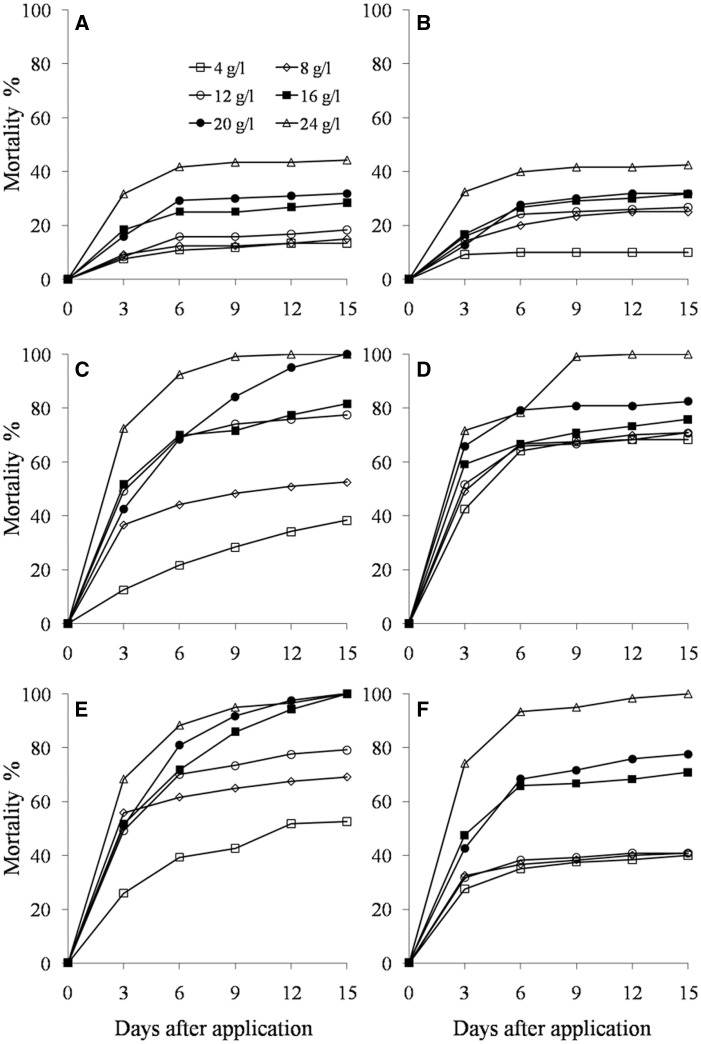
The homogeneous pattern of response to higher concentrations indicated that the Ci. sinensis, R. communis, N. tabacum, and Ca. annuum caused higher mortality of D. neivai and lethal effect on this insect with variable values as estimated by the regression model (Table 1). The best results were obtained with concentrations of 20 and 24 g/liter of afore mentioned extracts. The mortality of this insect between concentrations of the each extract showed adjustment by Probit (X2, P < 0.001). This value was lower with A. indica (X2 = 11.92, P < 0.0006) and Ar. absinthium (X2 = 81.77, P < 0.001). The mortality of D. neivai with the concentrations of Ci. sinensis, R. communis, N. tabacum, and Ca. annuum was lower compared with that of A. indica and Ar. absinthium. The mortality of D. neivai was higher and increased up to 3 days between the concentration tested with ∼90% from 72 to 144 h of exposure to the plant extracts Ci. sinensis, R. communis, N. tabacum, and Ca. annuum and 50% for Ar. absinthium and A. indica (Fig. 1 ).
Fig. 1.
Mortality of D. neivai (Coleoptera: Chrysomelidae) adults by concentration of Ar. absinthium (A), A. indica (B), C. annuum (C), C. sinensis (D), N. tabacum (E), and R. communis (F) during 15 days to calculate the LC50-90 (P < 0.0001).
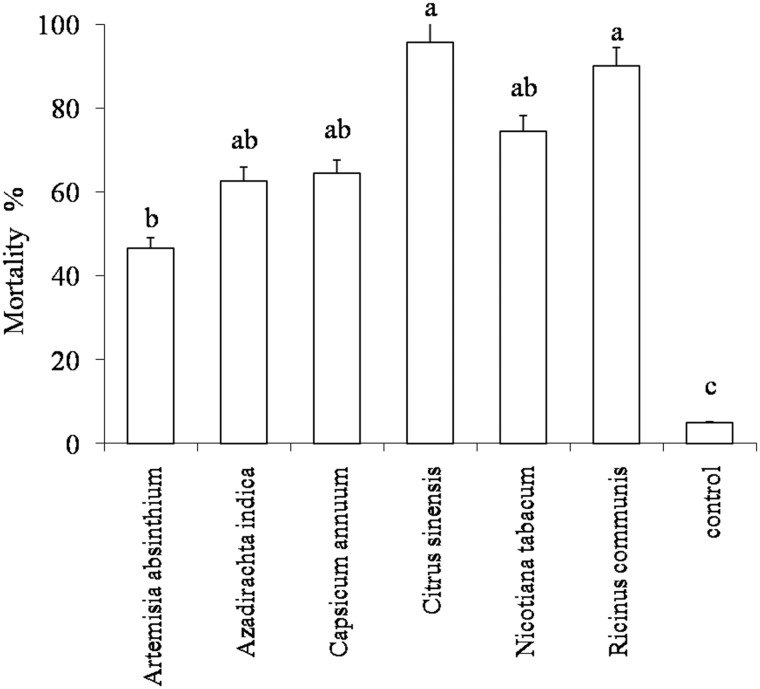
The mortality of D. neivai was similar in semi-controlled conditions in the field with the estimated concentration for the LC90 (F1,28 = 4.85, P < 0.05) by Tukey test. The Ci. sinensis and R. communis showed lethal effects on this insect with 95.5 and 89.9% mortality, respectively, followed by N. tabacum, Ca. annuum, and A. indica, 74.4, 64.4, and 62.6%. The mortality rate was lower with Ar. absinthium, 46.6% (Fig. 2 ).
Fig. 2.
Mortality in semi-controlled conditions of D. neivai (Coleoptera: Chrysomelidae) adults by plant extracts (Tukey P < 0.05).
Repellent Effect
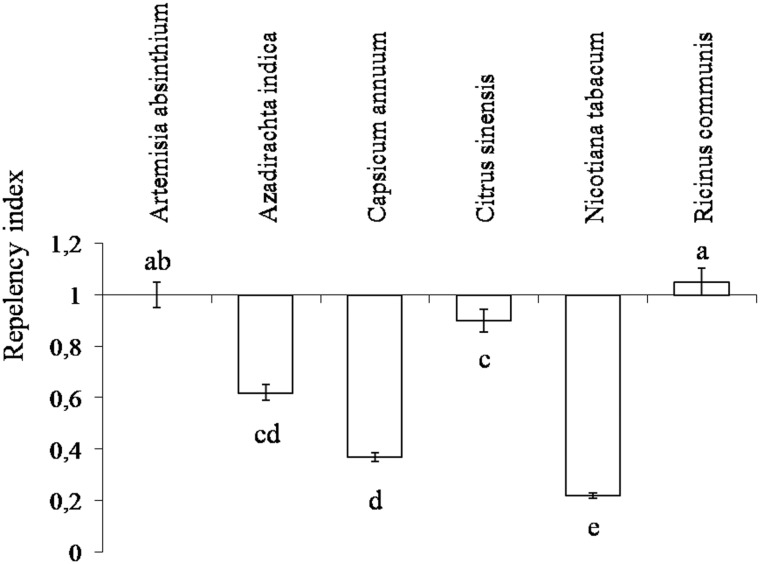
The repellency index was higher with N. tabacum (RI = 0.22), Ca. annuum (RI = 0.37), and A. indica (RI = 0.62). Ci. sinensis (RI = 0.9), Ar. absinthium (RI = 1), and R. communis (RI = 1.05) with values varying between treatments (F1,28 = 8.65, P < 0.05) and forming different groups by Tukey test (Fig. 3 ).
Fig. 3.
Repellency of D. neivai (Coleoptera: Chrysomelidae) adults by plant extracts (Tukey P < 0.05).
Antifeeding Effect
The plant extracts showed high antifeeding activity for D. neivai (Table 2 ) with the amount of food consumed by adults of this insect differing according to the concentration. The consumption of exocarp of oil palm was lower with the estimated lethal concentration LC50 and higher with the LC90 with variations up to 95% FII. The FII of R. communis and N. tabacum was higher than with Ar. absinthium. Adults of D. neivai had significant response (F1,28 = 8.65, P < 0.05) with Ca. annuum, Ci. Sinensis, and A. indica with moderate ingestion of these compounds (Table 3 ).
Table 2.
Activity and response of the lethal concentrations of plant extracts on D. neivai (Coleoptera: Chrysomelidae)
| Ar. absinthium | LC50 | 1.89 | 1.55–2.39 | 81.77 | <0.0001 |
| LC90 | 3.62 | 3.05–4.44 | |||
| LC99 | 6.57 | 5.54–8.07 | |||
| Az. indica | LC50 | 3.73 | 2.75–4.18 | 11.92 | 0.0006 |
| LC90 | 9.69 | 6.40–11.5 | |||
| LC99 | 14.5 | 9.34–20.9 | |||
| C. annuum | LC50 | 0.26 | 0.24–0.27 | 221.52 | <0.0001 |
| LC90 | 0.51 | 0.47–0.57 | |||
| LC99 | 1.09 | 0.93–1.33 | |||
| C. sinensis | LC50 | 0.22 | 0.48–3.54 | 12.09 | <0.00005 |
| LC90 | 0.70 | 0.41–1.14 | |||
| LC99 | 1.80 | 0.75–1.24 | |||
| N. tabacum | LC50 | 0.21 | 0.20–0.23 | 216.19 | <0.0001 |
| LC90 | 0.41 | 0.37–0.45 | |||
| LC99 | 0.83 | 0.71–1.00 | |||
| R. communis | LC50 | 0.32 | 0.30–0.35 | 183.64 | <0.0001 |
| LC90 | 0.84 | 0.74–0.98 | |||
| LC99 | 2.35 | 1.85–3.25 |
C, concentrations causing 50, 90, and 99% mortality; E, estimated value; IC, confidence interval; Chi, chi-square value. Significance level at P < 0.0001.
Table 3.
Index of food inhibition (FII) by plant extracts on D. neivai (Coleoptera: Chrysomelidae) adults (P < 0.05, compared by Wilcoxon)
| Extract | Concentration | P < 0.05 | FII (%) |
|---|---|---|---|
| Ar. absinthium | LC50 | 0.001 | — |
| LC90 | <0.0001 | 11.33 | |
| Az. indica | LC50 | <0.0001 | 35.85 |
| LC90 | <0.0001 | 70.49 | |
| Ca. annuum | LC50 | <0.0001 | 61.12 |
| LC90 | <0.0001 | 95.76 | |
| Ci. sinensis | LC50 | <0.0001 | 45.08 |
| LC90 | <0.0001 | 79.71 | |
| N. tabacum | LC50 | <0.0001 | 65.37 |
| LC90 | <0.0001 | 100.00 | |
| R. communis | LC50 | <0.0001 | 63.05 |
| LC90 | <0.0001 | 100.00 |
Discussion
Plant extracts have potential for integrated management of phytophagous insects in oil palm (Pérez and Iannacone 2006, 2008). The insecticidal activity of six plant extracts was evaluated against D. neivai, the lethal and sublethal effects were observed in the bioassays. The extracts Ci. sinensis, R. communis, Ca. annuum, and N. tabacum showed significant effect on adults of D. neivai with lethal effects in insects just after the exposure raising 100% mortality at 72 and 144 h. The dose– response bioassays showed increased toxicity of D. neivai with increasing concentrations and differing between Ar. absinthium and A. indica. Similar results were reported for beetles with increased concentration of plant extracts (Kim et al. 2003, Cerna-Chávez et al. 2010). Ci. sinensis was toxic to Callosobruchus chinensis (L.) and Sitophilus oryzae (L.) (Coleoptera: Curculionidae), R. communis to Sitophilus zeamais Motsch (Coleoptera: Curculionidae), Ca. annuum, and N. tabacum to larvae of Lepidoptera (Park et al. 2003, Vandenborre et al. 2010, Ahn et al. 2011). A. indica and Ar. absinthium extracts were not as active on D. neivai, but were lethal to many insect pests (Ahn et al. 1998, Scott et al. 2003, Kessler et al. 2006).
The plant extracts have repellent and attractive effects on D. neivai. N. tabacum and Ca. annuum showed repellent effect on D. neivai, while Ci. sinensis, Ar. Absinthium, and R. communis altered the behavior of this insect. The repellents compounds obtained of N. tabacum and Ca. annuum altered mating behavior, oviposition, and food preference of insects (Feeny et al. 1989, Jaenson et al. 2005, Delphia et al. 2007). The response to volatile plants can vary among arthropods. For example, volatile compounds found in orange fruit Citrus aurantium (L.) (Sapindales: Rutaceae) were attractive to Anastrepha ludens (Loew) (Diptera: Tephritidae) and repellent for Culex pipiens (Coquillett) (Diptera: Culicidae) (Won-Sik et al. 2002, Rasgado et al. 2009). In eucalipts leaves, the 1,8-cineol was not repellent to most insects (Pavela 2011). On the other hand, 1,8-cineol was attractive to orchid bees of the Euglossina genus (Williams and Whitten 1983). In our study, the repellent effect caused by N. tabacum and Ca. annuum can help disperse the populations of D. neivai and reduce damage on oil palm fruits. Different plants have been used as repellent against insect vectors of diseases, the use of N. tabacum is a powerful repellent against mosquito that causes malaria in humans while Ca. annuum is used to disperse weevil pest on stored products (Lale 1992, Karunamoorthi et al. 2009, Sõukand et al. 2010).
The extracts of N. tabacum, R. communis, and Ca. annuum had high antifeeding effect (P ≤ 0.05). Lethal concentration LC90 caused greater inhibition on D. neivai. Secondary metabolites of feeding deterrents are chemicals that inhibit food behavior of insects (Schoonhoven et al. 2005). Compounds such as alkaloids, terpenoids, and phenolic compounds found in nature can inhibit the absorption of food by insects (Wei et al. 2000, Koul 2008). Feeding reduction or inhibition by organic extracts or plant allelochemicals have been demonstrated for several insect orders. Aqueous extracts of R. communis leaves were active against C. chinensis (Upasani et al. 2003) and Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) (Pavela et al. 2010). R. communis mixed with fatty acids had an indirect antifeeding effect on Atta sexdens (Hymenoptera: Formicidae) in symbiosis with the fungus Leucoagaricus gongylophorus (A, Moller) (Agaricales: Agarocaceae) (Bigi et al. 2004). N. tabacum did not affect C. chinensis and C. maculatus (Khalequzzaman and Osman-Goni 2009), while the strongest effects were found on Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) (Musser et al. 2005). Antifeeding effect was found on Spodoptera frugiperda (Smith), Helicoverpa zea (Boddie), Heliothis virescens (F.), and Heliothis subflexa (Guenée) (Lepidoptera: Noctuidae) using Ca. annuum extracts (Ahn et al. 2011).
The selectivity of Ci. sinensis, R. communis, N. tabacum, and Ca. annuum may allow controlling one or more insect species or plants when applied simultaneously. The insecticide action of these plants can be due to synergism of compounds and its ability to penetrate the insect body through respiratory system. In addition, this too produced an enzymatic phagous-inhibition during digestion and as allelochemicals by interfering in chemical communication. Ci. sinensis, R. communis, N. tabacum, and Ca. annuum extracts have lethal and sublethal effects on D. neivai and with potential to manage populations of this insect.
Acknowledgments
We thank to Colombian Oil Palm Research Center (CENIPALMA), Oleaginosas Las Brisas (Colombia), Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brasil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Brasil), and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (Brasil).
References Cited
- Abbassy M. A., Abdelgaleil S. A. M., Belal A. S. H., Abdel Rasoul A. A. 2007. Insecticidal, antifeedant and antifungal activities of two glucosides isolated from the seeds of Simmondsia chinensis. Ind. Crop. Prod. 26: 345–350. [Google Scholar]
- Abbott W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–266. [Google Scholar]
- Abou-Fakhr H., Zournajian E. M. H., Talhouk S. 2001. Efficacy of extracts of Melia azedarach L. callus, leaves and fruits against adults of the sweet potato whitefly Bemisia tabaci. J. Appl. Entomol. 125: 483–488. [Google Scholar]
- Ahn Y. J., Lee S. B., Lee H. S., Kim G. H. 1998. Insecticidal and acaricidal activity of carvacrol and b-thujaplicine derived from Thujopsis dolabrata var. hondai sawdust. J. Chem. Ecol. 24: 81–90. [Google Scholar]
- Ahn S. J., Badenes-Pérez F. R., Heckel D. G. 2011. A host–plant specialist, Helicoverpa assulta, is more tolerant to capsaicin from Capsicum annuum than other noctuid species. J. Insect Physiol. 57: 1212–1219. [DOI] [PubMed] [Google Scholar]
- Akhtar Y., Isman M. 2004. Feeding responses of specialist herbivores to plant extracts and pure allelochemicals: effects of prolonged exposure. Entomologia Experimentalis et Applicata 111: 201–208. [Google Scholar]
- Aldana J. A., Calvache H., Cataño J. E., Hernández J. 2004. Aspectos biológicos y alternativas de control de Imatidium neivai Bondar (Coleoptera: Chrysomelidae). Palmas 25: 240–248. [Google Scholar]
- Bigi M. F., Torkomian V. L., De Groote S. T., Hebling M. J., Bueno O. C., Pagnocca F. C., Fernandes J. B., Vieira P. C., Da Silva M. F. 2004. Activity of Ricinus communis (Euphorbiaceae) and ricinine against the leafcutting ant Atta sexdens rubropilosa (Hymenoptera: Formicidae) and the symbiotic fungus Leucoagaricus gongylophorus. Pest Manage. Sci. 60: 933–938. [DOI] [PubMed] [Google Scholar]
- Bourguet D., Genissel A., Raymond M. 2000. Insecticide resistance and dominance levels. J. Econ. Entomol. 93: 1588–1595. [DOI] [PubMed] [Google Scholar]
- Cerna-Chávez E., Guevara L., Landeros J., Badii M. H., Ochoa Y. M., Olalde V. 2010. Evaluación de aceites y extractos vegetales para el control de Sitophilus zeamaiz y su efecto en la calidad de semilla de maíz. Revista de la Facultad de Ciencias Agrarias 42: 135–145. [Google Scholar]
- Chermenskaya T. D., Stepanycheva E. A., Shchenikova A. V., Chakaeva A. S. 2010. Insectoacaricidal and deterrent activities of extracts of Kyrgyzstan plants against three agricultural pests. Indu. Crop. Prod. 32: 157–163. [Google Scholar]
- Delphia C. M., Mescher M. C., De Moraes C. M. 2007. Induction of plant volatiles by herbivores with different feeding habits and the effects of induced defenses on host-plant selection by thrips. J. Chem. Ecol. 33: 997–1012. [DOI] [PubMed] [Google Scholar]
- Feeny P., Stadler E., Ahman I., Carter M. 1989. Effects of plant odor on oviposition by the black swallowtail butterfly, Papilio polyxenes (Lepidoptera, Papilionidae). J. Insect Behav. 2: 803–827. [Google Scholar]
- Genty P., Desmier de Chenon R., Morin J. 1978. Les ravages du palmier a huile en Ámerique Latine. Oléagineux 33: 326–420. [Google Scholar]
- Haouas D., Guido F., Monia B. H. K., Habib B. H. M. 2011. Identification of an insecticidal polyacetylene derivative from Chrysanthemum macrotum leaves. Ind. Crop. Prod. 34: 1128–1134. [Google Scholar]
- Jaenson T. G. T., Palsson K., Borg-Karlson A. K. 2005. Evaluation of extracts and oils of tick repellent plants from Sweden. Med. Veter. Entomol. 19: 345–352. [DOI] [PubMed] [Google Scholar]
- Karunamoorthi K., Ilango K., Endale A. 2009. Ethnobotanical survey of knowledge and usage custom of traditional insect/mosquito repellent plants among the Ethiopian Oromo ethnic group. J. Ethnopharmacol. 125: 224–229. [DOI] [PubMed] [Google Scholar]
- Kessler A., Halitschke R., Diezel C., Baldwin I. T. 2006. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 148: 280–292. [DOI] [PubMed] [Google Scholar]
- Khalequzzaman M., Osman-Goni S. H. M. 2009. Toxic potentials of some plant powders on survival and development of Callosobruchus maculatus (F.) and Callosobruchus chinensis L. J. Life Earth Sci. 3: 1–6. [Google Scholar]
- Kim S. I., Park C., Ohh M. H., Cho H. C., Ahn Y. J. 2003. Contact and fumigant activities of aromatic plants extracts and essential oils against Lasioderma serricorne (Coleoptera: Anobiidae). J. Stored Prod. Res. 39: 11–19. [Google Scholar]
- Koul O. 2008. Phytochemicals and insect control: an antifeedant approach. Crit. Rev. Plant Sci. 27: 1–24. [Google Scholar]
- Lale N. E. S. 1992. Oviposition-deterrent and repellent effects of products from dry chilli pepper fruits, Capsicum species on Callosobruchus maculatus. Postharvest Biol. Technol. 1: 343–348. [Google Scholar]
- Martín L., Julio L. F., Burillo J., Sanz J., Mainara A. M., González-Coloma A. 2011. Comparative chemistry and insect antifeedant action of traditional (Clevenger and 318 Soxhlet) and supercritical extracts (CO2) of two cultivated wormwood (Artemisia 319 absinthium L.) populations. Ind. Crop. Prod. 34: 1615–1621. [Google Scholar]
- Martínez L. C., Valencia C., Aldana R. C. 2008. Efecto letal y subletal causado por un extracto cítrico sobre Demotispa neivai (Coleoptera: Chrysomelidae). Palmas 29: 39–46. [Google Scholar]
- Martínez L. C., Plata-Rueda A., Zanuncio J. C., Leite G. L. D., Serrão J. E. 2013. Morphology and morphometry of Demotispa neivai (Coleoptera: Chrysomelidae) adults. Ann. Entomol. Soc. Am. 106: 164–169. [Google Scholar]
- Musser R. O., Cipollini D. F., Hum-Musser S. M., Williams S. A., Brown J. K., Felton G. W. 2005. Evidence that the caterpillar salivary enzyme glucose oxidase provides herbivore offense in solanaceous plants. Arch. Insect Biochem. Physiol. 58: 128–137. [DOI] [PubMed] [Google Scholar]
- Park I. K., Leeb S. L., Choib D. H., Park J. D., Ahna Y. J. 2003. Insecticidal activities of constituents identified in the essential oil from leaves of Chamaecyparis obtusa against Callosobruchus chinensis (L.) and Sitophilus oryzae (L.). J. Stored Prod. Res. 39: 375–384. [Google Scholar]
- Parmar V. S., Jain S. C., Bisht K. S., Jain R., Taneja P., Jha A., Tyagi O. M., Prasad A. K., Wengel J., Olsen C. E., et al. 1997. Phytochemistry of the genus Piper. Phytochemistry 46: 597–673. [Google Scholar]
- Pavela R. 2011. Insecticidal and repellent activity of selected essential oils against of the pollen beetle, Meligethes aeneus (Fabricius) adults. Ind. Crop. Prod. 34: 888–892. [Google Scholar]
- Pavela R., Sajfrtovab M., Sovovab H., Barnetc M., Karbanb J. 2010. The insecticidal activity of Tanacetum parthenium (L.) Schultz Bip extracts obtained by supercritical fluid extraction and hydrodistillation. Ind. Crop. Prod. 31: 449–454. [Google Scholar]
- Pérez D., Iannacone J. 2006. Efectividad de extractos botánicos de diez plantas sobre la mortalidad y repelencia de larvas de Rhynchophorus palmarum L., insecto plaga del pijuayo Bactris gasipaes Kunth en la Amazonía del Perú. Agricultura Técnica 343 66: 21–30. [Google Scholar]
- Pérez D., Iannacone J. 2008. Mortalidad y repelencia en Eupalamides cyparissias (Lepidoptera: Castniidae), plaga de la palma aceitera Elaeis guineensis, por efecto de diez extractos botánicos. Revista de la Sociedad Entomologica Argentina 67: 41–48. [Google Scholar]
- Rasgado M. A., Malo E. A., Cruz-López L., Rojas J. C., Toledo J. 2009. Olfactory response of the Mexican fruit fly (Diptera: Tephritidae) to Citrus aurantium volatiles. J. Econ. Entomol. 102: 585–594. [DOI] [PubMed] [Google Scholar]
- SAS. 2002. The SAS system for windows, release 9.0. SAS Institute Inc. Cary, N.C. [Google Scholar]
- Schoonhoven L. M., Van Loon J. J. A., Dicke M. 2005. Insect–plant interactions, 2nd ed. Oxford University Press, Oxford. [Google Scholar]
- Scott I. M., Jensen H., Scott J. G., Isman M. B., Arnason J. T., Philogène B. J. R. 2003. Botanical insecticides for controlling agricultural pests: Piperamides and the Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Arch. Insect Biochem. Physiol. 54: 212–225. [DOI] [PubMed] [Google Scholar]
- Simmonds M. S. J., Manlove J. D., Khambay B. P. S. 2002. Effects of selected botanical insecticides on the behavior and mortality of the glasshouse whitefly Trialeurodes vaporariorum and the parasitoid Encarsia formosa. Entomologia Experimentalis et Applicata 102: 39–47. [Google Scholar]
- Sõukand R., Kalle R., Svanberg I. 2010. Uninvited guests: traditional insect repellents in Estonia used against the clothes moth Tineola bisselliella, human flea Pulex irritans and bedbug Cimex lectularius. J. Insect Sci. 10: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines C. L. 1996. The Hispinae (Coleoptera: Chrysomelidae) of Nicaragua. Revista Nicaraguense de Entomología 37: 1–65. [Google Scholar]
- Staines C. L. 2002. The New World tribes and genera of hispines (Coleoptera: Chrysomelidae: Cassidinae). Proc. Entomol. Soc. Wash. 104: 721–784. [Google Scholar]
- Tavares W. S., Cruz I., Petacci F., Assis Júnior S. L., Freitas S., Zanuncio J. C., Serrão J. E. 2009. Potential use of Asteraceae extracts to control Spodoptera frugiperda (Lepidoptera: Noctuidae) and selectivity to their parasitoids Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) and Telenomus remus (Hymenoptera: Scelionidae). Ind. Crop. Prod. 30: 384–388. [Google Scholar]
- Tukey J. W. 1949. Comparing individual means in the analysis of variance. Biometrics 5: 99–114. [PubMed] [Google Scholar]
- Upasani S. M., Kotkar H. M., Mendki P. S., Maheshwari V. L. 2003. Partial characterization and insecticidal properties of Ricinus communis L foliage flavonoids. Pest Manage. Sci. 59: 1349–1354. [DOI] [PubMed] [Google Scholar]
- Vandenborre G., Groten K., Smagghe G., Lannoo N., Baldwin I. T., Van Damme E. J. M. 2010. Nicotiana tabacum agglutinin is active against Lepidopteran pest insects. J. Exp. Bot. 61: 1003–1014. [DOI] [PubMed] [Google Scholar]
- Wei Z., Baggerman G., Nachman R. J., Goldsworthy G., Verhaert P., De Loof A., Schoofs L. 2000. Sulfakinins reduce food intake in the desert locust, Schistocerca gregaria. J. Insect Physiol. 46: 1259–1265. [DOI] [PubMed] [Google Scholar]
- Williams N. H., Whitten W. M. 1983. Orchid floral fragrances and male euglossine bees: methods and advances in the last sesquidecade. Biol. Bull. 164: 355–395. [Google Scholar]
- Won-Sik C., Park B. S., Ku S. K., Lee S. E. 2002. Repellent activities of essential oils and monoterpenes against Culex pipiens pallens. J. Am. Mosquito Control Assoc. 18: 348–351. [PubMed] [Google Scholar]
- XLSTAT. 2004. XLSTAT-Pro v. 7.5, user’s manual. Addinsoft SARL. Addinsoft, UK. [Google Scholar]
- Yeoh C. B., Kuntom A., Dorasamy S., Omar M. R., Nor M. Y. M., Noh M. R. M. 2006. Determination of acephate, methamidophos and monocrotophos in crude palm oil. Eur. J. Lipid Sci. Technol. 108: 960–964. [Google Scholar]